Letter to the Editor
Â
Dr Hartung and Colleagues Reply
To the Editor: Stensland et al raise several methodological considerations related to our article.1 They are concerned that our selection criteria screened out individuals with schizophrenia and that our cutoffs for subtherapeutic dosing were too conservative.
In our evaluation, subjects were selected if they had no history of antipsychotic medication use in the 6 months prior to the index prescription. We noted in the first sentence of the abstract that this selection criterion was designed to focus the analysis on prescribing patterns in a cohort of new users. As Stensland et al suggest, and as we pointed out in the paragraph addressing study limitations, this selection criterion yielded a sample of patients not completely generalizable to individuals with schizophrenia, who tend to switch among antipsychotic medications. However, research has shown that medication switching is also frequent among patients with bipolar disorder, suggesting that this phenomenon is not unique to schizophrenia.2 As we noted in the discussion section, our selection criteria “may have reduced representation of individuals with certain disorders (eg, schizophrenia).”1(p1546) Moreover, we believe that any intervention aimed at improving prescribing practices would be most appropriate and easily implemented for patients new to therapy. Therefore, the focus of our research was on adult Medicaid clients (regardless of diagnosis) who were new to atypical antipsychotic medication. We acknowledge that our sample of subjects may not extrapolate to all patients with schizophrenia. This does not diminish the relevance of our findings to new adult users of atypical antipsychotics in a Medicaid program, who may be of concern to clinicians and policy makers.
Stensland et al also indicate that the definitions of subtherapeutic doses used in our analysis, which were largely based upon the adult prescribing information approved by the US Food and Drug Administration (FDA) and target doses from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, may not reflect randomized controlled trial evidence suggesting that favorable outcomes can be achieved using lower doses. Here, it is important to appreciate that our study pertained to adults (ages 20 through 64 years). For example, Stensland et al note that FDA- approved dosing for risperidone is only 0.5 to 3 mg/d for people with irritability associated with autistic disorder. This indication and dosing, however, pertain only to “children and adolescents aged 5-16 years,”3 which is not applicable to our adult-focused study.
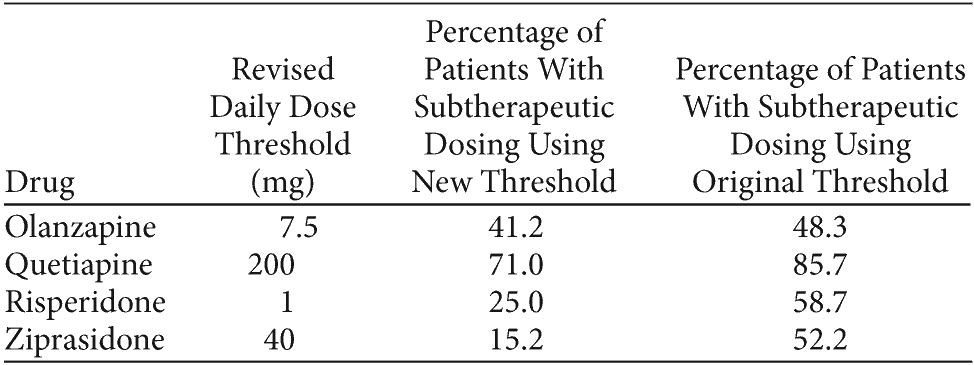
Table 1. Table 1. Percentage of Patients With Subtherapeutic DosingAfter Applying Lower Dose Thresholds
Click figure to enlarge
As we indicate in our discussion section,1 we agree with Stensland et al that the “correct” dose range for a drug depends on the condition being treated. Applying thresholds for treatment of people with schizophrenia to a drug used more broadly without regard to diagnosis could create false positives for subtherapeutic dosing. We also agree with the sentiment that the ranges selected for this evaluation might overestimate the prevalence of subtherapeutic prescribing. However, even allowing for less conservative definitions, the proportion of subjects receiving subtherapeutic doses would still be noteworthy. Table 1 shows the impact of reducing the adult subtherapeutic dose thresholds from those used in our article to very liberal values. Prevalence estimates generated for risperidone and ziprasidone changed the most but still represent nontrivial utilization and a matter of concern. Importantly, even with a very liberal dose threshold, the vast majority of quetiapine users are still in the subtherapeutic group.
We are pleased that Stensland et al are also concerned about subtherapeutic dosing. We agree that defining a precise therapeutic dose for an individual is a great challenge made difficult by disparate and conflicting efficacy data for people with different diagnoses. However, on the basis of our findings, we confirm that subtherapeutic prescribing of these agents is a prevalent phenomenon that needs further exploration.
References
1. Hartung DM, Wisdom JP, Pollack DA, et al. Patterns of atypical antipsychotic sub-therapeutic dosing among Oregon Medicaid patients. J Clin Psychiatry. 2008;69(10):1540-1547. PubMed
2. Garver D, Lazarus A, Rajagopalan K, et al. Racial differences in medication switching and concomitant prescriptions in the treatment of bipolar disorder. Psychiatr Serv. 2006;57(5):666-672. PubMed doi:10.1176/appi.ps.57.5.666
3. Risperdal (risperidone): full prescribing information. Titusville, NJ: Janssen Division of Ortho-McNeil-Janssen Pharmaceuticals; August 2008.
Author affiliations: College of Pharmacy, Oregon State University, Campus of Oregon Health & Science University, Portland (Drs Hartung, Hamer, and Haxby and Mr Middleton); New York State Psychiatric Institute, New York (Dr Wisdom); and Department of Psychiatry, Oregon Health & Science University, Portland (Drs Pollack and McFarland). Financial disclosure: Dr McFarland has received grant/research support from Eli Lilly and Abbott. Drs Hartung, Wisdom, Pollack, Hamer, and Haxby and Mr Middleton report no additional financial or other relationships relevant to the subject of this letter. Funding/support: The study discussed in this letter was funded by grant number R24 MH074912 from the National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland.
doi:10.4088/JCP.08lr04936a
© Copyright 2009 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!