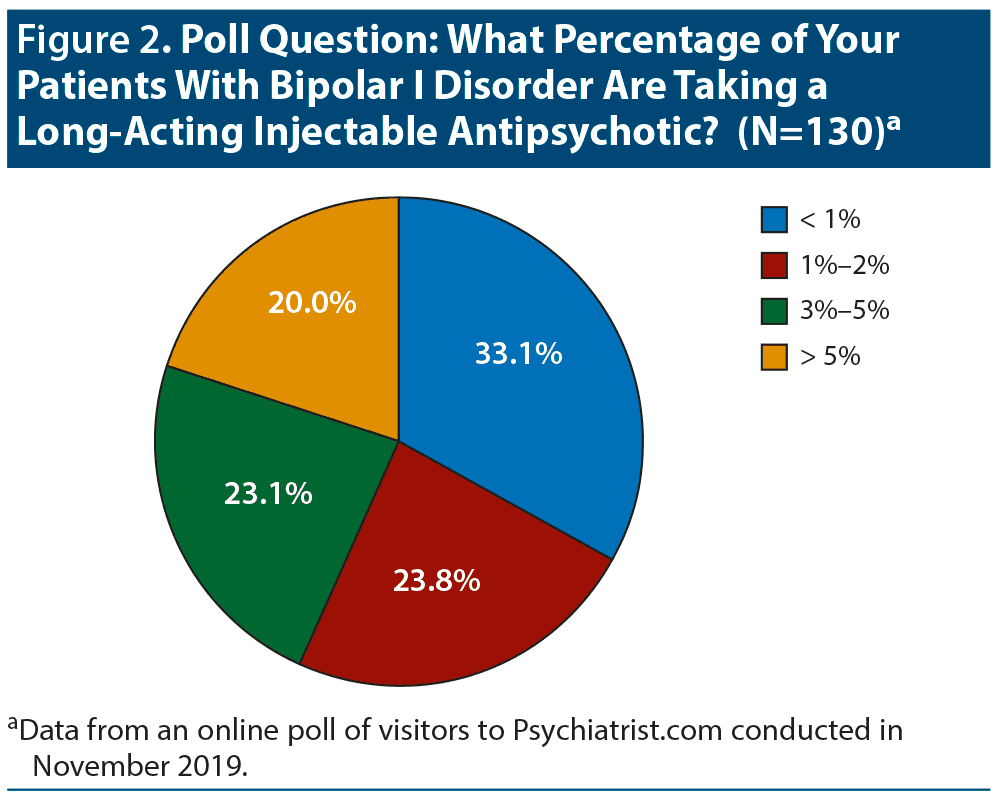Abstract
People with bipolar I disorder experience an illness course marked by potentially disastrous manic episodes, disabling depressive episodes, and functional impairment. A frequent obstacle to wellness in these individuals is nonadherence to treatment. Long-acting injectable (LAI) antipsychotics have the potential to address nonadherence and thereby increase patients’ chances at sustained recovery and normal psychosocial functioning. LAI formulations of 2 second-generation antipsychotics—aripiprazole monohydrate and risperidone—have received approval from the US Food and Drug Administration as monotherapy or adjunctive therapy to lithium or valproate for the maintenance treatment of bipolar I disorder in adult patients. In a recent roundtable meeting, a panel of 4 experts discussed the use of these medications in bipolar I disorder. This Academic Highlights summarizes their discussion, which included the impact of functional impairment, the potential benefits of employing an LAI antipsychotic at earlier stages of bipolar illness, and the characteristics of patients who may be good candidates for treatment with an LAI antipsychotic.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the expert roundtable meeting “Identifying Profiles of Patients With Bipolar I Disorder Who Would Benefit From Maintenance Therapy With a Long-Acting Injectable Antipsychotic,” which was held December 6, 2019.
The roundtable was chaired by Mauricio Tohen, MD, DrPH, MBA, Department of Psychiatry and Behavioral Sciences, University of New Mexico, Albuquerque, New Mexico. The faculty were Joseph F. Goldberg, MD, Department of Psychiatry, Icahn School of Medicine, Mount Sinai, New York City, New York; Youssef Hassoun, MD, Department of Psychiatry, South Oaks Hospital, The Zucker School of Medicine at Hofstra/Northwell, Amityville, New York; and Suresh Sureddi, MD, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas.
Financial disclosures: Dr Tohen has received honoraria from or consulted for Alkermes, Allergan, Otsuka, Minerva, and Sunovion. Dr Goldberg has been a consultant for BioXcel, Neurocrine, Medscape, Otsuka, SAGE Pharmaceuticals, Sunovion, and WebMD; received honoraria from Allergan and Otsuka; participated in speakers bureaus for Allergan, Neurocrine, Otsuka, and Sunovion; and received royalties from American Psychiatric Publishing, Inc. Dr Hassoun has been a consultant for and participated in speakers/advisory boards for Otsuka, Lundbeck, and Alkermes. Dr Sureddi has participated in speakers/advisory boards for Otsuka, Lundbeck, Sunovion, Alkermes, IntraCellular, Allergan, Neurocrine, and Teva.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this Academic Highlights was provided by Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, Inc. The faculty acknowledges Sarah Brownd, MA, ELS, for editorial assistance in developing the manuscript. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc., the publisher, or the commercial supporters. This article is distributed by Otsuka Pharmaceutical Development & Commercialization, Inc., and Lundbeck, Inc., for educational purposes only.
J Clin Psychiatry 2020;81(4):OT19046AH1
To cite: Tohen M, Goldberg JF, Hassoun Y, et al. Identifying profiles of patients with bipolar I disorder who would benefit from maintenance therapy with a long-acting injectable antipsychotic. J Clin Psychiatry. 2020;81(4):OT19046AH1
To share: https://doi.org/10.4088/JCP.OT19046AH1
© Copyright 2020 Physicians Postgraduate Press, Inc.
Meet the Faculty
People with bipolar I disorder experience an illness course marked by potentially disastrous manic episodes, disabling depressive episodes, and functional impairment. A frequent obstacle to long-term wellness in these individuals is nonadherence to treatment. Long-acting injectable (LAI) antipsychotics have the potential to address the problem of nonadherence and thereby increase patients’ chances at achieving sustained recovery and normal psychosocial functioning. To date, LAI formulations of 2 second-generation antipsychotics (SGAs)—aripiprazole monohydrate1 and risperidone2—have received approval from the US Food and Drug Administration (FDA) as monotherapy or adjunctive therapy to lithium or valproate for the maintenance treatment of bipolar I disorder in adult patients.
In a recent roundtable meeting, a panel of 4 experts discussed the use of these medications in bipolar I disorder. This Academic Highlights summarizes their discussion, which included the impact of functional impairment, the potential benefits of employing an LAI antipsychotic at earlier stages of bipolar illness, and the characteristics of patients who may be good candidates for treatment with an LAI antipsychotic.
FUNCTIONAL IMPAIRMENT IN BIPOLAR DISORDER
The meeting began with a discussion of functional impairment in bipolar disorder. The substantial functional impairment that is characteristic of bipolar illness is well documented,3 and patients experience impairment in both occupational4 and social/interpersonal5 domains. Functional recovery is a high priority for patients, as it allows a return to their normal lives, and it should therefore be a priority for clinicians and a main goal of maintenance treatment of bipolar disorder.
Many patients who achieve “syndromal” recovery from their symptoms, which may be clinically defined as 8 weeks of wellness,6 unfortunately continue to experience functional impairment between episodes. Dr Goldberg cited the McLean-Harvard First Episode project,6 which found that, 2 years after initial hospitalization for a manic or mixed episode, 98% of bipolar I patients had achieved syndromal recovery and 72%, symptomatic recovery, but only 43% had achieved functional recovery (defined in this study as regaining premorbid occupational and residential status). Another 13% of patients had achieved, and then lost, functional recovery within 2 years.
