Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Cepeda et al reported lower levels of (fractional exhaled) nitric oxide (NO) and higher levels of C-reactive protein (CRP) in 1,325 major depressive disorder (MDD) patients in comparison with 12,951 nondepressed subjects. The authors of this remarkable study considered their finding "paradoxical" because both CRP and NO are markers of chronic low-grade inflammation associated with MDD. However, their very important finding might be expected according to the interferon-γ (IFNG)-inducible kynurenines/pteridines inflammation cascade hypothesis.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
See article by Cepeda et al
Low Nitric Oxide and High C-Reactive Protein in Depression: Not a Paradox!
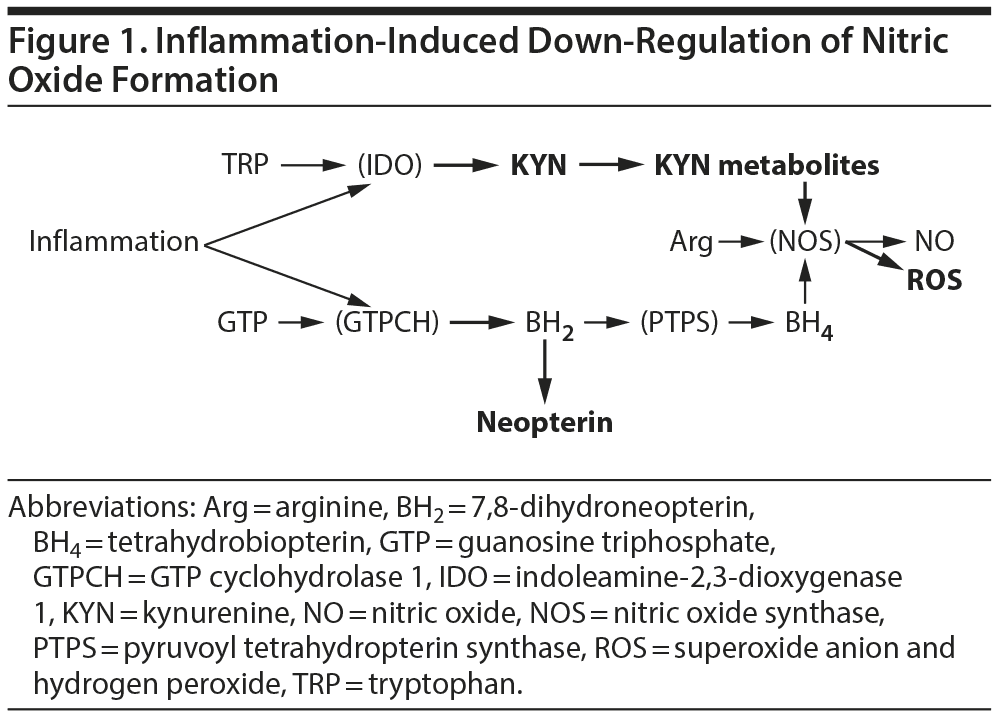
To the Editor: Cepeda et al1 reported lower levels of (fractional exhaled) nitric oxide (NO) and higher levels of C-reactive protein (CRP) in 1,325 major depressive disorder (MDD) patients in comparison with 12,951 nondepressed subjects. The authors of this remarkable study considered their finding "paradoxical" because both CRP and NO are markers of chronic low-grade inflammation associated with MDD. However, their very important finding might be expected according to the interferon-γ (IFNG)-inducible kynurenines/pteridines inflammation cascade hypothesis.2 Inflammatory factors such as IFNG, a key proinflammatory cytokine, activate indoleamine-2,3-dioxygenase 1, a first and rate-limiting enzyme of tryptophan-kynurenine metabolism, catalyzing formation of kynurenine from tryptophan. Concurrently, IFNG activates guanosine triphosphate (GTP) cyclohydrolase 1 (GTPCH), a first and rate-limiting enzyme of pteridine metabolism, catalyzing formation of 7,8-dihydroneopterin (BH2) from GTP (Figure 1).
In humans, IFNG-induced stimulation of GTPCH does not result in correspondent up-regulation of pyruvoyl tetrahydropterin synthase (PTPS), catalyzing BH2 conversion into tetrahydrobiopterin (BH4). Hence, PTPS becomes the rate-limiting enzyme with consequent accumulation of BH2 and its stable metabolite, neopterin.3 Therefore, the enhanced production of neopterin occurs at the expense of BH4 formation.4 Downstream kynurenine metabolites are able to activate NOS, thus increasing demand for BH4.5,6 Up-regulation of nitric oxide synthase (NOS) activity (induced by kynurenine downstream metabolites) and decreased formation of the NOS mandatory cofactor, BH4, result in uncoupling of NOS and shifting of arginine metabolism from formation of NO to production of reactive oxygen species (eg, superoxide anion and hydrogen peroxide).7,8
Although systemic inflammation is, by and large, associated with elevated production of both CRP and neopterin, it should be considered that neopterin and kynurenine are produced by monocyte-derived macrophages, dendritic cells, and astrocytes, while CRP is produced by liver.9 Up-regulation of neopterin formation is, therefore, more specific than CRP as a marker of inflammation (eg, IFNG)-induced activation of tryptophan-kynurenine metabolism. Increased formation of kynurenines from tryptophan at the expense of serotonin biosynthesis10 is one of the major factors contributing to mechanisms of depression in predisposed individuals.11
Dr Cepeda was shown this letter and declined to reply.
References
1. Cepeda MS, Stang P, Makadia R. Depression is associated with high levels of C-reactive protein and low levels of fractional exhaled nitric oxide: results from the 2007-2012 National Health and Nutrition Examination Surveys. J Clin Psychiatry. 2016;77(12):1666-1671. PubMed doi:10.4088/JCP.15m10267
2. Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J Neural Transm (Vienna). 2011;118(1):75-85. PubMed doi:10.1007/s00702-010-0475-7
3. Schoedon G, Troppmair J, Adolf G, et al. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986;6(6):697-703. PubMed doi:10.1089/jir.1986.6.697
4. Fuchs D, Avanzas P, Arroyo-Espliguero R, et al. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem. 2009;16(35):4644-4653. PubMed doi:10.2174/092986709789878247
5. Melillo G, Cox GW, Biragyn A, et al. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem. 1994;269(11):8128-8133. PubMed
6. Pérez-Severiano F, Escalante B, R×os C. Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem Res. 1998;23(10):1297-1302. PubMed doi:10.1023/A:1020700401678
7. Pou S, Pou WS, Bredt DS, et al. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267(34):24173-24176. PubMed
8. Oxenkrug G. Interferon-gamma-inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging Dis. 2011;2(6):474-486. PubMed
9. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805-1812. PubMed doi:10.1172/JCI18921
10. Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1(7586):132-136. PubMed doi:10.1016/S0140-6736(69)91140-4
11. Oxenkrug G. Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets. 2013;14(5):514-521. PubMed doi:10.2174/1389450111314050002
aPsychiatry and Inflammation Program, Department of Psychiatry, Tufts University School of Medicine/Tufts Medical Center, Boston, Massachusetts
Potential conflicts of interest: None.
Funding/support: None.
J Clin Psychiatry 2017;78(6):732
https://doi.org/10.4088/JCP.17lr11532
© Copyright 2017 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!