Antipsychotic medication does not always result in the remission of positive symptoms in patients with schizophrenia. Furthermore, in many patients in whom positive symptoms substantially or completely remit, negative symptoms, cognitive disturbances, and other impairments often remain. Antipsychotic augmentation is one of many strategies to which clinicians turn in an attempt to reduce the burden of these residual symptoms, and famotidine is one of many unconventional augmentation agents that has been studied in this context.
Histamine mechanisms may play a role in schizophrenia.1,2 Famotidine is a potent, selective histamine H2 receptor antagonist that poorly penetrates the blood-brain barrier.3,4 Although H2 receptors are present in the brain, there does not seem to be a strong argument made for the involvement of the H2 receptor in schizophrenia; nevertheless, many case reports and small open studies suggest that famotidine may improve outcomes in patients with schizophrenia.
Famotidine Augmentation in Schizophrenia: Case Reports and Open Studies
Interest in the field began serendipitously when Kaminsky et al5 reported a patient who was treated with famotidine monotherapy (40 mg/d) for peptic ulcer disease. This patient also had schizophrenia, and the deficit symptoms that were present improved after the initiation of famotidine, worsened when famotidine was withdrawn, and improved again when famotidine was reintroduced. Rosse et al6 reported that a treatment-resistant patient with chronic undifferentiated schizophrenia also improved dramatically with famotidine (40-100 mg/d) across 10 months of treatment.
In an open-label study,7 famotidine (40 mg/d) was added to the current antipsychotic regimen of 10 schizophrenia and schizoaffective disorder patients who had responded poorly to drugs. There was a small but statistically significant improvement during famotidine treatment relative to before treatment and after treatment withdrawal. In another open-label study, Whiteford et al8 administered famotidine (40 mg/d) to 5 patients with chronic schizophrenia. Only 1 of 4 study completers showed improvement. Famotidine did not influence the levels of the concurrent antipsychotic medications.
In a study9 of 12 treatment-resistant schizophrenia patients, all of whom had significant negative symptoms, famotidine augmentation in the dose of 40 mg/d for 6 weeks or longer resulted in sufficient improvement in negative symptoms for 7 patients to be discharged; benefits were observed within 2-3 weeks of the onset of treatment. Rosenberg et al10 also reported improvement with famotidine (100 mg/d) after just 3 weeks of augmentation therapy in 19 patients with schizophrenia. Rosse et al11 administered famotidine (100 mg/d) to 18 patients with schizophrenia or schizoaffective disorder; ongoing neuroleptic medications were continued. After 3 weeks of treatment, there were significant improvements in negative symptoms and overall psychopathology, as well as global ratings. Dannon et al12 also reported improvement with famotidine augmentation of neuroleptic drugs in 11 schizophrenia patients who received treatment for 4 weeks.
In all reports and studies, famotidine was generally well tolerated. There were, however, a few exceptions. For example, Whiteford et al8 reported that 1 patient had to be withdrawn because of new-onset aggression.
Famotidine Augmentation in Schizophrenia: Randomized Controlled Trials
Four small randomized controlled trials (RCTs) of famotidine augmentation in schizophrenia have been conducted. Abhari and Mohtasham13 randomized 38 chronic schizophrenia patients to receive famotidine (40 mg/d) or placebo for 6 weeks. All patients also received haloperidol (20 mg/d). Positive and Negative Syndrome Scale (PANSS) total scores decreased significantly more with famotidine than with placebo, but there was no significant difference in negative symptom outcomes. The full text of this study could not be evaluated because it was not in English.
In the only study of patients who were not medication-refractory, Poyurovsky et al14 described a 6-week RCT of famotidine (40 mg/d) versus placebo in 14 olanzapine-treated patients with first-episode schizophrenia. Famotidine did not attenuate olanzapine-related weight gain, nor did it influence treatment response to olanzapine on measures of positive symptoms, negative symptoms, or global severity of illness.
Farzin et al15 recruited 30 schizophrenia patients who had not responded to at least 2 previous antipsychotic trials. These patients were randomized to receive famotidine (60 mg/d) or placebo for 6 weeks. All patients also received perphenazine (40 mg/d). At the end of the study, mean PANSS total scores dropped by 49% (from 133 to 68) in famotidine-treated patients and by 22% (from 118 to 92) in placebo patients. The advantage for famotidine was also significant for the PANSS general psychopathology measure and for a measure of aggression. Results for other outcomes were not presented and were presumably not significant.
Famotidine was well tolerated in these RCTs, as far as could be judged from the limited adverse effect data that were provided.
A Recent RCT
A very recent RCT2 received wide publicity in the scientific as well as lay press. The study was a small, 4-week, investigator-initiated trial in which 30 patients with chronic schizophrenia were randomized to receive famotidine (n = 16; 100 mg twice daily) or placebo (n = 14) along with their ongoing antipsychotic medication. The dose of famotidine was carefully chosen on the basis of its known pharmacokinetics, so as to ensure a 50%-80% occupancy of H2 receptors in the brain during most of the day.
