This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: A mild, ongoing inflammatory process may be involved in the pathophysiology of a subgroup schizophrenia. Meta-analyses found add-on anti-inflammatory treatment to be effective in at least early stages of schizophrenia. Immunologically, a blunted type 1 (acute) immune response and shift to the type 2 (chronic) response have been described in schizophrenia before.
Adjunctive Recombinant Human Interferon Gamma-1b for Treatment-Resistant Schizophrenia in 2 Patients
To the Editor: A mild, ongoing inflammatory process may be involved in the pathophysiology of a subgroup schizophrenia.1 Meta-analyses found add-on anti-inflammatory treatment to be effective in at least early stages of schizophrenia.2,3 Immunologically, a blunted type 1 (acute) immune response and shift to the type 2 (chronic) response have been described in schizophrenia before. Serum levels of the proinflammatory type 1 cytokine interferon gamma (IFN-γ) and in vitro IFN-γ production after stimulation were lower in samples from unmedicated schizophrenia patients than in those from healthy controls,4,5 although the findings are in part controversial.6 Therefore, the type 1 response stimulant IFN-γ was hypothesized to have a therapeutic effect. We describe the effects of adjunctive IFN-γ in 2 treatment-resistant schizophrenia inpatients.
Case reports. Mr A, a 47-year-old man with a 26-year history of DSM-IV-defined paranoid schizophrenia, was admitted to the hospital in 2012 because of an exacerbation of paranoid-hallucinatory symptoms. Inpatient antipsychotic treatment for 31 weeks with a combination of clozapine 500 mg/d (mean plasma level = 758 ng/mL), amisulpride 600 mg/d (mean plasma level = 670 ng/mL), and haloperidol 2.5 mg/d had no effect, and a series of 20 electroconvulsive therapy (ECT) treatments yielded only marginal effect.
Mr B, a 36-year-old man with a 13-year history of paranoid schizophrenia (DSM-IV), was admitted to the hospital in 2012 because of an exacerbation of auditory hallucinations and paranoid thinking. Inpatient antipsychotic treatment for 20 weeks with clozapine 700 mg/d (mean plasma level = 655 ng/mL), benperidol 25 mg/d (mean plasma level = 11.8 ng/mL), and prothipendyl 80 mg/d had no effect, and 25 ECT treatments improved symptoms only briefly.
Treatment with IFN-γ-1b was started in these patients, and their antipsychotic combinations were kept nearly constant during IFN-γ-1b therapy. Three injections of 0.5 mL recombinant human IFN-γ-1b were administered subcutaneously every week for 4 weeks to both patients as adjunctive treatment. The IFN-γ-1b dose was then tapered down over 2 and 3 weeks in Mr A and Mr B, respectively.
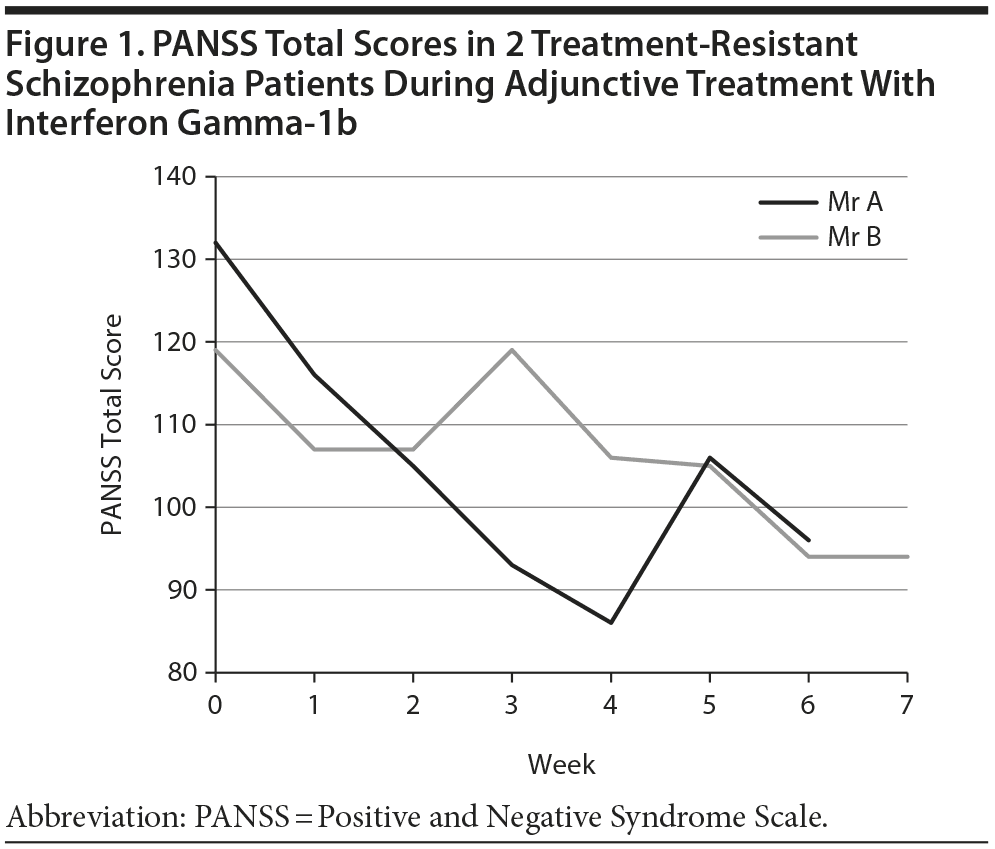