The body of research that is focused on functional outcomes has grown in recent years, with the Global Assessment of Functioning and the Functional Assessment Short Test often used as outcome measures.7 A number of correlates associated with poorer functional outcomes have been identified. Demographic characteristics include male sex8 and older age.9 Clinical characteristics include treatment nonadherence, residual symptoms, comorbid alcohol abuse,10 and neurocognitive impairment.3 Other predictors include high levels of familial expressed emotion and negative affective style.11 Regarding contributors to functional impairment, two major areas that have been studied are symptom- or illness-related factors and neurocognitive factors.
Functional Impairment and Illness Stage
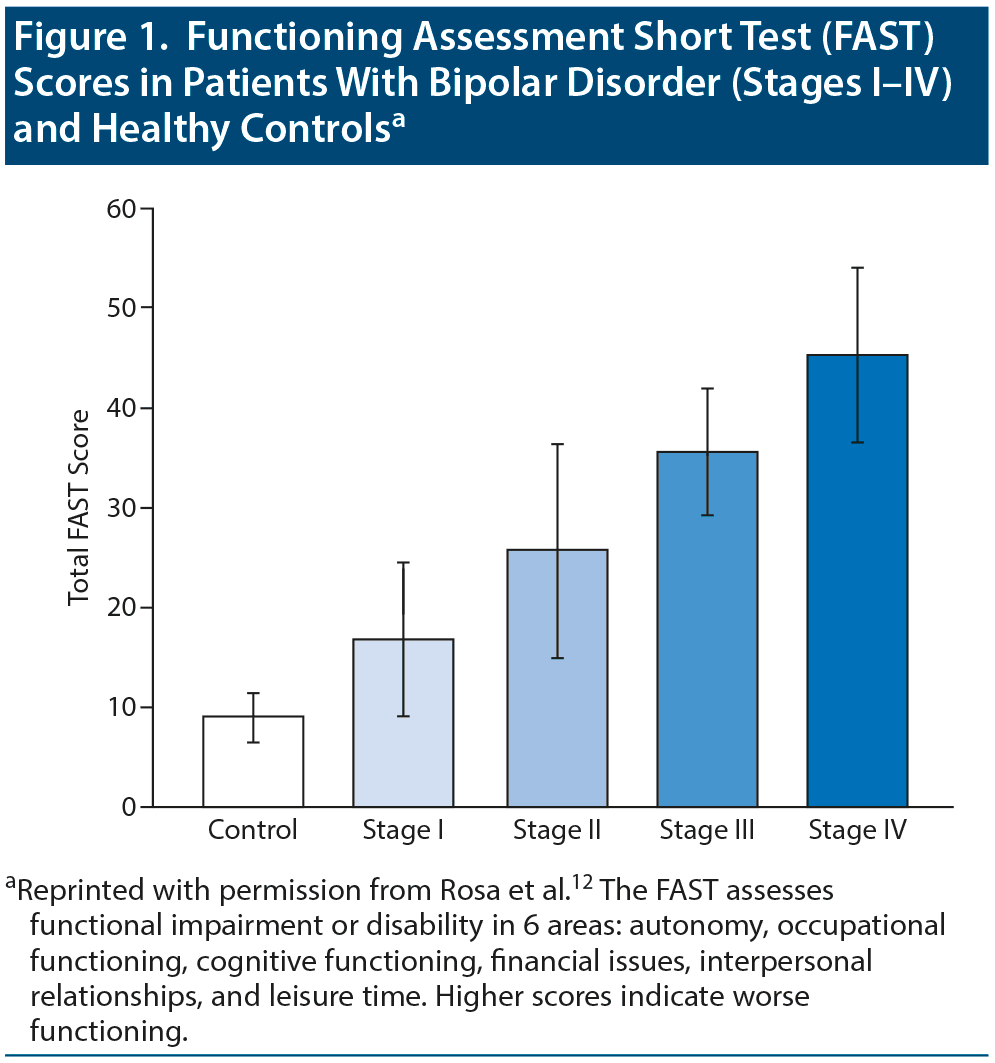
Patients’ level of functioning worsens with advancing illness stage. Rosa et al12 used a staging model to demonstrate functional changes from stage I (same status between episodes as before the onset of bipolar disorder) to stage IV (individuals who cannot maintain self-care or live autonomously). Patients in stage I showed significantly (P < .001) better functioning than those in stages III and IV (Figure 1). In the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study,13 number of episodes was associated with more severe symptoms and with poorer functioning and quality of life. With each recurring episode, patients experience demoralization, and their confidence to go back to work, resume relationships, and go on with their lives dwindles.
Cognitive Impairment
Cognitive impairment is seen in individuals with bipolar disorder not only during episodes but also during euthymic periods.14 Intuitively, cognitive impairment contributes to functional impairment, as poor memory and concentration make it more difficult for patients to return to work and resume their usual activities. Jensen et al15 showed that bipolar patients with global cognitive impairment (in verbal learning, working memory, and executive skills) or selective impairment (processing speed deficits) had greater perceived stress and subjective cognitive complaints, poorer work and social adjustment, and reduced quality of life compared to patients who were cognitively intact. Further, a meta-analysis by Wingo et al3 found that neurocognitive impairment was significantly associated with impaired psychosocial functioning in 6 of 8 studies of euthymic bipolar disorder patients and 5 of 5 studies of non-euthymic patients, even after adjusting for residual mood symptoms.
 Patient Perspectives
Patient Perspectives
“The number 1 goal I wanted to accomplish was having a long-term career—and with the ups and downs with my condition, I’ ve had problems continuing with work. I’ ve had a lot of jobs here and there, just bouncing around.”
“The biggest issue I have with manic episodes is spending money on things I don’ t need. It left me homeless in November. Bad decision-making. I didn’ t pay a bill, and that ended up leaving me homeless, jobless, and car-less. It took me a couple of months to recover from that . . . I still haven’ t really recovered.”
In thinking about cognitive impairment, the panelists discussed neuroprogression, or pathological changes that occur in the brain over time in association with a mental disorder. In November 2019, a series of online polls were conducted via the Psychiatrist.com website regarding clinicians’ experiences with treating bipolar I disorder, and one question asked if the respondent believes that bipolar I disorder is neuroprogressive. Of the 120 respondents, 83.3% indicated that they believe it is. Dr Goldberg stated that the structural-neuroanatomical data are currently stronger than the functional-cognitive data regarding frank neuroprogression in bipolar disorder. A key goal regarding the neuroprogression question will be the conduct of long-term studies of the same patients, and longitudinal studies that track within-subject changes over time are needed.
Neuroimaging data. Some neuroimaging data suggest that brain structure remains relatively unchanged early in the illness course but that progressive changes are seen over time.16 Regarding episode recurrence, Kozicky et al17 looked at change in gray matter volume in bipolar I patients who were within 3 months of recovery from a first manic/mixed episode and compared patients who did versus did not have a mood episode recurrence between baseline and 1-year follow-up. The recurrent episode patients had more gray matter volume loss in bilateral frontal, temporal, and left parietal regions as compared to patients with sustained wellness, and the study therefore indicated that the neuroprogression seen with a mood episode recurrence was not seen in those with sustained remission.
Assessments of cognitive function. Some data indicate poorer cognitive performance even from early stages of the disorder. A meta-analysis18 found that first-episode bipolar disorder is associated with deficits in many domains, including attention and verbal and visual memory. Torres and colleagues19 found, in the first year after an initial manic episode, poorer performance than healthy subjects in most domains but improvement in processing speed and executive function from baseline to 1 year (relative to controls).
Multiple manic episodes may contribute to worsening of cognition. In a recent study20 of euthymic patients, no differences were found between patients and controls on change in neurocognitive composite index or subdomains at 5-year follow-up. However, occurrence of 2 or more manic/hypomanic episodes (versus 0 or 1) was associated with decline in global cognitive function (P = .005), working memory (P = .032), and visual memory (P = .012). A previous study of euthymic patients by López-Jaramillo et al21 also showed that number of manic episodes was associated with worse performance on cognitive tests.