All patients had shown unsatisfactory response to previous and current psychotropic medications, and all required assisted living. The mean age of the sample was about 52 years. The sample was 60% female. Some patients were receiving antipsychotic polytherapy, and 11 (37%) were receiving clozapine. The mean PANSS score was 77.6 at baseline. No patient dropped out after randomization.
After 4 weeks of treatment, the PANSS total score decreased significantly more with famotidine than with placebo (by 11% vs 1%, respectively). Improvement in PANSS general psychopathology and Clinical Global Impressions scale scores was also significantly greater with famotidine than with placebo; the advantage for famotidine over placebo was 13% and 11%, respectively. However, improvement in PANSS positive symptom, PANSS negative symptom, and Scale for Assessment of Negative Symptoms scores did not differ significantly between the 2 groups. Famotidine was well tolerated.
The findings of this study suggest that, in chronic, medication-refractory, positive symptom schizophrenia, 4 weeks of treatment with famotidine (200 mg/d) results in a small (10%) but statistically significant improvement in the overall severity of illness.
Synthesis and Critical Appraisal
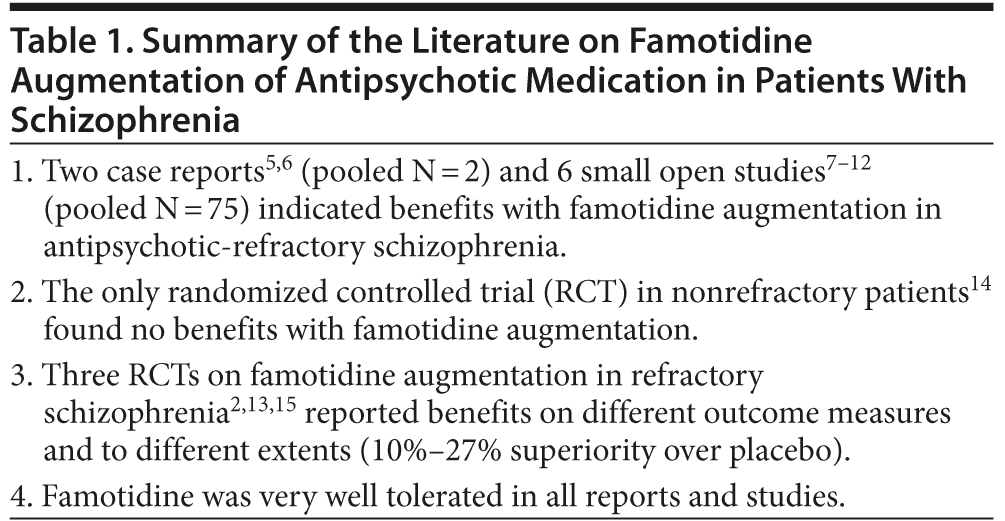
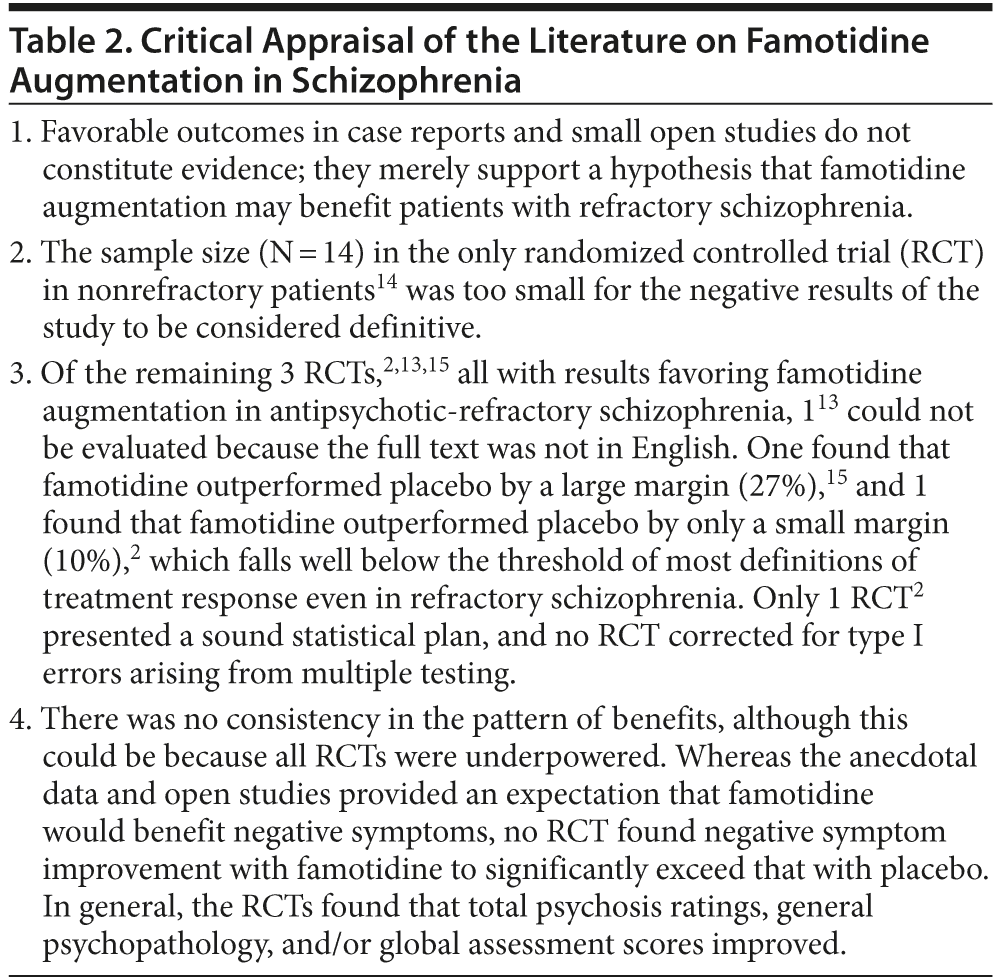
Table 1 provides a synthesis of the literature on famotidine augmentation of antipsychotic drugs in patients with schizophrenia. A critical appraisal of this literature is presented in Table 2.
There are some additional matters to be considered. Authors who have studied famotidine augmentation have speculated that the benefits arise from H2 receptor antagonism. This hypothesis is but natural, given that H2 antagonism is the principal and possibly sole action of the drug. In support of the H2 antagonism hypothesis is the recent finding that clozapine is a potent H2 receptor inverse agonist.16 However, asenapine is a potent H2 receptor antagonist,17 and, unlike clozapine, no especial therapeutic advantage for asenapine has so far been postulated in schizophrenia. Furthermore, tricyclic and nontricyclic antidepressant drugs both block H2 receptors,18 which is why these drugs were found effective in peptic ulcer disease.19,20 Even alprazolam blocks H2 receptors.21 Although these drugs may benefit some patients with schizophrenia, it is generally considered that mechanisms such as monoamine reuptake inhibition or muscarinic receptor blockade (rather than H2 receptor blockade) mediate their therapeutic action.
