The International Society of Psychiatric Genetics (ISPG) created a Residency Education Committee with the purpose of identifying key genetic knowledge that should be taught in psychiatric training programs. Thirteen committee members were appointed by the ISPG Board of Directors, based on varied training, expertise, gender, and national origin. The Committee has met quarterly for the past 2 years, with periodic reports to the Board and to the members of the Society. The information summarized includes the existing literature in the field of psychiatric genetics and the output of ongoing large genomics consortia. An outline of clinically relevant areas of genetic knowledge was developed, circulated, and approved. This document was expanded and annotated with appropriate references, and the manuscript was developed. Specific information regarding the contribution of common and rare genetic variants to major psychiatric disorders and treatment response is now available. Current challenges include the following: (1) Genetic testing is recommended in the evaluation of autism and intellectual disability, but its use is limited in current clinical practice. (2) Commercial pharmacogenomic testing is widely available, but its utility has not yet been clearly established. (3) Other methods, such as whole exome and whole genome sequencing, will soon be clinically applicable. The need for informed genetic counseling in psychiatry is greater than ever before, knowledge in the field is rapidly growing, and genetic education should become an integral part of psychiatric training.
See letter by de leon and reply by Nurnberger et al
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

What Should a Psychiatrist Know About Genetics?
Review and Recommendations From the Residency Education Committee of the International Society of Psychiatric Genetics

ABSTRACT
The International Society of Psychiatric Genetics (ISPG) created a Residency Education Committee with the purpose of identifying key genetic knowledge that should be taught in psychiatric training programs. Thirteen committee members were appointed by the ISPG Board of Directors, based on varied training, expertise, gender, and national origin. The Committee has met quarterly for the past 2 years, with periodic reports to the Board and to the members of the Society. The information summarized includes the existing literature in the field of psychiatric genetics and the output of ongoing large genomics consortia. An outline of clinically relevant areas of genetic knowledge was developed, circulated, and approved. This document was expanded and annotated with appropriate references, and the manuscript was developed. Specific information regarding the contribution of common and rare genetic variants to major psychiatric disorders and treatment response is now available. Current challenges include the following: (1) Genetic testing is recommended in the evaluation of autism and intellectual disability, but its use is limited in current clinical practice. (2) Commercial pharmacogenomic testing is widely available, but its utility has not yet been clearly established. (3) Other methods, such as whole exome and whole genome sequencing, will soon be clinically applicable. The need for informed genetic counseling in psychiatry is greater than ever before, knowledge in the field is rapidly growing, and genetic education should become an integral part of psychiatric training.
J Clin Psychiatry 2019;80(1):17nr12046
To cite: Nurnberger JI Jr, Austin J, Berrettini WH, et al. What should a psychiatrist know about genetics? review and recommendations from the Residency Education Committee of the International Society of Psychiatric Genetics. J Clin Psychiatry. 2019;80(1):17nr12046.
To share: https://doi.org/10.4088/JCP.17nr12046
© Copyright 2018 Physicians Postgraduate Press, Inc.
aIndiana University School of Medicine, Indianapolis, Indiana
bUniversity of British Columbia, Vancouver, British Columbia, Canada
cUniversity of Pennsylvania School of Medicine, Philadelphia, Pennsylvania
dUniversity of California Los Angeles Semel Institute of Neuroscience and Human Behavior, Los Angeles, California
eVA Boston Healthcare System and Department of Psychiatry, Harvard Medical School, Boston, Massachusetts
fMt Sinai School of Medicine, New York, New York
gCentre for Addiction and Mental Health and University of Toronto, Toronto, Ontario, Canada
hWarren Alpert Medical School of Brown University, Providence, Rhode Island
iJohns Hopkins University School of Medicine, Baltimore, Maryland
jYale University School of Medicine, Hartford, Connecticut
kInstitute of Psychiatric Phenomics and Genomics, University Hospital, LMU Munich, Munich, Germany
*Corresponding author: John I. Nurnberger Jr, MD, PhD, 320 W 15th St, Indianapolis, IN 46202 ([email protected]).
