Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: A single subanesthetic intravenous (IV) dose of ketamine leads to rapid antiobsessional effects in obsessive-compulsive disorder (OCD) patients with near-constant intrusive obsessions, but these effects usually do not persist. We tested whether a brief course of exposure-based cognitive-behavioral therapy (CBT) could extend ketamine’s effects in a 2-week pilot open trial and if this effect was maintained (without additional treatment) 2 weeks later. Our rationale was (1) ketamine is reported to enhance plasticity and extinction learning in rodents, and (2) enhanced extinction learning may facilitate CBT gains, as reported in trials that combined CBT with medication thought to facilitate extinction learning (eg, D-cycloserine).
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Can Exposure-Based CBT Extend the Effects of Intravenous Ketamine in Obsessive-Compulsive Disorder? An Open-Label Trial
To the Editor: A single subanesthetic intravenous (IV) dose of ketamine leads to rapid antiobsessional effects in obsessive-compulsive disorder (OCD) patients with near-constant intrusive obsessions,1 but these effects usually do not persist.1-3 We tested whether a brief course of exposure-based cognitive-behavioral therapy (CBT) could extend ketamine’s effects in a 2-week pilot open trial and if this effect was maintained (without additional treatment) 2 weeks later. Our rationale was (1) ketamine is reported to enhance plasticity and extinction learning in rodents,4-6 and (2) enhanced extinction learning may facilitate CBT gains, as reported in trials that combined CBT with medication thought to facilitate extinction learning (eg, d-cycloserine).7,8 Mimicking those trials, our study design included CBT that was abbreviated (ie, 10 one-hour exposure sessions) but delivered during the putative time interval when ketamine facilitates extinction learning (within 14 days).4
Methods. With institutional review board approval, 10 unmedicated OCD outpatients (ages 18-55 years) with near-constant intrusive obsessions (> 8 hours/d) were recruited (March 2014-March 2015). They provided written informed consent. Participants met DSM-IV and DSM-5 criteria for OCD with at least moderate symptoms (Yale-Brown Obsessive Compulsive Scale [YBOCS]9,10 score ≥ 16). Exclusion criteria included severe depression (Hamilton Depression Rating Scale [HDRS]11 > 25), current CBT, and comorbid psychiatric or medical conditions that made participation unsafe.
In an open-label design, participants received a single 40-minute IV infusion of ketamine (dose = 0.5 mg/kg), followed by 10 one-hour exposure sessions delivered over 2 weeks. The CBT treatment was planned in a 90-minute session the day before the ketamine infusion. All CBT sessions were administered by the same therapist (M.W.) and followed standard procedures.12
At baseline, during the infusion, and at 20, 90, 110, and 230 minutes postinfusion, patients rated their obsessional severity using the OCD Visual Analog Scale (OCD-VAS).1,13-15 We focused on obsessions because the patients were supine and connected to stationary monitoring equipment during the infusion. At baseline and weekly for 4 weeks postketamine, an independent evaluator, blind to study design, evaluated patients using the YBOCS (primary outcome measure), which appraises obsessive and compulsive symptoms over the prior week. Treatment response was defined a priori as ≥ 35% YBOCS reduction at week 2.16 YBOCS outcomes were analyzed using mixed-effects regression to model symptoms as a function of time.
