Objective: The aim of this systematic review and meta-analysis was to determine the effect of antidepressant discontinuation on the risk of relapse of depression during pregnancy.
Data Sources: MEDLINE, EMBASE, CINAHL, and PsycInfo were searched from the inception of each database through March 2019 using keywords such as antidepressants, pregnancy, preconception, discontinuation, stop, recurrence, reintroduction, and relapse.
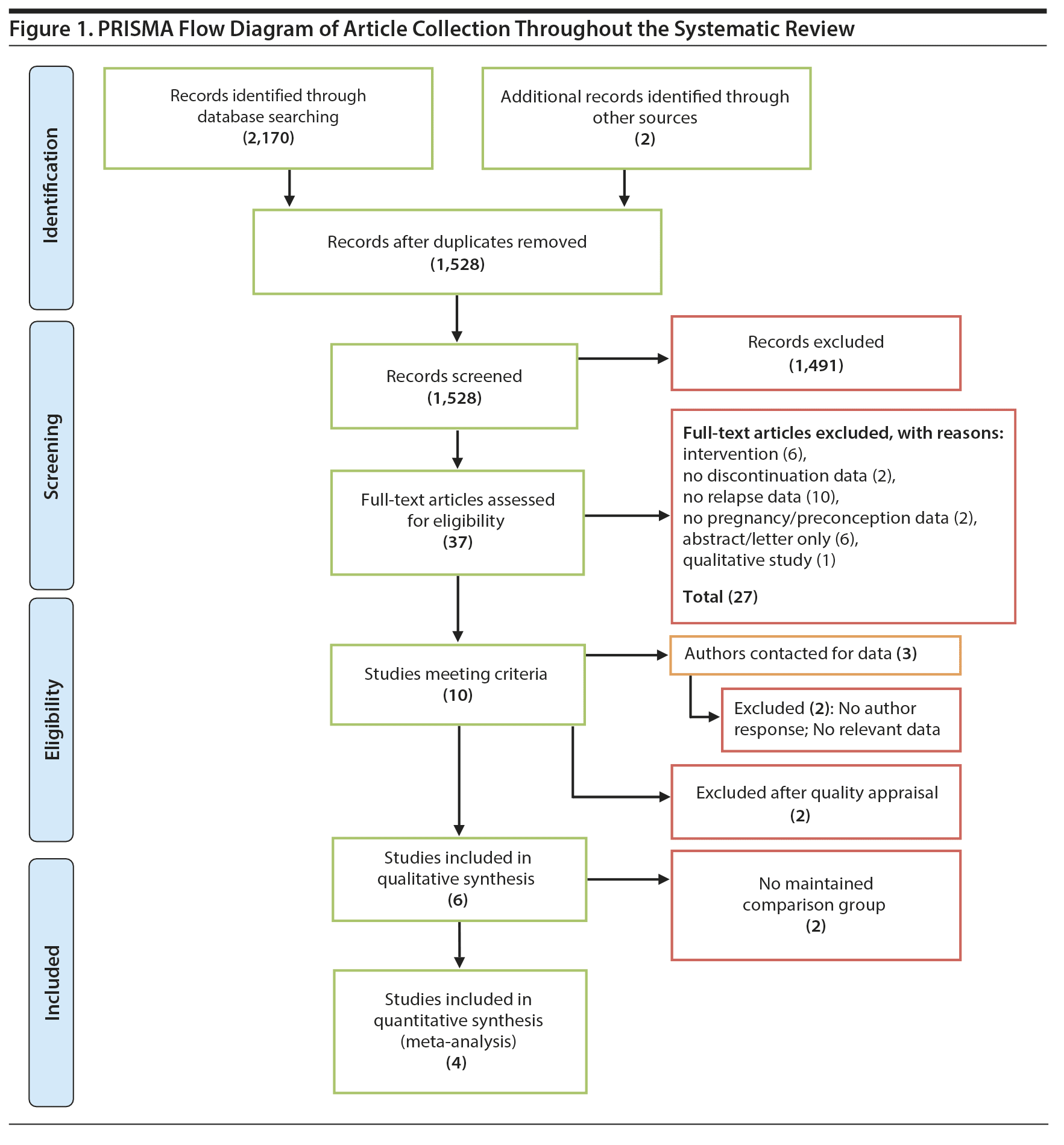
Study Selection: Original studies that involved pregnant women who discontinued antidepressants during preconception (ie, 3 months prior to pregnancy) or pregnancy and examined the relapse of depression during pregnancy (ie, the reemergence of depression or reintroduction of medication) and published in English were included. A total of 2,172 records were identified, and the full texts of 37 articles were reviewed. Eight studies met the inclusion criteria, 6 of which fulfilled the quality criteria, with 4 studies providing data for the meta-analysis.