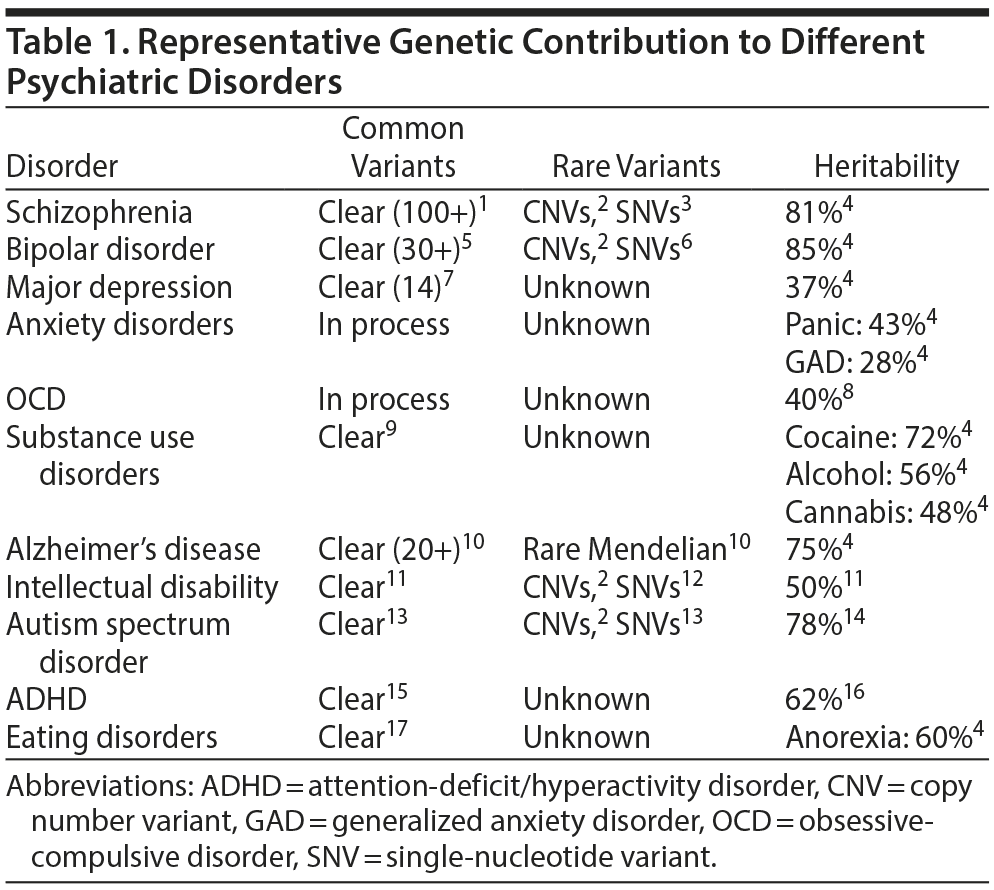
Many of the major psychiatric disorders have a high heritability, reflecting a strong genetic component. The genetic architecture of these disorders is becoming clearer (Table 1).1-17
Psychiatrists are already confronted with questions about genetics in their daily work. Patients want to know the risks of passing on illness to offspring. They may ask about genetic testing for pharmacologic treatment selection. Sometimes they will bring such information to their clinicians from the internet or from DNA test results.
Psychiatrists may be uncomfortable responding, since formal genetics training from college or medical school may be poorly remembered and no longer current.18 Most residents have less than 3 hours of genetics training during the residency itself.19 Residency training programs frequently do not include faculty with genetics expertise. Some psychiatrists and trainees may erroneously believe that genetics is peripheral to the understanding and treatment of psychiatric illness.20 A related issue is the limited time for didactics in residency programs and the need for prioritization of subspecialty topics.
The Accreditation Council for Graduate Medical Education (ACGME) indicates that “biological, genetic, psychological, sociocultural, economic, ethnic, gender, religious/spiritual, sexual orientation, and family factors that significantly influence physical and psychological development throughout the life cycle” should be part of the residency training experience.21 The relative emphasis for these factors differs substantially depending on the needs and resources of the individual institution. The American Board of Psychiatry and Neurology (ABPN) includes genetics in its content specifications22; however, in practice, the examinations may not reflect current knowledge and progress in psychiatric genetics. We would encourage both the ACGME and the ABPN to increase emphasis on neuroscience and genetics in their guidance to the field. Since these institutions are critical to the future development of psychiatric training and trainees, we would encourage academic psychiatrists to become involved in these organizations and to encourage constructive change.
The need for knowledge of genetics in psychiatric practice is likely to increase over time. The work done by the Psychiatric Genomics Consortium and other groups has given rise to many different gene associations with psychiatric disorders and associated brain and behavioral phenotypes. Sequencing consortiums are now established for many disorders as well. New analytic methods, such as the polygenic risk score and pathway analysis, are increasingly applied in research and may eventually become useful clinically. Pharmacogenomic tests provide the basis for precision medicine in psychiatry and, with careful development through clinical trials, should improve patient care.