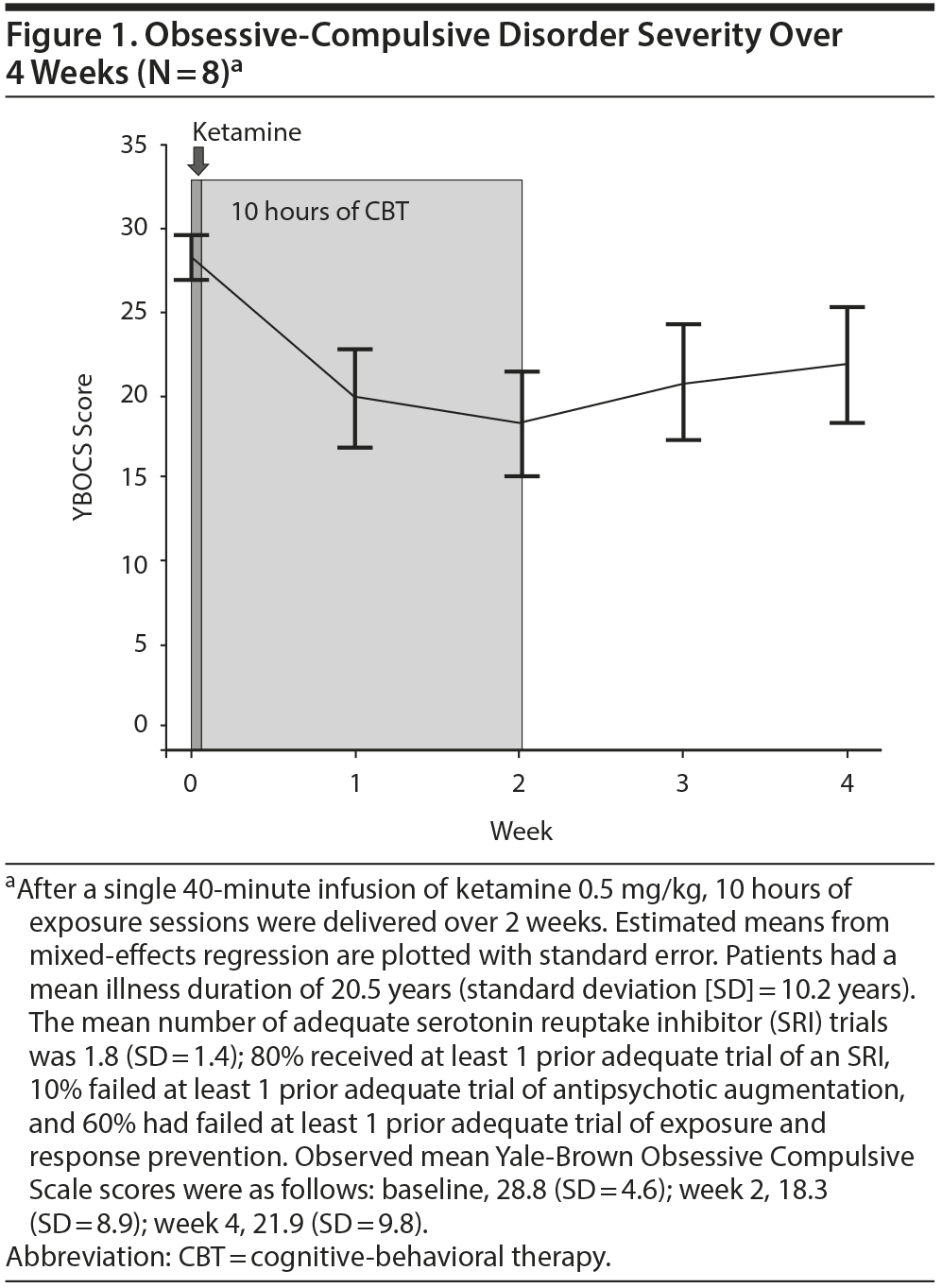
Results. Of the 10 patients who started ketamine, 9 completed the infusion. Eight reported a rapid reduction in obsessive severity as measured by the OCD-VAS, which persisted up to 230 minutes post-infusion in 7 patients; all 8 completed the 10 hours of exposure and the 2-week follow-up and were included in the YBOCS analyses. From baseline to 4 weeks postinfusion, OCD severity, as measured by the YBOCS, was significantly decreased over time (F4,28 = 14.36, P < .0001; Figure 1). Compared to baseline, the mean estimated YBOCS score was significantly lower at week 2 (difference = −10.75 points, SE = 1.44, P < .0001) and at week 4 (difference = −6.88, SE = 2.61, P = .01); there was a trend-level increase between weeks 2 and 4 (difference = 3.63, SE = 1.97, P = .07). At the end of CBT (week 2), 63% of patients demonstrated treatment response (≥ 35% YBOCS reduction). Importantly, individuals varied in their response, with 1 subject having no benefit, the majority benefitting for up to 2 weeks, and 1 no longer meeting criteria for OCD (ie, achieving minimal symptoms postinfusion that persisted throughout the CBT and up to 6 months in naturalistic follow-up).