Further, given that antidepressant drugs cross the blood-brain barrier better than does famotidine, one would expect H2 receptor blockade in the brain to be better with antidepressants than with famotidine (the subject, however, has not been formally studied); if so, given the demonstrated efficacy of antidepressant augmentation in schizophrenia,22 the possible role for famotidine augmentation stands diminished.
About a decade ago, 2 studies23,24 suggested lamotrigine as a possible antipsychotic augmentation agent in atypical antipsychotic- and clozapine-refractory schizophrenia. Whereas benefits were small in both studies, one may argue that in such refractory samples, even small benefits could represent a clinically meaningful reduction in illness burden. Hopes were, however, dashed when 2 large (N = 217 and N = 212), industry-driven RCTs found that lamotrigine augmentation was no better than placebo in schizophrenia patients with stable, residual psychotic symptoms.25 One wonders whether the famotidine experience will follow the lamotrigine story.
However, if famotidine is indeed a safe and effective augmenting agent in antipsychotic-refractory schizophrenia, many questions remain to be answered. No famotidine RCT has extended beyond 6 weeks; do benefits with famotidine increase with continued therapy, or are they at least maintained in the intermediate term? Studies have examined doses as low as 40 mg/d and as high as 200 mg/d; what is the ideal dose of famotidine? Although the efficacy of famotidine for different outcome measures has been inconsistent, might a specific symptom cluster be famotidine-responsive? Is famotidine effective even in patients who are already receiving potent H2 inverse agonists or antagonists such as clozapine and asenapine? What are the long-term safety and efficacy of the drug in schizophrenia?
For the moment, the safest conclusion is that the use of famotidine as an antipsychotic augmentation agent is emphatically experimental.