Major developments in psychiatric genetics affecting clinical practice may be expected within the next 20 years, the period during which today’s residents will be professionally active (Table 2). There has been an acceleration of genetic findings relevant to psychiatric illness over the past 10 years, and previous timelines no longer apply.23 Changes in clinical practice based on genetic findings may be expected to occur from one year to the next. Patients will increasingly expect understanding of new advances in psychiatric genetics and appropriate application by their clinicians. An up-to-date psychiatric genetics curriculum will be required to enable psychiatrists in training to respond to the challenges posed by current and emerging findings in genomics.
In 2015, the International Society of Psychiatric Genetics (ISPG) established a Residency Education Committee. The committee members had general expertise in psychiatric genetics, as well as specific expertise in related fields. The manuscript for this article was developed during 2015-2017 based on review of the literature and a series of discussions among the 13 committee members in person, on quarterly conference calls, and in periodic e-mail exchanges. We were also attentive to the findings of ongoing consortia and consulted regularly with the members of the Society and the ISPG Board of Directors. Notably, the committee has coordinated efforts with the Inter-Society Coordinating Committee for Practitioner Education in Genomics24-26 and the National Neuroscience Curriculum Initiative (NNCI),27-31 a National Institute of Mental Health-funded program that interacts with the American Association of Directors of Residency Training in Psychiatry. This effort has resulted in a set of resources that are specifically intended to enhance genetics training (publicly available on the NNCI website: www.nncionline.org; Supplementary Table 1). Other online resources are available through the National Human Genome Research Institute-maintained website https://genomicseducation.net.
This article describes the rationale, methods, goals, and recommendations for the incorporation of genetics into psychiatric training. Points that are important for current clinical practice are underlined.

- Psychiatric genetics knowledge and its application are not well covered in current residency programs.
- Residents should consider genetic testing as a standard part of the diagnostic workup for patients with autism spectrum disorders or intellectual disability.
- Pharmacogenomic testing is not generally indicated but may be helpful in certain situations (eg, carbamazepine, citalopram).
- Direct-to-consumer testing results may be misleading.
BASIC PRINCIPLES OF MEDICAL GENETICS
Every physician should have a basic grounding in the process by which genetic information is transmitted between generations, the cell cycle in which germ cells are created and recombine, and the relationship between genetic distance on a chromosome and statistical association due to linkage. What has become more critical in recent years is the application of these principles in methods such as genome-wide association studies (GWAS) and sequencing studies. New knowledge of multiple types of RNA and the role of transcription factors32 is also important for the physician who wishes to keep up with the genetic literature.
FAMILY STUDIES AND HERITABILITY
The evidence for heritability of the major psychiatric disorders comes from the collection of family, twin, and adoption studies, extending throughout the 20th century. This evidence is substantial for schizophrenia, bipolar disorder, major depression, alcoholism, antisocial personality disorder, and autism spectrum disorders, among other conditions. These data are summarized in many standard psychiatric texts (eg, The Medical Basis of Psychiatry33). Heritability is classically calculated as the comparison of monozygotic to dizygotic twin concordance rates, although it can be estimated from comparing other family relationships as well. Also important is the concept of relative risk (quantified by lambda), that is, the increased vulnerability of some individuals with a specific risk factor for a disorder compared with the general population. The lambda for the relationship between risk in first-degree relatives of a proband and individuals in the general population34 for schizophrenia is about 10 and for bipolar I disorder, 8-10.
Empirical information regarding lifetime risk for illness in relatives (morbid risk) gives a basis for estimates of likelihood of illness. This information may be useful to provide when questions arise about risk to an individual or to his/her family members. For most complex diseases, including major psychiatric disorders, empirical risk figures33 still form the best basis for deriving risk estimates for families.
COMMON GENETIC VARIANTS
Common gene variants (those with a prevalence of 1% or greater in the general population) play a large role in psychiatric disorders. Common variants are likely to have quite small effects (relative risk of ~1.1, or an increase in risk of 10% for carriers), whereas rare variants may have larger effects. The effects of common variants may be combined, and it is likely that the major psychiatric disorders result from the combination of variants in hundreds or even thousands of genes.