Other studies have indicated relative stability of cognitive function. A meta-analysis22 indicated that performance on 14 cognitive measures (including global cognitive function) remained stable after a mean follow-up of 4.6 years. Further, a study of 68 bipolar patients followed for a mean of 8.9 years found that while executive function worsened, other cognitive measures remained stable.23 Dr Goldberg pointed out that the preserved cognition demonstrated in some studies may be because the study subjects are more—perhaps much more—adherent to treatment than real-world patients by virtue of the regular monitoring that is part of the study procedures. Nonadherence and cognitive impairment are related, but the causal relationship is unclear. Poor adherence may worsen the illness course and so indirectly worsen cognitive performance; at the same time, cognitive impairment may contribute to poor treatment adherence and reflect more severe illness.24
It may seem like a tautology, but the longer you’ re well, the longer you’ re well. —Dr Joseph F. Goldberg
Prevent Episodes, Preserve Functioning
An ideal study would follow patients from their first episode and include functional and neurocognitive assessments as well as neuroimaging. In the absence of such a study, though, one can still connect the existing evidence. Dr Goldberg summarized:
We extrapolate from the studies of multiepisode patients compared to first-episode patients and can say that if you’ ve had many episodes, you are prone to have more episodes—more does beget more. The patient is more likely to have more disability and collateral damage from more episodes, so we will see more work impairment and persistent difficulties in multiepisode patients. Even if we don’ t know with certainty whether a neuroprogressive, degenerative process is occurring, we do know that multiepisode patients fare more poorly.
Video 1. Preventing Episodes and Preserving Functioning
For him, this awareness critically informs the aim of ongoing management: “My objective in first-episode manic patients, therefore, is for them to never have a second episode.” Dr Tohen characterized the aims of bipolar disorder treatment as 2-fold: to prevent episodes, which can be catastrophic, or even lethal, and to improve long-term outcomes by preserving social and work functioning.
As emphasized by the panelists, the aim of maintenance treatment is to prevent further episodes and thereby help patients maintain healthy functioning; one way of doing this is to encourage adherence to treatment. The next part of the discussion focused on LAI antipsychotics as a tool that can help prevent further episodes by improving adherence.
LAI ANTIPSYCHOTICS AS MAINTENANCE TREATMENT
Medications currently used to treat bipolar I disorder include lithium and anticonvulsant mood stabilizers, as well as some SGAs. Pharmacologic treatment of bipolar I disorder has generally consisted of orally administered medication. However, LAI antipsychotic formulations may increase adherence rates,25,26 provide more consistent dosing, and foster more regular contact between patients and their health care providers (via regular injection visits).27 In terms of adverse events, including extrapyramidal side effects and metabolic parameters, meta-analysis data suggest that LAI and oral antipsychotics do not differ.28 Although a number of injectable SGAs have been studied for use in bipolar disorder, the following sections will focus on aripiprazole and risperidone, the 2 agents that are FDA-approved for maintenance treatment of bipolar I disorder.
Efficacy and Tolerability
Risperidone LAI. Several studies have demonstrated efficacy and tolerability of risperidone LAI for bipolar I disorder.29 A large study29 comparing risperidone LAI with placebo injections (following an open-label study of oral risperidone) demonstrated longer time to recurrence of any mood episode with risperidone LAI versus placebo (P < .001); the difference was significant for elevated mood episodes but not depressive episodes. In line with the metabolic side effect profile of risperidone, weight gain ≥ 7% occurred in 15% of patients at 26-week follow-up.29
A double-blind, placebo-controlled study by Macfadden et al30 of risperidone LAI, administered every 2 weeks adjunctive to treatment as usual (mood stabilizers, antidepressants, anxiolytics), found that at 1 year, the relapse rate was 23.1% in the risperidone LAI group compared to 45.8% in the placebo group (P = .01). Time to relapse was also longer in the risperidone LAI group (P = .01). The most common side effects in the risperidone group were tremor, insomnia, muscle rigidity, weight gain, and hypokinesia.
Aripiprazole monohydrate LAI. Efficacy results from maintenance studies of aripiprazole LAI have been comparable to those from risperidone LAI studies. A 52-week randomized controlled trial (RCT) by Calabrese et al31 found that aripiprazole once monthly (AOM) 400 mg significantly delayed the time to recurrence of any mood episode compared with placebo (P < .0001). Fewer patients had a recurrence of any mood episode with AOM (26.5%) versus placebo (51.1%) (P < .0001), with the effects seen predominantly for manic episodes (P < .0001). Mean weight gain was low, and no clinically meaningful alterations in prolactin were seen.31 On the basis of change in Functioning Assessment Short Test scores, the AOM group was more likely than the placebo group to maintain functional improvement (P = .0279).32
An open-label study33 with patients from the earlier RCT31 (n = 85) and patients who were naive to AOM treatment (n = 379) found that improvements in Young Mania Rating Scale and Clinical Global Impressions for Bipolar Disorder-Severity scale scores were maintained, and the majority of AOM-naive patients (87.0%) and those from the RCT (97.6%) maintained stability through their last visit. Patient satisfaction levels were high, and both patient groups rated the side effect burden of AOM as greatly improved relative to previous medications. In line with the adverse event profile of aripiprazole, the most commonly occurring treatment-emergent adverse event was akathisia.33 Aripiprazole is associated with moderate weight gain and is prolactin-sparing, whereas a number of other antipsychotics, including risperidone, elevate prolactin.31
 Patient Perspectives
Patient Perspectives
“My psychiatrist brought up [treatment with an injectable formulation]. He didn’ t like the fact that I was using CBD and marijuana, but he was the first one to suggest Abilify Maintena. He gave it to me in pill form to start, and I did it for a short time . . . he told me that there is an injectable, and I wouldn’ t have to take pills anymore. I’ m against pills because I’ ve taken over 100,000 of them in the 10 years of medications—9 pills a day, 3 times a day, is 27 pills—it’s a lot of medicine. It took about a year to be able to get the injectable every month and actually stick to it. Once I stuck to it and saw the changes, it made a world of difference in my self-confidence on certain things. It did change things a lot, and I’ m happy with it. So, I’ m glad he made that call and offered it.”