Discussion. These results corroborate prior findings1,2 that IV ketamine can rapidly reduce obsessions in unmedicated OCD patients and advance the growing literature on enhancing CBT with agents that facilitate extinction learning.7,8,17 Limitations typical of an open-label trial include lack of randomization to a comparison group, which may lead to allocation and ascertainment (response) bias. The data suggest that a brief course of CBT may help some individuals maintain the improvement they experienced from ketamine; however, this needs to be formally tested in a randomized controlled trial to determine whether the improvement seen after 2 weeks of CBT is due to the addition of CBT, or whether the effects of ketamine persist longer in some than previously described.
References
1. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483. PubMed doi:10.1038/npp.2013.150
2. Rodriguez CI, Kegeles LS, Flood P, et al. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(4):567-569. PubMed doi:10.4088/JCP.10l06653
3. Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964-970. PubMed
4. Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31(4):291-296. PubMed doi:10.1002/da.22227
5. Liu RJ, Lee FS, Li XY, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996-1005. PubMed doi:10.1016/j.biopsych.2011.09.030
6. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649-8654. PubMed doi:10.1073/pnas.1323920111
7. Craske MG, Treanor M, Conway CC, et al. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther. 2014;58:10-23. PubMed doi:10.1016/j.brat.2014.04.006
8. Hofmann SG. D-cycloserine for treating anxiety disorders: making good exposures better and bad exposures worse. Depress Anxiety. 2014;31(3):175-177. PubMed doi:10.1002/da.22257
9. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed doi:10.1001/archpsyc.1989.01810110048007
10. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry. 1989;46(11):1012-1016. PubMed doi:10.1001/archpsyc.1989.01810110054008
11. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
12. Foa EB, Yadin E, Lichner TK. Exposure and Response (Ritual) Prevention for Obsessive Compulsive Disorder: Therapist Guide. New York, NY: Oxford University Press; 2012. doi:10.1093/med:psych/9780195335286.001.0001
13. Murphy DL, Mueller EA, Hill JL, et al. Comparative anxiogenic, neuroendocrine, and other physiologic effects of m-chlorophenylpiperazine given intravenously or orally to healthy volunteers. Psychopharmacology (Berl). 1989;98(2):275-282. PubMed doi:10.1007/BF00444705
14. Greenberg BD, Benjamin J, Martin JD, et al. Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl). 1998;140(4):434-444. PubMed doi:10.1007/s002130050787
15. Abrantes AM, Strong DR, Cohn A, et al. Acute changes in obsessions and compulsions following moderate-intensity aerobic exercise among patients with obsessive-compulsive disorder. J Anxiety Disord. 2009;23(7):923-927. PubMed doi:10.1016/j.janxdis.2009.06.008
16. Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown Obsessive Compulsive Scale. J Clin Psychiatry. 2005;66(12):1549-1557. PubMed doi:10.4088/JCP.v66n1209
17. Grassi G, Godini L, Grippo A, et al. Enhancing cognitive-behavioral therapy with repetitive transcranial magnetic stimulation in refractory obsessive-compulsive-disorder: a case report. Brain Stimulat. 2015;8(1):160-161. PubMed doi:10.1016/j.brs.2014.10.007
aDepartment of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California
bVeterans Affairs Palo Alto Health Care System, Palo Alto, California
cNew York State Psychiatric Institute, New York, New York
dDepartments of Psychiatry and eBiostatistics, College of Physicians and Surgeons, Columbia University, New York, New York
Potential conflicts of interest: Drs Rodriguez, Wheaton, Steinman, and Galfalvy and Mss Zwerling and Sonnenfeld report no additional financial or other relationships relevant to the subject of this letter. Dr Simpson has received royalties from Cambridge University Press and UpToDate, Inc.
Funding/support: This study was supported by the National Institute of Mental Health (K23MH092434 [Dr Rodriguez], K24MH09155 [Dr Simpson]), the Black Family Foundation, and the New York State Psychiatric Institute.
Role of the sponsor: The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, or preparation or final approval of the manuscript prior to publication.
Acknowledgment: The authors thank the individuals who generously donated their time to participate in this research study. They also thank Roberto Lewis-Fernández, MD, and John C. Markowitz, MD, for helpful comments on the manuscript. Drs Lewis-Fernández and Markowitz report no potential conflict of interest related to the subject of this letter.
Trial registration: ClinicalTrials.gov identifier: NCT02062658
J Clin Psychiatry 2016;77(3):408-409
dx.doi.org/10.4088/JCP.15l10138
© Copyright 2016 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!