The polygenicity of psychiatric disorders has certain implications. One is that the variants that influence illness may be expected to be widely distributed in the general population. It is only when the number and effect size of these variants exceed a threshold that a subject will be affected. Another is that the genetic profile of any affected person may be expected to be different from the genetic profile of most other affected individuals. That is, genetic heterogeneity will be very substantial.
Common variant effects may be detected using a GWAS, which tests such effects at markers spaced across the entire genome. Current platforms for GWAS include thousands to millions of individual single-nucleotide polymorphism markers. Each marker is tested for variant frequency differences between cases and controls. Such analyses must be corrected for multiple testing. The currently accepted significance threshold for GWAS is 5 ×— 10−8, and this threshold is generally reached only by using very large sample sizes. A recent schizophrenia GWAS1 included more than 30,000 cases and resulted in the identification of more than 100 independent genetic loci, which are now being analyzed for clues to pathophysiology.
One may also use GWAS data to improve our understanding of psychiatric disorders in additional ways. Common variant heritability of disorders may be understood quantitatively by genome-wide complex trait analysis.35 This method has demonstrated substantial common variant heritability for multiple disorders,15 along with surprising demonstrations of coheritability (eg, a 70% overlap in common genetic vulnerability markers between bipolar disorder and schizophrenia). Other methods now becoming useful in research, and with the potential for clinical utility, include polygenic risk scores,36 which combine the effects of multiple loci in a single estimate of risk, and pathway analyses, which combine effects of multiple loci to understand pathophysiology. Recent data suggest emerging clinical utility for polygenic risk scores.37,38
RARE GENETIC VARIANTS
Rare variants are, by definition, present in < 1% of the population. While the vast majority of rare variants will have small effect, some rare variants have large effects on vulnerability. Some of the most prominent findings involve copy number variants (CNVs), small deletions or duplications of genetic material detectable with molecular techniques. Some, such as the 22q11 deletion, are associated with major psychiatric disorder.39 Chromosomal microarray (CMA) analyses can detect many of these CNVs. The CMA technique (along with fragile X testing) is now widely recommended as a first-line evaluation in the assessment and treatment of autism spectrum disorders.40-44
Rare single-nucleotide variants (SNVs) have also been associated with autism, schizophrenia, and bipolar disorder. Unlike CNVs, SNVs require sequencing to be detected. Sequencing can be targeted to a specific gene, to all the coding portions of the genome (whole exome), or to the entire coding and non-coding parts of the genome (whole genome). These techniques have now become an important diagnostic tool in the evaluation of neurodevelopmental disorders, and they may eventually take the place of microarrays. Both CNVs and SNVs may be inherited from a parent or they may occur de novo (ie, not present in either parent).
Rare variants appear to be implicated in 10%-30% of cases of autism spectrum disorder and up to 3%-5% of cases of bipolar illness and schizophrenia.39 When present, they may be associated with specific medical syndromes.13 If present in the parents, they also may be associated with an increased risk for illness in siblings. Psychiatrists should be aware of the substantial risk that some rare variants confer, when and how to test for them, and the implications of these variants on clinical management of psychiatric conditions.
Epigenetics/Gene Expression
Although the constitutional genome sequence tends to be quite stable, gene expression may change with age, gender, diet, season, time of day, drug and medication use, and exposure to a wide variety of environmental stimuli. Epigenetic events include methylation of genes, modifications of the histone proteins that interact with genes, and other chemical changes influencing gene expression; these effects may persist throughout life and in some cases may even be passed on to the next generation.45 It is possible to measure gene expression in many peripheral tissues including blood and saliva. Blood gene expression appears to parallel brain gene expression for some genes,46 but there are many differences as well.47 We can also observe gene expression changes in cultured cells obtained from the tissue of patients with psychiatric disorders (skin or blood cells). Such cells may offer the opportunity for in vitro models of psychiatric disorders.48
Psychiatrists should be aware of the difference between genetic variation and epigenetic changes, understand the relationship of such changes to gene expression, and be able to evaluate potential biomarker studies employing gene expression. An understanding of epigenetics can be a very helpful tool in counseling patients about the interaction of genetics and environment in the risk of developing a psychiatric disorder.
Pharmacogenetics/ Pharmacogenomics
Both therapeutic response and adverse side effects of pharmacologic treatments are modulated by genetic factors. The US Food and Drug Administration (FDA) recommends that patients of Asian descent be screened for specific human leukocyte antigen variants that put them at elevated risk of developing Stevens-Johnson syndrome when taking carbamazepine.49 Genetic screening for those at high risk for clozapine-induced agranulocytosis may soon be possible.