Increased Treatment Adherence
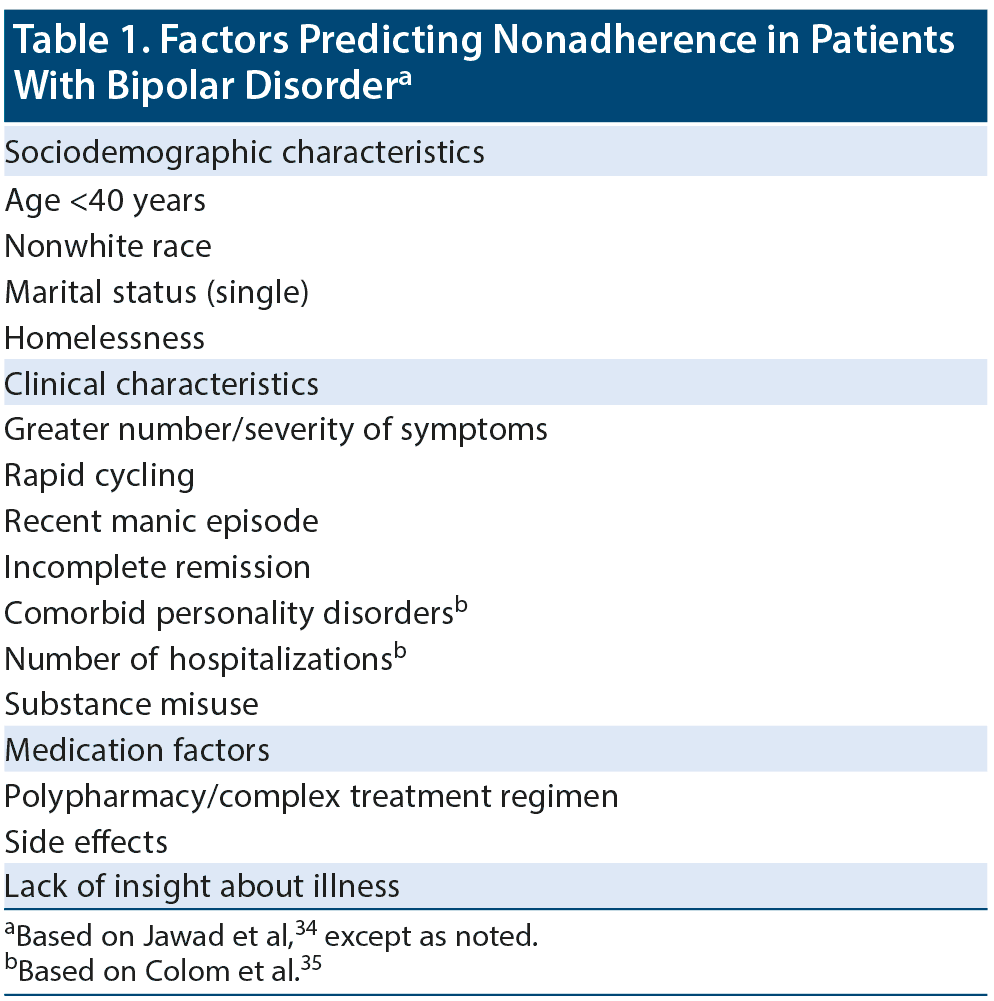
Dr Tohen stated that nonadherence is the best predictor of relapse in bipolar disorder. A number of factors can increase the likelihood of nonadherence,34,35 as shown in Table 1. The panelists agreed that, unfortunately, nonadherence to oral medication can be tricky to detect, even to the trained eye. In an expert consensus survey by Sajatovic et al36 of physicians with expertise in prescribing or studying LAIs, 45% indicated they were “only somewhat confident” and another 33% said they were “not very confident” in assessing bipolar patients’ adherence to oral medication.
All patients should receive psychosocial interventions (educating about symptoms, promoting lifestyle regularity, identifying triggers and early warning signs37) and cognitive-behavioral therapy (CBT) to increase adherence and engagement. Dr Sureddi mentioned that patients’ families, as well as support networks such as the Depression and Bipolar Support Alliance and the National Alliance on Mental Illness, can be supportive of adherence; he also stated that he encourages each patient to engage in CBT. For some patients, though, these efforts may not be enough. LAI formulations can be a good choice in these cases because they ensure that as long as the patient is attending injection appointments, he or she is receiving a consistent dose of medication. In fact, the panelists noted that, in general, patients do better if they are monitored regularly. If a lapse in the treatment regimen occurs, the clinician can work with the patient to identify and address any issues.
Video 2. Adherence
Cost-Effectiveness
The societal costs of bipolar I disorder are high: Cloutier and colleagues38 estimated the total US costs at $202.1 billion in 2015, with an average cost of $81,559 per individual. Dr Sureddi noted that in the past, cost and availability may have been considered barriers to use of LAIs but that, in fact, the reverse may now be true. An analysis39 of Medicaid claims from 2010 to 2015 among beneficiaries with schizophrenia indicated not only that treatment with LAI SGAs was associated with better adherence and persistence to therapy over 12 months than oral atypical antipsychotics but also that the LAI agents were associated with lower medical costs that offset over half of the higher pharmacy costs relative to the oral agents. The improved adherence associated with use of LAI antipsychotics could reduce cost by preventing rehospitalization, as nonadherence after discharge predicts rehospitalization.40
Current Use by Clinicians
The panelists felt that LAI antipsychotics are currently underused for bipolar I disorder. The Psychiatrist.com poll confirmed infrequent use; only one-fifth of the respondents treat more than 5% of their bipolar I patients with an LAI antipsychotic (Figure 2). One-third replied that they treat less than 1% of their bipolar I patients with one of these medications.
A number of factors contribute to underuse of LAI medications,41 including lack of clinician awareness. Clinicians also may overestimate, perhaps significantly, their patients’ adherence and therefore underestimate the advantage that LAI antipsychotics could provide in this area.42 Further, Dr Goldberg cited a sense of demoralization on the part of both clinicians and patients that can accompany repeat episodes in bipolar I disorder, in which they fail to consider that a treatment option exists that is fundamentally different and could break the episode cycle. Dr Tohen noted that treating patients with LAI antipsychotics seems to be more prevalent among nurse practitioners than among psychiatrists and that this may be because they are more up-to-date on treatment options and are focused on prevention of illness as well as treatment of current illness.
 Patient Perspectives
Patient Perspectives
“In a way, I just feel like I get just a little bit of time, since I’ m kind of a longer-term patient—I mean, the appointments may only be 10 or 15 minutes where they’ re basically writing a script and kind of moving on. So, I don’ t feel like I get to express myself as much with continued care.”
Current Treatment Guidelines
Among respondents to the Psychiatrist.com poll, 69% felt that the current guidelines do not adequately address all treatment options. The most recent full guidelines43 from the American Psychiatric Association (APA) are from 2002 and reflect the data available at that time, advising that “for patients treated with an antipsychotic medication during the preceding acute episode, antipsychotics should be discontinued unless they are required for control of persistent psychosis [first-level recommendation] or prophylaxis against recurrence [third-level recommendation]. While maintenance therapy with atypical antipsychotics may be considered, there is as yet no definitive evidence that their efficacy in maintenance treatment is comparable to that of agents such as lithium or valproate.”43(p11) An APA Guideline Watch published in 200544 outlines more evidence on use of SGAs for manic episodes, including risperidone, ziprasidone, aripiprazole, and quetiapine, although it is limited to oral formulations (risperidone LAI received its first FDA approval, for schizophrenia, in 2003; aripiprazole LAI was first approved in 2013).
More recent guidance comes from the 2017-2018 Florida Best Practice Psychotherapeutic Medication Guidelines.45 For continuation and maintenance therapy of bipolar I disorder, long-acting injectable forms of both aripiprazole and risperidone as monotherapy are included among treatments with “level 1” efficacy; other level-1 options include lithium, quetiapine, or asenapine monotherapy. These guidelines emphasize use of measurement-based care and comprehensive assessment for all patients and cite the role of multimodality therapies including pharmacotherapy, psychosocial treatment, cognitive remediation, and lifestyle modification (eg, exercise) in forestalling recurrence.
Dr Hassoun stated that, even for recent guidelines, better guidance is needed regarding the role of LAI antipsychotics, particularly in early intervention, and a review46 of guidelines published up to 2018 cited specifically a lack of recommendations for early-stage bipolar disorder. Current guidelines do not adequately describe the point at which LAIs may be started—or the potential preventive role they may play. Dr Goldberg also mentioned the lack of guidance regarding how long clinicians should continue an oral antipsychotic in order to gauge efficacy before switching to an LAI formulation.
TIMING OF LAI ANTIPSYCHOTIC USE
Usual practice has been to reserve LAI antipsychotic formulations for patients with established nonadherence who have experienced multiple episodes. A question in the Psychiatrist.com poll asked at what point in the treatment algorithm the clinician would use an LAI, and the most common response was “after multiple hospitalizations within the last year” (72%), with only 11% of respondents using them at initial diagnosis. However, contrary to current practice patterns, the panelists felt that LAI use is sometimes appropriate early in the disease course and in younger patients.