Psychopathology was assessed weekly with the Positive and Negative Syndrome Scale (PANSS).7 Both patients showed a marked clinical improvement: Mr A’s PANSS total score decreased from 119 to 93 after 7 weeks, and Mr B’s decreased from 132 to 96 after 6 weeks (Figure 1). The patients were carefully monitored for side effects, since short-term side effects such as fever, headache, muscle pains, or malaise are often found. In Mr A, a transient increase to double normal liver enzyme values was observed after 2 weeks; values returned to normal in the fourth week. No fever, fatigue, decreased drive, or appetite occurred.
Despite the availability of numerous antipsychotics, the long-term course of schizophrenia is more or less the same as in the pre-neuroleptic era, and new therapeutic approaches are needed. Immune-based therapies—successfully introduced in psychiatry by the Nobel laureate Julius Wagner von Jauregg8 and later forgotten—are a promising new field. Stimulating the blunted part of the immune response and down-regulation of the (up-regulated) immune response with anti-inflammatory medication might be 2 sides of the same coin. For ethical reasons, immune-based therapies currently can be evaluated only as add-on therapies in psychiatry, mostly in treatment-resistant patients. A further limitation is the uncontrolled open-label treatment of these patients. Despite these limitations, the effects are promising, and studies of immune-based approaches are urgently required.
References
1. Müller N, Bechter K. The mild encephalitis concept for psychiatric disorders revisited in the light of current psychoneuroimmunological findings. Neurol Psychiatry Brain Res. 2013;19(3):87-101. doi:10.1016/j.npbr.2013.04.004
2. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39(6):1230-1241. PubMed doi:10.1093/schbul/sbt070
3. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? a meta-analysis. J Clin Psychiatry. 2012;73(4):414-419. PubMed doi:10.4088/JCP.10r06823
4. Rothermundt M, Arolt V, Leadbeater J, et al. Cytokine production in unmedicated and treated schizophrenic patients. Neuroreport. 2000;11(15):3385-3388. PubMed doi:10.1097/00001756-200010200-00024
5. Schwarz MJ, Chiang S, Müller N, et al. T-helper-1 and T-helper-2 responses in psychiatric disorders. Brain Behav Immun. 2001;15(4):340-370. PubMed doi:10.1006/brbi.2001.0647
6. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. PubMed doi:10.1016/j.biopsych.2011.04.013
7. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
8. Wagner von Jauregg J. Fieberbehandlung bei psychosen. Wien Med Wochenschr. 1926;76:79-82.
Author affiliations: Department of Psychiatry and Psychotherapy, Ludwig-Maximilian University, Munich, Germany.
Potential conflicts of interest: Dr. Müller has received grant/research support from Novartis and honoraria from Takeda. The other authors report no potential conflict of interest.
Funding/support: None reported.
Acknowledgment: The authors thank Jacquie Klesing, ELS, for editing assistance. Ms Klesing has no potential conflict of interest to report.
J Clin Psychiatry 2014;75(11):1266-1267 (doi:10.4088/JCP.14l09005).
© Copyright 2014 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!