The cytochrome P450 (CYP) enzyme system influences metabolism of psychotropic medications including antidepressants and antipsychotics. Several companies now market tests that utilize multiple genetic markers reflecting the status of these metabolic enzymes. Academic laboratories and genetic testing companies have designed proprietary algorithms to predict the combined effect of these variants on drug response and side effects.50 The efficacy of these pharmacogenomic profiles requires further investigation in controlled studies. However, several hundred thousand tests have already been performed commercially, and the costs may be covered by third-party payers. Two specific enzymes have figured in FDA warnings. Patients who are slow metabolizers based on CYP2C19 should not receive doses of citalopram greater than 20 mg daily.51 There is a similar warning for doses of vortioxetine greater than 10 mg for slow metabolizers based on CYP2D6.52
The quality and utility of pharmacogenomic tests should increase over time. Psychiatrists in training will need to know how to consider whether these products are a useful guide to the management of their patients. They will need to respond to patients who may request such genetic testing or bring results of pharmacogenetic tests to their appointments. The NNCI recently released an educational module that highlights a commentary on this topic.53
ETHICAL AND SOCIAL ISSUES
During the early 20th century, observations of the familial nature of behavioral disorders led some scientists and social theorists to develop the now discredited eugenics movement. Eugenics was a misguided attempt to implement social control over human reproduction. People with a variety of psychiatric and developmental conditions were encouraged to undergo voluntary sterilization in the United States and Europe.54 Under the Nazis in the 1930s and 1940s,55,56 eugenics was used to justify programs of euthanasia in psychiatric hospitals.
There are still many misconceptions about genetic factors in medical disorders. Patients may be concerned that “If it’s genetic, my children will have it” or “If it’s genetic, it can’ t be treated.” There may be reasonable concern about discrimination based on genetic information.
Social use of individual genetic information is fraught with ethical and political problems. African-Americans have been particularly sensitized to issues of genetic stigma since restrictive regulations have been applied to (mostly African-American) carriers of sickle cell trait (HbS).57,58 More generally, screening for the XYY karyotype was carried out among newborns in Boston (1968-1975), following data indicating an association between XYY and antisocial personality disorder. The program was stopped because of justifiable concern regarding stigmatization and inadequate consent procedures.59
Misconceptions about such use of genetic data need to be addressed. Our trainees require the knowledge necessary to approach such issues with sensitivity to the ethical issues involved.
PRINCIPLES OF RISK COMMUNICATION
Addressing families’ concerns about risks for psychiatric disorders still currently relies heavily on empirical risk figures derived from family studies; efforts should be made to adapt the data to the unique family situation.60,61 Psychiatrists should be able to talk with patients and families about the hereditary nature of many psychiatric disorders and how this varies in the setting of a given genetic variant. They should be comfortable with explaining probabilistic concepts of inheritance. In the area of autism and intellectual disability, they will increasingly be expected to refer patients for genetic testing and to help them understand the results of such tests.
There are psychological issues inherent in the reception and interpretation of genetic data. Patients may find knowledge regarding a genetic condition in their family either liberating or burdensome. Self-concept may be affected. Decisions about childbearing are intensely personal and emotional. Guilt, shame, fear, and stigma are often attached to people’s explanations for cause of illness.62 Although addressing emotions such as these lies squarely within the domain of expertise of psychiatrists, genetic counselors are also uniquely equipped to address these challenges,63 and thus collaboration is important. Formal exposure to genetic counselors and to their work with patients and families dealing with neuropsychiatric syndromes would be an excellent training experience for psychiatric residents.
Key points from the text for psychiatric clinicians are summarized in Table 2. A glossary of commonly used genetic terms is available from the National Human Genome Research Institute (https://www.genome.gov/glossary).