Early Intervention
Prompt diagnosis of bipolar disorder is of the essence. Delay to diagnosis can unfortunately be substantial and was mentioned by Dr Hassoun as a barrier to early intervention. Manifestation of bipolar symptoms is heterogeneous, giving rise to early diagnostic uncertainty,47 and initial presentation of depressive symptoms can lead to incorrect initial diagnosis. Although these stages are the time at which early intervention has the best chance of containing the illness, diagnosis and treatment cannot commence until symptoms reach a certain threshold.47 Parental early onset is the strongest predictor of conversion to bipolar I disorder,48 and screening for mania-like symptoms in young patients is important, particularly in those with a family history of bipolar illness.48
In a review, Vieta and colleagues48 note that the existence of an at-risk state characterized by prodromal manic symptoms lays the foundation for work on early intervention, but also that more studies, including identification of biomarkers, are needed to help differentiate genuinely at-risk individuals from those with benign states. Positive effects have been demonstrated for psychotherapeutic interventions in young patients with bipolar disorder,49,50 and these can be a well-received first step in early intervention.48
More longitudinal data are needed on the impact of earlier pharmacologic treatment. Some evidence from staging models suggests that intervening with medication earlier in the illness course would be instrumental in preventing disease progression,12 and starting lithium treatment early versus later in the illness course has been associated with greater probability of response.51 Dr Hassoun stated that lessons can perhaps also be learned from data on schizophrenia, which have shown that treatment earlier in the illness course (shorter duration of untreated psychosis) is more effective. A recent meta-analysis by Correll et al52 found significantly lower relapse rates and greater quality of life, improvement in global functioning, and symptom improvement in patients with first-episode psychosis or early-phase schizophrenia who received early intervention services (pharmacologic and psychosocial) versus treatment as usual. Further, in another study, LAI initiation within 1 year of a new schizophrenia episode (compared with LAI initiation after 1 year) was shown to be associated with significantly (P < .001) lower hospitalization rates and health care costs.53
How Early Should an LAI Be Offered?
Drs Tohen and Sureddi cited the second mood episode as the key episode at which the disorder is confirmed to be recurrent and therefore the point at which a need for maintenance therapy is affirmed. They felt that for patients with certain risk factors (see Which Patients Are Good Candidates for LAI Antipsychotics?), though, the first episode may even be an appropriate time to initiate use of an LAI formulation.
Video 3. Inverting the Order
The panelists linked their awareness of the progressive nature of bipolar disorder to the way in which they proceed with treatment. Many clinicians resort to an LAI medication only after numerous detrimental events have occurred in the patient’s illness course, but Dr Goldberg felt that in light of the progression seen in bipolar disorder, the usual order of when to start an LAI medication should be inverted. If a reliable treatment such as an LAI antipsychotic could be started early and maintained, and if relapses and the consequences of episodes could be averted, he said, the patient’s medication regimen may be simpler in the long run. According to Dr Tohen, “We should assume that every patient with bipolar disorder we see is at risk of nonadherence and therefore at risk of progressive functional deterioration, so we should start from the very beginning to try to intervene in a preventive way.”
Mania prevention is especially important right from the beginning of the illness because of the high stakes associated with manic episodes. Stopping a cycle of repeating episodes could prevent a cascade of potentially disastrous outcomes. Dr Goldberg emphasized that, while residual depression is undoubtedly a significant source of morbidity, just one manic episode has the potential to destroy a career, cause financial ruin, or have serious legal implications. Intervening early could also save lives by reducing suicide risk, as bipolar disorder is associated with a particularly high suicide rate. Large epidemiologic samples report that 3.4%-5.9% of suicide deaths occur in people with bipolar disorder; further, evidence suggests that longer duration of untreated bipolar disorder is associated with increased risk of suicide attempts.54
How Long Should an Oral Formulation Be Used Before an LAI Formulation Can Be Started?
If a patient has not taken a particular antipsychotic before, tolerability and efficacy should first be established with the oral formulation.2 Dr Hassoun mentioned a study55 of aripiprazole LAI in schizophrenia that suggested a lengthy trial of the oral formulation was not necessary to ensure response. An expert consensus survey by Sajatovic et al56 preferred a duration of 4-14 days, agreeing that they would not continue it for more than 6 weeks before beginning the LAI treatment. Establishing efficacy is more difficult than establishing tolerability, and guidelines are lacking. According to Dr Goldberg, for antimanic agents, a noticeable effect may be seen within the first week or two. When introducing a patient to the concept of an injectable medication, it is helpful to be able to point to the established effectiveness of the oral formulation for the patient. For hospitalized patients, the first LAI antipsychotic injection may be given in the hospital. Dr Goldberg mentioned that hospitalists sometimes think in the short term, focusing primarily on getting patients to the point where they are dischargeable. Initiation of a long-acting medication in the hospital reflects a longer-term strategy that can help prevent episodes down the road, in the weeks and months after discharge.
How Long Should LAI Antipsychotic Treatment Be Continued?
Dr Tohen said that when patients ask him how long they’ ll have to take medication, he tells them, “Until we find a cure.” Dr Sureddi advised that if a patient has had many episodes over his or her lifetime, and seems to tolerate and respond well to an LAI antipsychotic, he would continue treatment with it indefinitely. Although he would be open to the possibility of a patient’s switching to an oral formulation if they wish to do so after being treated with an LAI medication for a few years, he said he would still prefer that the patient remain with the formulation that is currently working. Dr Hassoun related that in his personal experience, nearly every time a patient has decided to switch to an oral medication from an LAI formulation, the outcome was not successful. Patients who have been well for a few years may be susceptible to nonadherence if they believe that they are cured and no longer need medication. For this subpopulation, switching to an oral formulation could mean discontinuation of the medication and possible relapse.
Dr Goldberg raised the concept of a “foundational medication” in bipolar disorder: a reliable agent that has demonstrated value in preventing mood episodes. He said that although lithium works well in the long term for a small percentage of patients, particularly those with “pure” euphoric mania, most patients do not fit the ideal profile for lithium treatment. A robust treatment that will help minimize nonadherence and can prevent mood episodes, such as an LAI antipsychotic, might work better as a long-term “foundational” treatment, even if there are periods of time in which the patient may also need other treatments to address specific situations.
WHICH PATIENTS ARE GOOD CANDIDATES FOR LAI ANTIPSYCHOTICS?
Many clinicians reserve injectable medications for patients with severe illness, multiple hospitalizations or medication trials, or well-established nonadherence. The Psychiatrist.com poll asked clinicians what primary factor would make them consider an LAI in a bipolar I patient presenting with manic symptoms, and the 2 most common responses were “number of prior manic episodes that led to hospitalization” (66%) and “number of prior antipsychotic treatments” (20%). In considering which patients may benefit from LAI antipsychotics, it is also relevant to consider characteristics that increase risk of nonadherence, relapse, and poorer outcomes.
Video 4. Characteristics of Candidates for LAIs
In addition to poor functioning and repeat episodes, Dr Sureddi mentioned a couple of other key characteristics that might make a patient a good candidate for an LAI antipsychotic:
- Family history of bipolar illness. Particularly if the affected relatives have significant functional deterioration or many repeat episodes, Dr Sureddi said he might consider prescribing an LAI antipsychotic for these patients from the beginning. High level of familial expressed emotion was also mentioned as a factor that predicts poor outcome and might influence early choice of an LAI antipsychotic.
- Anosognosia. Anosognosia, which Dr Sureddi noted is quite common in bipolar disorder, is linked to nonadherence.57 Patients may believe that they were never ill or that their illness is cured or fail to recognize signs of impending relapse.
Other characteristics discussed by the panelists as suggesting the choice of an LAI antipsychotic include the following:
- Substance use disorder. It was noted that substance use comorbidity is very common in patients with bipolar disorder. It predicts poorer functioning10,58 and is a particularly strong predictor of nonadherence and therefore relapse.10,57,59 Selecting a medication that helps with adherence increases the likelihood that the patient will remain well, which could decrease the likelihood of substance abuse and in turn decrease the likelihood of relapse. Dr Tohen observed that the clinician is therefore doing secondary prevention of the primary condition.