© Copyright 2013 Physicians Postgraduate Press, Inc.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Arrang JM. Histamine and schizophrenia. Int Rev Neurobiol. 2007;78:247-287. PubMed doi:10.1016/S0074-7742(06)78009-6
2. Meskanen K, Ekelund H, Laitinen J, et al. A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia. J Clin Psychopharmacol. 2013;33(4):472-478. PubMed doi:10.1097/JCP.0b013e3182970490
3. Chremos AN. Clinical pharmacology of famotidine: a summary. J Clin Gastroenterol. 1987;9(suppl 2):7-12. PubMed doi:10.1097/00004836-198707002-00003
4. Langtry HD, Grant SM, Goa KL. Famotidine: an updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1989;38(4):551-590. PubMed doi:10.2165/00003495-198938040-00005
5. Kaminsky R, Moriarty TM, Bodine J, et al. Effect of famotidine on deficit symptoms of schizophrenia. Lancet. 1990;335(8701):1351-1352. PubMed doi:10.1016/0140-6736(90)91237-5
6. Rosse RB, Kendrick K, Tsui LC, et al. Famotidine adjunctive pharmacotherapy of schizophrenia: a case report. Clin Neuropharmacol. 1995;18(4):369-374. PubMed doi:10.1097/00002826-199508000-00009
7. Deutsch SI, Rosse RB, Kendrick KA, et al. Famotidine adjunctive pharmacotherapy for schizophrenia: preliminary data. Clin Neuropharmacol. 1993;16(6):518-524. PubMed doi:10.1097/00002826-199312000-00005
8. Whiteford HA, Stedman TJ, McGrath JJ, et al. An open-label study of famotidine as a treatment for schizophrenia. J Psychiatry Neurosci. 1995;20(3):239-240. PubMed
9. Oyewumi LK, Vollick D, Merskey H, et al. Famotidine as an adjunct treatment of resistant schizophrenia. J Psychiatry Neurosci. 1994;19(2):145-150. PubMed
10. Rosenberg PB, Rosse RB, Johri SK, et al. Smooth pursuit eye movements in the evaluation of famotidine adjunctive therapy of schizophrenia: a preliminary report. Clin Neuropharmacol. 1996;19(3):276-281. PubMed doi:10.1097/00002826-199619030-00011
11. Rosse RB, Kendrick K, Fay-McCarthy M, et al. An open-label study of the therapeutic efficacy of high-dose famotidine adjuvant pharmacotherapy in schizophrenia: preliminary evidence for treatment efficacy. Clin Neuropharmacol. 1996;19(4):341-348. PubMed doi:10.1097/00002826-199619040-00007
12. Dannon P, Lepkifker E, Iancu I, et al. Famotidine: a supplemental drug for the treatment of schizophrenia. Eur Psychiatry. 1997;12(5):263-264. doi:10.1016/S0924-9338(97)83302-0
13. Abhari SAA, Mohtasham S. Clinical trial of H2 blocker: augmentation treatment of schizophrenia. Andeesheh va Raftar. 2000;5(3):10-17.
14. Poyurovsky M, Tal V, Maayan R, et al. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. Eur Neuropsychopharmacol. 2004;14(4):332-336. PubMed doi:10.1016/j.euroneuro.2003.10.004
15. Farzin D, Hosseini SH, Shafaat A. A randomized double blind clinical trial in famotidine adjuvant therapy in schizophrenia. Iran J Med Sci. 2005;30(2):59-62.
16. Humbert-Claude M, Davenas E, Gbahou F, et al. Involvement of histamine receptors in the atypical antipsychotic profile of clozapine: a reassessment in vitro and in vivo. Psychopharmacology (Berl). 2012;220(1):225-241. PubMed doi:10.1007/s00213-011-2471-5
17. Shahid M, Walker GB, Zorn SH, et al. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23(1):65-73. PubMed doi:10.1177/0269881107082944
18. Alvarez FJ, Casas E, Franganillo A, et al. Effects of antidepressants on histamine H2 receptors in rat isolated uterus. J Pharmacol. 1986;17(3):351-354. PubMed
19. Goodman WK, Charney DS. Therapeutic applications and mechanisms of action of monoamine oxidase inhibitor and heterocyclic antidepressant drugs. J Clin Psychiatry. 1985;46(10 pt 2):6-24. PubMed
20. Orsulak PJ, Waller D. Antidepressant drugs: additional clinical uses. J Fam Pract. 1989;28(2):209-216. PubMed
21. Alvarez FJ, Velasco A, Palomares JL. Blockade of muscarinic, histamine H1 and histamine H2 receptors by antidepressants. Pharmacology. 1988;37(4):225-231. PubMed doi:10.1159/000138470
22. Kishi T, Iwata N. Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia [published online ahead of print July 3, 2013]. Int J Neuropsychopharmacol. 2013;1-12. PubMed doi:10.1017/S1461145713000667
23. Tiihonen J, Hallikainen T, Ryynפnen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry. 2003;54(11):1241-1248. PubMed doi:10.1016/S0006-3223(03)00524-9
24. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):441-446. PubMed doi:10.1016/j.biopsych.2004.06.029
25. Goff DC, Keefe R, Citrome L, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin Psychopharmacol. 2007;27(6):582-589. PubMed doi:10.1097/jcp.0b013e31815abf34
J Clin Psychiatry 2013;74(9):e855-e858 (doi:10.4088/JCP.13f08707)