CONCLUSIONS
Every psychiatrist should know the basic principles of genetics, the role of genes in psychiatric disorders and their treatment, the ways in which environment affects gene expression, ethical issues in the use of genetic information, and how to talk to patients and families about genetics. These should no longer be thought of as optional or special-interest areas in psychiatric training. They are essential to current psychiatric patient care. We face multiple challenges in meeting these essential training goals. There is a paucity of faculty with the necessary expertise in many departments of psychiatry; there are severe time limitations for didactic sessions in residency programs with many competing needs; and, historically, there has been a tendency for some psychiatrists to regard genetics and neurobiology as secondary to other aspects of clinical practice. We would encourage residency programs to emphasize experiential problem-solving exercises (such as those available from the NNCI) in addition to a limited number of overview classroom lectures. Informal seminars and journal clubs can also be used to tackle genetic topics in a setting that allows for more questions and discussion than the lecture format. Residents should be encouraged to attend meetings that include sophisticated presentations of genetic data (such as Biological Psychiatry, American College of Neuropsychopharmacology, and the World Congress of Psychiatric Genetics). It should be expected that departments of psychiatry will increasingly appreciate the need for faculty with specialized expertise in this area as new findings emerge and the intersection of genetics with clinical practice increases. Genetics education among psychiatric residents should become an essential part of the emerging agenda for the development of a new cadre of psychiatric professionals for the 21st century.
Submitted: November 29, 2017; accepted June 1, 2018.
Published online: November 27, 2018.
Potential conflicts of interest: Dr Nurnberger is an investigator for Assurex and for Janssen. Dr Kennedy is on the scientific advisory board for Assurex (unpaid) and has received honoraria from Shire and Novartis for lectures. The other authors report no potential conflict of interest.
Funding/support: Support was provided by Parthenon Management Company, the National Human Genome Institute’s Inter-Society Coordinating Committee for Practitioner Education in Genomics (www.genome.gov/27554614), and the National Neuroscience Curriculum Initiative (R25 MH10107602S1 and R25 MH086466 07S1, to Dr Ross and colleagues).
Role of the sponsor: The content of the manuscript for this article was solely determined by the International Society of Psychiatric Genetics through its Residency Education Committee. No company contributed to the content of this manuscript or to the interpretation of data contained herein.
Previous presentation: World Congress of Psychiatric Genetics, October 30-November 4, 2016, Jerusalem, Israel, and October 13-17, 2017, Orlando, Florida; and Psychiatric Research Society, February 2016, Park City, Utah.
Acknowledgments: Helpful and critical input was provided by interactions with J. Raymond De Paulo, Jr, MD, and the ISPG Genetic Testing and Global Diversity Committees.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. PubMed CrossRef
2. Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223-1241. PubMed CrossRef
3. Foley C, Corvin A, Nakagome S. Genetics of schizophrenia: ready to translate? Curr Psychiatry Rep. 2017;19(9):61. PubMed CrossRef
4. Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric “diseases” versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41(1):33-40. PubMed CrossRef
5. Stahl E, Forstner A, McQuillin A, et al. Bipolar Disorder Working Group of the PGC. Genomewide association study identifies 30 loci associated with bipolar disorder [published online August 8, 2018]. bioRxiv. CrossRef
6. Ament SA, Szelinger S, Glusman G, et al; Bipolar Genome Study. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci U S A. 2015;112(11):3576-3581. PubMed CrossRef
7. Howard DM, Adams MJ, Shirali M, et al. Genome-wide association study of depression phenotypes in UK Biobank (n=322,580) identifies the enrichment of variants in excitatory synaptic pathways [published online August 1, 2018]. bioRxiv. CrossRef
8. Pauls DL, Abramovitch A, Rauch SL, et al. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15(6):410-424. PubMed CrossRef
9. Gelernter J, Kranzler HR, Sherva R, et al. Genome-wide association study of alcohol dependence: significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19(1):41-49. PubMed CrossRef
10. Nicolas G, Charbonnier C, Campion D. From common to rare variants: the genetic component of Alzheimer disease. Hum Hered. 2016;81(3):129-141. PubMed CrossRef
11. Le Hellard S, Steen VM. Genetic architecture of cognitive traits. Scand J Psychol. 2014;55(3):255-262. PubMed CrossRef
12. Baker K, Raymond FL, Bass N. Genetic investigation for adults with intellectual disability: opportunities and challenges. Curr Opin Neurol. 2012;25(2):150-158. PubMed CrossRef
13. Vorstman JAS, Parr JR, Moreno-De-Luca D, et al. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18(6):362-376. PubMed CrossRef
14. Tick B, Bolton P, Happé F, et al. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585-595. PubMed CrossRef
15. Lee SH, Ripke S, Neale BM, et al; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. PubMed CrossRef
16. Larsson H, Anckarsater H, R×¥stam M, et al. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J Child Psychol Psychiatry. 2012;53(1):73-80. PubMed CrossRef
17. Boraska V, Franklin CS, Floyd JAB, et al; Wellcome Trust Case Control Consortium 3. A genome-wide association study of anorexia nervosa. Mol Psychiatry. 2014;19(10):1085-1094. PubMed CrossRef
18. Finn CT, Wilcox MA, Korf BR, et al. Psychiatric genetics: a survey of psychiatrists’ knowledge, opinions, and practice patterns. J Clin Psychiatry. 2005;66(7):821-830. PubMed CrossRef
19. Winner JG, Goebert D, Matsu C, et al. Training in psychiatric genomics during residency: a new challenge. Acad Psychiatry. 2010;34(2):115-118. PubMed CrossRef
20. Hoop JG, Savla G, Roberts LW, et al. The current state of genetics training in psychiatric residency: views of 235 US educators and trainees. Acad Psychiatry. 2010;34(2):109-114. PubMed CrossRef
21. ACGME Program Requirements for Graduate Medical Education in Psychiatry. Accreditation Council for Graduate Medical Education website. http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/400_psychiatry_2017-07-01.pdf?ver=2017-05-25-083803-023. Effective July 1, 2017.