- Initial manic presentation, rapid cycling, and residual manic symptoms. Manic and mixed states and rapid cycling are associated with nonadherence.57 Initial manic presentation increases the likelihood of a manic recurrence,6,60 and long-term wellness seems more difficult to achieve in patients with a greater preponderance of manic symptoms. As demonstrated in the NIMH Collaborative Depression Study,61 residual affective symptoms put individuals at a more than a 3-fold increased risk for syndromal relapse. The greater adherence facilitated by LAI antipsychotics may be helpful in combating symptom recurrence in these patients.
- Recent incarceration. People whose bipolar illness was stable due to consistent medication use while in prison are at risk of nonadherence after their release. Suddenly stopping medication would greatly increase their risk of relapse, and resumption of substance use upon release could make the situation worse. Dr Tohen mentioned that clinical encounters that focus on reintegration and coincide with the individual’s release from prison (eg, New Mexico’s “welcoming centers”) would be a good setting in which to determine if a patient would be at risk of nonadherence and therefore a candidate for LAI antipsychotic use (voluntary, unless court-ordered).
- Polypharmacy. Dr Goldberg theorized that polypharmacy is sometimes a “flag” for nonadherence. Prescribed medications may have accumulated due to repeated attempts to address poor response that, paradoxically, resulted from a failure to take medication in the first place. Switching to an LAI antipsychotic might reduce the total number of medications, perhaps decreasing side effects and increasing the chances of wellness. He said that although multiple antipsychotics are sometimes prescribed, the evidence for doing so is weak in bipolar disorder,62 and streamlining to a single “foundational” medication that fosters long-term adherence may be more beneficial. Dr Sureddi observed that in his experience, LAI antipsychotics have often helped minimize the number of medications that patients are taking. It was also observed by Dr Tohen that, for patients who are benefiting from multiple medications, the consistent treatment provided by an LAI antipsychotic may increase the likelihood of adherence to the other medications.
Video 5. Polypharmacy and “Foundational Treatment”
Are There Patients in Whom LAI Antipsychotics Should Not Be Used?
Use of LAI antipsychotics would be inappropriate in patients with a history of nonresponse to the medication. Current or past intolerance to the medication in oral or injectable form would also preclude its use. Certain side effects may not necessarily require the end of LAI antipsychotic use, though, depending on the situation, and Dr Goldberg stated that clinicians should monitor closely for metabolic changes so that they may be managed promptly. A history of neuroleptic malignant syndrome (NMS) might indicate against using an LAI antipsychotic; however, Dr Sureddi said that if the antipsychotic that caused NMS was first-generation, he might still consider a second-generation agent. In such cases, he would monitor the patient closely and apprise the patient and family of the risk.
Other patient groups in whom LAI antipsychotic use may not be appropriate include individuals with existing tardive dyskinesia for whom alternatives to antipsychotics, if feasible, might be preferable. Dr Goldberg also noted that for patients who are more prone to depressive than manic recurrences, aripiprazole LAI or risperidone LAI may be not be the best choices because use of an SGA with proven efficacy for acute bipolar depression (eg, cariprazine, lurasidone, or olanzapine-fluoxetine combination) may be required.
 Case Practice Question
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 1. Dominic is a 30-year-old computer technician who has had 1 manic episode for which he was hospitalized; this episode occurred soon after he began his first year of community college. About a year ago, he had a first depressive episode. Six months later, he had his second lifetime manic episode, for which he was treated as an outpatient with divalproex plus quetiapine. Since then, he has had low-grade mixed features (impulsivity, irritability, and sleeping only a few hours nightly without next-day fatigue). He used alcohol heavily during high school until about the age of 20 but in the past year reports only “social” drinking and once-monthly cannabis use.
All of the following statements about Dominic’s prognosis are true except:
a. His residual manic symptoms pose more than a 3-fold increased risk for syndromal relapse
b. Male sex increases his likelihood for psychosocial recovery
c. His history of comorbid alcohol use disorder both increases the likelihood of poor psychosocial outcome and speeds the time until affective relapse
DISCUSSING TREATMENT WITH PATIENTS
Educating the Patient and Inquiring About Goals
Video 6. Discussing LAIs with Patients and Families
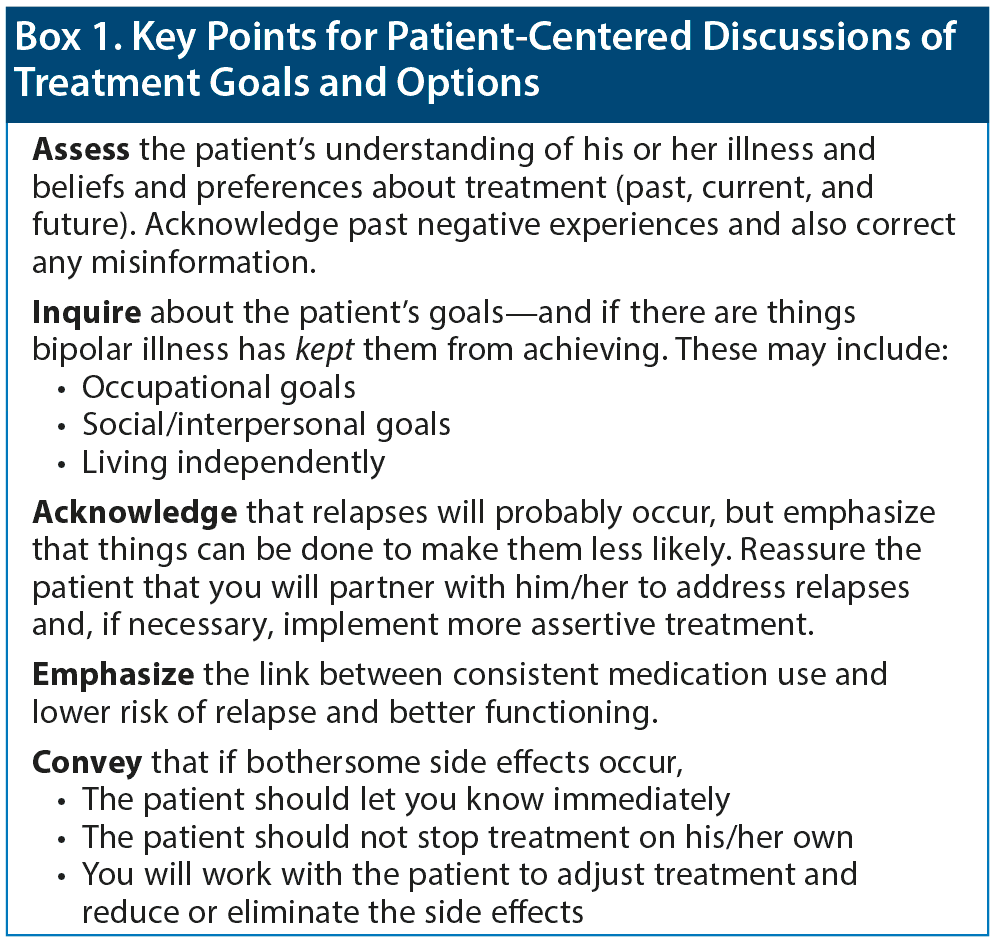
Strategies for discussing treatment goals and options in a patient-focused way are summarized in Box 1. Educating patients about their illness is paramount: what makes the illness better, what makes it worse, what may trigger an episode. Keeping patients’ priorities at the forefront is also key. Critical questions to ask are, “Is your current medication helpful for you? What is it doing for you? What things in your life do you want to see improve?” A patient may identify mood, or functioning, or sleep, and then the question is, “How well does this medicine accomplish that? What are the downsides?” If a patient is having a problem with his or her medication, such questions will help pinpoint whether the problem is due to side effects, lack of efficacy, or something else.
Dr Hassoun related that when inquiring about patients’ goals, he also asks what the illness has kept them from achieving. The conversation can be framed in a positive way: “Here’s what we can do about that; here are measures we can take to improve your chances of achieving those goals.” Then, the clinician can advise the patient that use of an appropriate medication, on a consistent basis, can help the patient achieve goals such as returning to work or school and resuming interpersonal relationships. Emphasizing specific steps that can be taken empowers patients in an illness over which they feel out of control.