22. Certification Examination in Psychiatry. American Board of Psychiatry and Neurology website. https://www.abpn.com/wp-content/uploads/2017/10/2018_Psychiatry_CERT_Content_Specifications.pdf. Published October 31, 2017.
23. GWAS Catalog: the NHGRI-EPI catalog of published genome-wide association studies. http://www.ebi.ac.uk/gwas/downloads/summary-statistics.
24. Korf BR, Berry AB, Limson M, et al. Framework for development of physician competencies in genomic medicine: report of the Competencies Working Group of the Inter-Society Coordinating Committee for Physician Education in Genomics. Genet Med. 2014;16(11):804-809. PubMed CrossRef
25. Manolio TA, Murray MF; Inter-Society Coordinating Committee for Practitioner Education in Genomics. The growing role of professional societies in educating clinicians in genomics. Genet Med. 2014;16(8):571-572. PubMed CrossRef
26. Musunuru K, Haspel RL; Innovative Approaches to Education Working Group of the Inter-Society Coordinating Committee for Practitioner Education in Genomics. Improving genomic literacy among cardiovascular practitioners via a flipped-classroom workshop at a national meeting. Circ Cardiovasc Genet. 2016;9(3):287-290. PubMed CrossRef
27. Ross DA, Travis MJ, Arbuckle MR. The future of psychiatry as clinical neuroscience: why not now? JAMA Psychiatry. 2015;72(5):413-414. PubMed CrossRef
28. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415. PubMed CrossRef
29. Arbuckle MR, Travis MJ, Ross DA. Integrating a neuroscience perspective into clinical psychiatry today. JAMA Psychiatry. 2017;74(4):313-314. PubMed CrossRef
30. Olfson E, Ross DA. Genes orchestrating brain function. Biol Psychiatry. 2017;82(3):e17-e19. PubMed CrossRef
31. Hirschtritt ME, Besterman AD, Ross DA. Psychiatric pharmacogenomics: how close are we? Biol Psychiatry. 2016;80(8):e63-e65. PubMed CrossRef
32. Gagliano SA. It’s all in the brain: a review of available functional genomic annotations. Biol Psychiatry. 2017;81(6):478-483. PubMed CrossRef
33. Fatemi SH, Clayton PJ. The Medical Basis of Psychiatry. 3rd ed. New York, NY: Humana Press; 2016.
34. Risch N. Linkage strategies for genetically complex traits, I: multilocus models. Am J Hum Genet. 1990;46(2):222-228. PubMed
35. Yang J, Lee SH, Goddard ME, et al. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76-82. PubMed CrossRef
36. Fullerton JM, Koller DL, Edenberg HJ, et al; Bipolar High Risk Study Group, BiGS Consortium. Assessment of first and second degree relatives of individuals with bipolar disorder shows increased genetic risk scores in both affected relatives and young at-risk individuals. Am J Med Genet B Neuropsychiatr Genet. 2015;168(7):617-629. PubMed CrossRef
37. Amare AT, Schubert KO, Hou L, et al; International Consortium on Lithium Genetics (ConLi+Gen). Association of polygenic score for schizophrenia and HLA antigen and inflammation genes with response to lithium in bipolar affective disorder: a genome-wide association study. JAMA Psychiatry. 2017;75(1):65-74. PubMed CrossRef
38. Zheutlin AB, Ross DA. Polygenic risk scores: what are they good for? Biol Psychiatry. 2018;83(11):e51-e53. PubMed CrossRef
39. Gershon ES, Alliey-Rodriguez N. New ethical issues for genetic counseling in common mental disorders. Am J Psychiatry. 2013;170(9):968-976. PubMed CrossRef
40. Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86(5):749-764. PubMed CrossRef
41. Schaefer GB, Mendelsohn NJ; Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013;15(5):399-407. PubMed CrossRef
42. Volkmar F, Siegel M, Woodbury-Smith M, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237-257. PubMed CrossRef
43. American Academy of Neurology. Chromosomal Microarray Analysis for Intellectual Disabilities. American Academy of Neurology website. https://www.aan.com/siteassets/home-page/tools-and-resources/practicing-neurologist—administrators/billing-and-coding/model-coverage-policies/15microarrayanalysismodel_tr.pdf. Effective August 20, 2013.