Dr Goldberg recommended discussing functional deterioration early and from a positive standpoint. Rather than telling patients that their functioning will definitely deteriorate over time—implying a hopeless situation—one can lay groundwork along the lines of, “Should it happen that more episodes occur, there are more aggressive and assertive modes of treatment we can try.” As a clinician, one cannot control for all of the variables that contribute to relapse and functional decline. However, one can identify and target certain variables, such as poor adherence or substance use or psychosis, and tell the patient, “I can improve your chances of not having the next episode by addressing these issues, but I can’ t guarantee it. But our objective is to try to get you well and prevent the next episode.”
Finally, patients should be encouraged to report any side effects that arise. They should be assured that the clinician will work with them to address any that are unacceptable; this may keep them from abruptly stopping medication due to side effects.
Presenting an LAI Antipsychotic as an Option
Clinicians may assume that their patients will reject the idea of injectable medication. Dr Sureddi reported that in his experience, patients and families are very receptive to LAI antipsychotics once the possible pros and cons are delineated. Evidence indicates that physicians’ beliefs regarding acceptability of injectable formulations to patients are inaccurate63; further, a 2017 review64 noted that the majority of bipolar disorder patients have positive attitudes toward antipsychotic treatment.
Video 7. Is It Patients/Families or Clinicians Who Are More Resistant to LAIs?
To maximize the chances that a patient will accept an injectable medication, Dr Tohen recommended pointing out the response achieved with the oral medication and then the convenience of the injectable form: “Before we say, ‘ Let’s take this efficacious drug and put it in a long-acting form,’ I also want to be able to say to the patient, ‘Clearly you’ ve responded to this treatment, and now I have a vehicle to administer it in a way that will be more consistent and convenient.’ “
Family education and involvement. From the first visit following an episode, ideally within a week, clinicians should seize an early opportunity to educate family members about the illness and to involve them in discussions about treatment. If the patient has been hospitalized after an acute episode, discussions with the family can take place right from the hospital setting. These discussions can include what to do, what not to do, what the follow-up will be like, and what the expectations are regarding outcomes. This can also be a good time to elicit family history. The value of engaging patients and families in this way, according Dr Hassoun, is that it can help stop them from thinking of bipolar disorder in the way one would think of an infection—that is, that it can be treated with a course of medication, and when the antibiotic course is finished, the illness is resolved. This can also be a good time to focus on the risk of relapse and the importance of treatment adherence; LAI antipsychotic treatment can be a natural extension of that discussion.
 Case Practice Question
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 2. Carlos is a 25-year-old security officer recently discharged from an inpatient unit after recovering from his first episode of mania. He had a good response to an atypical antipsychotic agent. He is willing to take maintenance treatment but is concerned that he may forget his medication, especially during weekends because he frequently goes camping. Is Carlos a good candidate for treatment with an LAI antipsychotic?
a. No, because he has suffered only 1 episode
b. No, because LAI antipsychotics are utilized only in schizophrenia, not in bipolar disorder
c. Yes, because due to lifestyle factors, the patient is at risk of nonadherence
d. Yes, because the risk of tardive dyskinesia is lower with LAI versus oral formulations
CONCLUSION
Video 8. Summary: Nonadherence and Functional Deterioration
Bipolar I disorder is a chronic disorder, marked by relapses and functional deterioration. Clinicians should think in the long term with regard to treatment, keeping in mind that relapses and functional deterioration are related. Mood stabilizers and antipsychotics are the primary pharmacologic treatments used, typically in oral formulations. For patients in whom adherence is a concern, LAI antipsychotic formulations can provide consistent, assured dosing, and 2 LAI antipsychotics, risperidone and aripiprazole, are FDA-approved as monotherapy for bipolar I disorder.
Besides established nonadherence, some characteristics that may make patients good candidates for LAI antipsychotics include a family history of bipolar illness (particularly with poor functioning), lack of illness insight, comorbid substance use, recent incarceration, and complex treatment regimens. A patient-centered approach should be taken when discussing treatment; this includes inquiring about the patient’s goals and challenges, correcting misinformation, and assuring patients that the clinician will partner with them to adjust treatment should problems arise.
 Discussion of Case Practice Questions
Discussion of Case Practice Questions
Case 1: Preferred response is b.
Male sex is associated with poorer psychosocial outcomes in bipolar disorder.8 Residual manic symptoms were associated with a 3.4-fold greater risk for affective relapse (as compared to the absence of residual symptoms) in the NIMH Collaborative Depression Study.61 Substance abuse predicts poorer functional outcomes and is a predictor of nonadherence and therefore relapse.10
Case 2: Preferred response is c. Increased risk of nonadherence, which can result from numerous patient characteristics and individual situations, can be addressed with LAI antipsychotics. A history of multiple episodes is not necessarily a prerequisite for use of LAI antipsychotics. They are effective in both schizophrenia and bipolar disorder,29,31 and there is no evidence that tardive dyskinesia risk is lower with LAI formulations.28
 Clinical Points
Clinical Points
- Bipolar disorder is associated with substantial functional impairment that often persists beyond episodes and worsens with each episode.
- When formulating a treatment plan, clinicians should think long-term and focus on prevention. Long-acting injectable (LAI) antipsychotics can support adherence and ensure that patients receive a consistent medication dose.
- Some characteristics, including substance use disorder, family history of bipolar illness, and use of multiple medications, may make patients particularly good candidates for LAI antipsychotic treatment.
- When discussing LAI antipsychotics with patients, clinicians should inquire about the patient’s goals, address concerns and any misinformation, and emphasize the link between adherence and better functioning.
Published online: June 16, 2020.
REFERENCES
1. Abilify Maintena [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co, Ltd; 2020.
2. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2007.
3. Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009;11(2):113-125. PubMed CrossRef
4. Michalak EE, Yatham LN, Maxwell V, et al. The impact of bipolar disorder upon work functioning: a qualitative analysis. Bipolar Disord. 2007;9(1-2):126-143. PubMed CrossRef
5. MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatr Scand. 2001;103(3):163-170. PubMed CrossRef
6. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107. PubMed CrossRef
7. Chen M, Fitzgerald HM, Madera JJ, et al. Functional outcome assessment in bipolar disorder: a systematic literature review. Bipolar Disord. 2019;21(3):194-214. PubMed CrossRef
8. Tohen M, Waternaux CM, Tsuang MT. Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry. 1990;47(12):1106-1111. PubMed CrossRef
9. Rosa AR, Reinares M, Franco C, et al. Clinical predictors of functional outcome of bipolar patients in remission. Bipolar Disord. 2009;11(4):401-409. PubMed CrossRef
10.Treuer T, Tohen M. Predicting the course and outcome of bipolar disorder: a review. Eur Psychiatry. 2010;25(6):328-333. PubMed CrossRef
11.O’ Connell RA, Mayo JA, Flatow L, et al. Outcome of bipolar disorder on long-term treatment with lithium. Br J Psychiatry. 1991;159(1):123-129. PubMed CrossRef
12.Rosa AR, Magalh×£es PVS, Czepielewski L, et al. Clinical staging in bipolar disorder: focus on cognition and functioning. J Clin Psychiatry. 2014;75(5):e450-e456. PubMed CrossRef
13.Magalh×£es PV, Dodd S, Nierenberg AA, et al. Cumulative morbidity and prognostic staging of illness in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Aust N Z J Psychiatry. 2012;46(11):1058-1067. PubMed CrossRef
14.Cullen B, Ward J, Graham NA, et al. Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: a systematic review. J Affect Disord. 2016;205:165-181. PubMed CrossRef
15.Jensen JH, Knorr U, Vinberg M, et al. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: associations with functional abilities. J Affect Disord. 2016;205:378-386. PubMed CrossRef
16.Lisy ME, Jarvis KB, DelBello MP, et al. Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disord. 2011;13(4):396-405. PubMed CrossRef
17.Kozicky J-M, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519. PubMed CrossRef
18.Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. 2015;41(5):1095-1104. PubMed CrossRef
19.Torres IJ, Kozicky J, Popuri S, et al. 12-month longitudinal cognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord. 2014;16(2):159-171. PubMed CrossRef
20.Sánchez-Morla EM, López-Villarreal A, Jiménez-López E, et al. Impact of number of episodes on neurocognitive trajectory in bipolar disorder patients: a 5-year follow-up study. Psychol Med. 2019;49(8):1299-1307. PubMed CrossRef
21.López-Jaramillo C, Lopera-Vásquez J, Gallo A, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12(5):557-567. PubMed CrossRef
22.Samamé C, Martino DJ, Strejilevich SA. Longitudinal course of cognitive deficits in bipolar disorder: a meta-analytic study. J Affect Disord. 2014;164:130-138. PubMed CrossRef
23.Torrent C, Martinez-Arán A, del Mar Bonnin C, et al. Long-term outcome of cognitive impairment in bipolar disorder. J Clin Psychiatry. 2012;73(7):e899-e905. PubMed CrossRef
24.Martinez-Aran A, Scott J, Colom F, et al. Treatment nonadherence and neurocognitive impairment in bipolar disorder. J Clin Psychiatry. 2009;70(7):1017-1023. PubMed CrossRef
25.Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed CrossRef
26.Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612-1625. PubMed CrossRef
27.Boyce P, Irwin L, Morris G, et al. Long-acting injectable antipsychotics as maintenance treatments for bipolar disorder: a critical review of the evidence. Bipolar Disord. 2018;20(suppl 2):25-36. PubMed CrossRef
28.Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2-3):220-230. PubMed CrossRef
29.Quiroz JA, Yatham LN, Palumbo JM, et al. Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry. 2010;68(2):156-162. PubMed CrossRef
30.Macfadden W, Alphs L, Haskins JT, et al. A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord. 2009;11(8):827-839. PubMed CrossRef
31.Calabrese JR, Sanchez R, Jin N, et al. Efficacy and safety of aripiprazole once-monthly in the maintenance treatment of bipolar I disorder: a double-blind, placebo-controlled, 52-week randomized withdrawal study. J Clin Psychiatry. 2017;78(3):324-331. PubMed CrossRef
32.Calabrese JR, Sanchez R, Jin N, et al. Symptoms and functioning with aripiprazole once-monthly injection as maintenance treatment for bipolar I disorder. J Affect Disord. 2018;227:649-656. PubMed CrossRef
33.Calabrese JR, Jin N, Johnson B, et al. Aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a 52-week, multicenter, open-label study. Int J Bipolar Disord. 2018;6(1):14. PubMed
34.Jawad I, Watson S, Haddad PM, et al. Medication nonadherence in bipolar disorder: a narrative review. Ther Adv Psychopharmacol. 2018;8(12):349-363. PubMed CrossRef
35.Colom F, Vieta E, Mart×nez-Arán A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61(8):549-555. PubMed CrossRef
36.Sajatovic M, Ross R, Legacy SN, et al. Identifying patients and clinical scenarios for use of long-acting injectable antipsychotics: expert consensus survey part 1. Neuropsychiatr Dis Treat. 2018;14:1463-1474. PubMed CrossRef
37.Crowe M, Porter R, Inder M, et al. Effectiveness of interventions to improve medication adherence in bipolar disorder. Aust N Z J Psychiatry. 2012;46(4):317-326. PubMed CrossRef
38.Cloutier M, Greene M, Guerin A, et al. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. 2018;226:45-51. PubMed CrossRef
39.Pilon D, Tandon N, Lafeuille M-H, et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39(10):1972-1985.e2. PubMed CrossRef
40.Hassan M, Lage MJ. Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm. 2009;66(4):358-365. PubMed CrossRef
41.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. PubMed CrossRef
42.Heres S, Hamann J, Kissling W, et al. Attitudes of psychiatrists toward anti psychotic depot medication. J Clin Psychiatry. 2006;67(12):1948-1953. PubMed CrossRef
43.Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice Guideline for the Treatment of Patients With Bipolar Disorder. 2nd ed. Arlington, VA: American Psychiatric Association; 2002:82.
44.Hirschfeld RMA. Guideline Watch: Practice Guideline for the Treatment of Patients With Bipolar Disorder. 2nd ed. Arlington, VA: American Psychiatric Association; 2005.
45. 2017-2018 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults. Tampa, FL: University of South Florida; 2018.
46.Chia MF, Cotton S, Filia K, et al. Early intervention for bipolar disorder: do current treatment guidelines provide recommendations for the early stages of the disorder? J Affect Disord. 2019;257:669-677. PubMed CrossRef
47.Malhi GS, Morris G, Hamilton A, et al. Is “early intervention” in bipolar disorder what it claims to be? Bipolar Disord. 2017;19(8):627-636. PubMed CrossRef
48.Vieta E, Salagre E, Grande I, et al. Early intervention in bipolar disorder. Am J Psychiatry. 2018;175(5):411-426. PubMed CrossRef
49.Macneil CA, Hasty M, Cotton S, et al. Can a targeted psychological intervention be effective for young people following a first manic episode? results from an 18-month pilot study. Early Interv Psychiatry. 2012;6(4):380-388. PubMed CrossRef
50.Scott J, Paykel E, Morriss R, et al. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry. 2006;188(4):313-320. PubMed CrossRef
51.Kessing LV, Vradi E, Andersen PK. Starting lithium prophylaxis early vs late in bipolar disorder. Br J Psychiatry. 2014;205(3):214-220. PubMed CrossRef
52.Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565. PubMed CrossRef
53.Munday J, Greene M, Chang E, et al. Early initiation of long-acting injectable antipsychotic treatment is associated with lower hospitalization rates and healthcare costs in patients with schizophrenia: real-world evidence from US claims data. Curr Med Res Opin. 2019;35(7):1231-1239. PubMed CrossRef
54.Schaffer A, Isometsפ ET, Tondo L, et al. Epidemiology, neurobiology and pharmacological interventions related to suicide deaths and suicide attempts in bipolar disorder: Part I of a report of the International Society for Bipolar Disorders Task Force on Suicide in Bipolar Disorder. Aust N Z J Psychiatry. 2015;49(9):785-802. PubMed CrossRef
55.Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254-1260. PubMed CrossRef
56.Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder: expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492. PubMed CrossRef
57.Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord. 2013;149(1-3):247-252. PubMed CrossRef
58.Mazza M, Mandelli L, Di Nicola M, et al. Clinical features, response to treatment and functional outcome of bipolar disorder patients with and without co-occurring substance use disorder: 1-year follow-up. J Affect Disord. 2009;115(1-2):27-35. PubMed CrossRef
59.Manwani SG, Szilagyi KA, Zablotsky B, et al. Adherence to pharmacotherapy in bipolar disorder patients with and without co-occurring substance use disorders. J Clin Psychiatry. 2007;68(8):1172-1176. PubMed CrossRef
60.Simhandl C, König B, Amann BL. A prospective 4-year naturalistic follow-up of treatment and outcome of 300 bipolar I and II patients. J Clin Psychiatry. 2014;75(3):254-262, quiz 263. PubMed CrossRef
61.Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65(4):386-394. PubMed CrossRef
62.Brooks JO 3rd, Goldberg JF, Ketter TA, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry. 2011;72(2):240-247. PubMed CrossRef
63.Samalin L, Charpeaud T, Blanc O, et al. Clinicians’ attitudes toward the use of long-acting injectable antipsychotics. J Nerv Ment Dis. 2013;201(7):553-559. PubMed CrossRef
64.Sajatovic M, DiBiasi F, Legacy SN. Attitudes toward antipsychotic treatment among patients with bipolar disorders and their clinicians: a systematic review. Neuropsychiatr Dis Treat. 2017;13:2285-2296. PubMed CrossRef
This PDF is free for all visitors!