44. American Academy of Pediatrics. Initial Medical Evaluation of a Child Diagnosed With an Autism Spectrum Disorder [Council on Children with Disabilities fact sheet]. American Academy of Pediatrics website. https://www.aap.org/en-us/Documents/cocd_fact_sheet_initialmedicine.pdf. Published 2013.
45. Champagne FA. Epigenetic legacy of parental experiences: dynamic and interactive pathways to inheritance. Dev Psychopathol. 2016;28(4pt2):1219-1228. PubMed CrossRef
46. Glatt SJ, Everall IP, Kremen WS, et al. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102(43):15533-15538. PubMed CrossRef
47. Walton E, Hass J, Liu J, et al. Correspondence of DNA methylation between blood and brain tissue and its application to schizophrenia research. Schizophr Bull. 2016;42(2):406-414. PubMed CrossRef
48. Mertens J, Wang QW, Kim Y, et al; Pharmacogenomics of Bipolar Disorder Study. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527(7576):95-99. PubMed CrossRef
49. Dean L. Carbamazepine therapy and HLA genotypes. In: Pratt V, McLeod H, Dean L, et al, eds. Medical Genetics Summaries. Bethesda, MD: National Center for Biotechnology Information; 2012-2015.
50. Altar CA, Carhart J, Allen JD, et al. Clinical utility of combinatorial pharmacogenomics-guided antidepressant therapy: evidence from three clinical studies. Mol Neuropsychiatry. 2015;1(3):145-155. PubMed CrossRef
51. Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses [FDA Drug Safety Communication]. US Food and Drug Administration website. https://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012.
52. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204447s007lbl.pdf.
53. Hirschtritt ME, Besterman AD, Ross DA. Psychiatric pharmacogenomics: how close are we? Biol Psychiatry. 2016;80(8):e63-e65. PubMed CrossRef
54. Kevles DK. In the Name of Eugenics. 2nd ed. Cambridge, MA: Harvard University Press; 1995.
55. Lifton RJ. German doctors and the final solution. N Y Times Mag. 1986; 21:64-65, 70-75.
56. Lifton RJ. The Nazi Doctors: Medical Killing in the Psychology of Genocide, New Edition. Bethesda, MD: Basic Books; 2017.
57. Diggs LW. The sickle cell trait in relation to the training and assignment of duties in the armed forces, IV: considerations and recommendations. Aviat Space Environ Med. 1984;55(6):487-492. PubMed
58. Kark JA, Posey DM, Schumacher HR, et al. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317(13):781-787. PubMed CrossRef
59. Kopelman L. Ethical controversies in medical research: the case of XYY screening. Perspect Biol Med. 1978;21(2):196-204. PubMed CrossRef
60. Austin JC, Palmer CG, Rosen-Sheidley B, et al. Psychiatric disorders in clinical genetics II: individualizing recurrence risks. J Genet Couns. 2008;17(1):18-29. PubMed CrossRef
61. Austin JC, Peay HL. Applications and limitations of empiric data in provision of recurrence risks for schizophrenia: a practical review for healthcare professionals providing clinical psychiatric genetics consultations. Clin Genet. 2006;70(3):177-187. PubMed CrossRef
62. Inglis A, Morris E, Austin J. Prenatal genetic counselling for psychiatric disorders. Prenat Diagn. 2017;37(1):6-13. PubMed CrossRef
63. Austin J, Inglis A, Hadjipavlou G. Genetic counseling for common psychiatric disorders: an opportunity for interdisciplinary collaboration. Am J Psychiatry. 2014;171(5):584-585. PubMed CrossRef
This PDF is free for all visitors!