Data Extraction: Data were extracted using a data extraction form developed for the purpose of this study. The Cochrane Collaboration Review Manager software version 5.3 was used to conduct the meta-analysis.
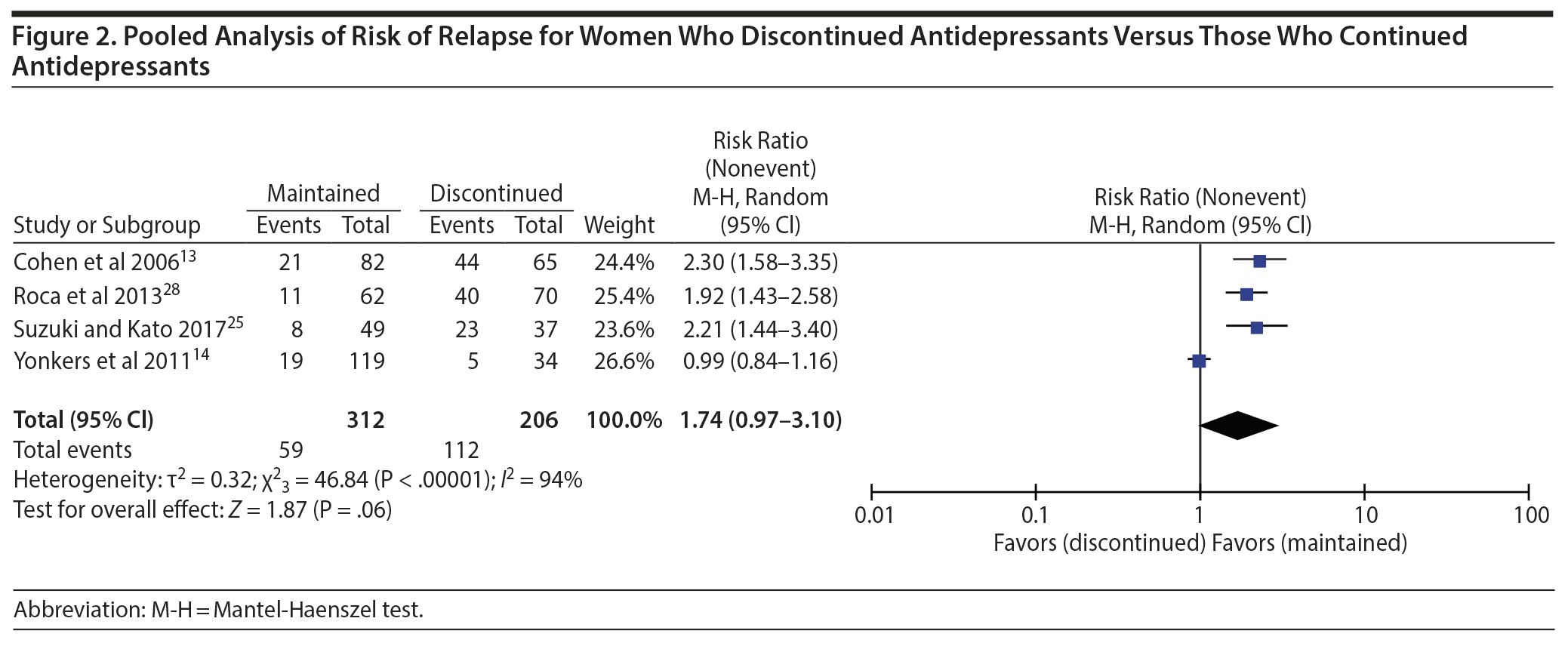
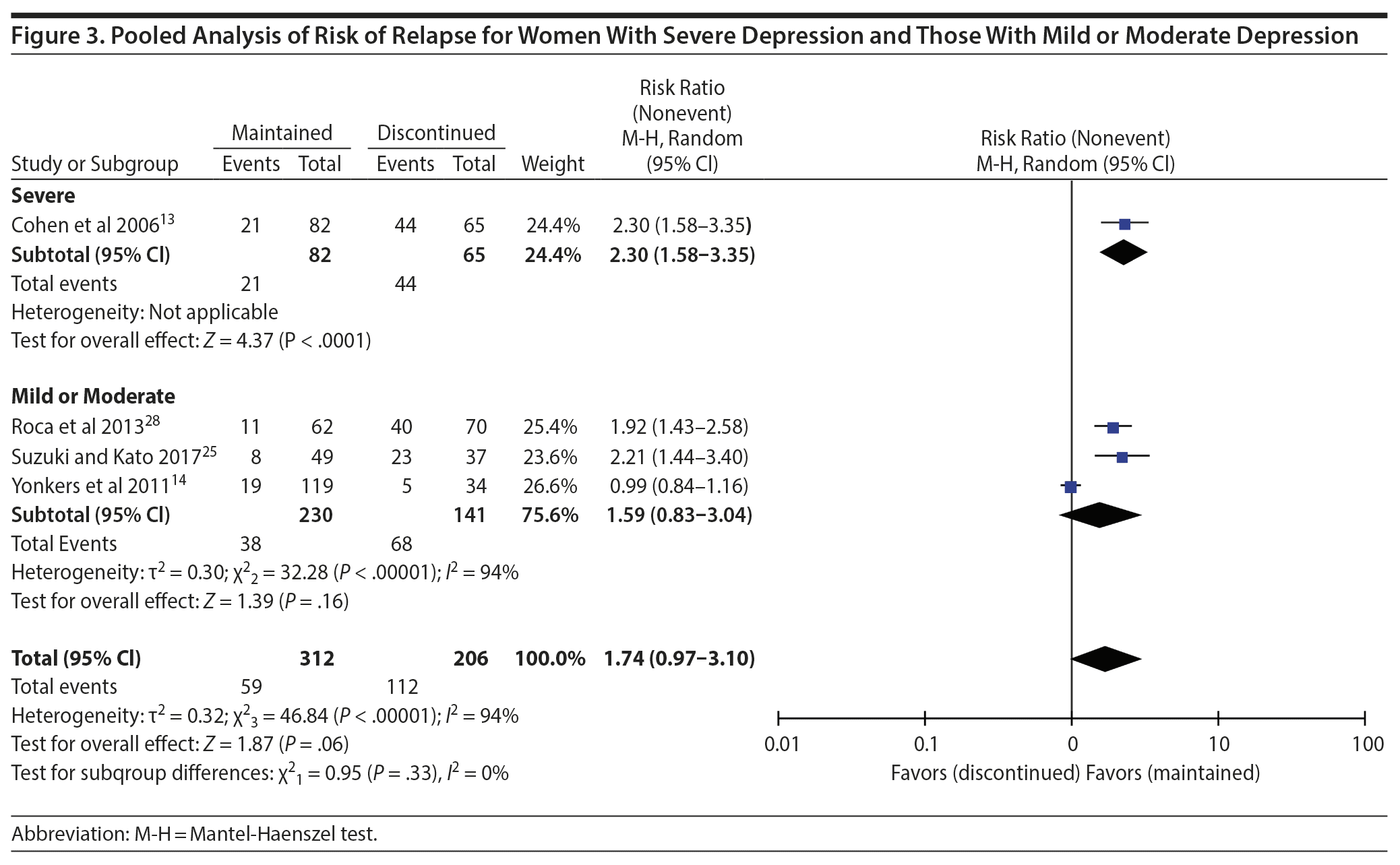
Results: Pooled data did not show higher risk of relapse of depression during pregnancy for women who discontinued antidepressants than for those who continued antidepressants (risk ratio [RR] = 1.74; 95% CI, 0.97 to 3.10; P = .06). In the subanalysis based on the severity and recurrence of depression in the study populations, the risk of relapse was significantly higher for populations suggestive of severe or recurrent depression (RR = 2.30; 95% CI, 1.58 to 3.35) but not for populations suggestive of mild or moderate depression severity (RR = 1.59; 95% CI, 0.83 to 3.04).
Conclusions: Women with severe or recurrent depression should be informed about the increased risk of relapse following antidepressant discontinuation, and those who discontinue antidepressants should be monitored for relapse.

ABSTRACT
Objective: The aim of this systematic review and meta-analysis was to determine the effect of antidepressant discontinuation on the risk of relapse of depression during pregnancy.
Data Sources: MEDLINE, EMBASE, CINAHL, and PsycInfo were searched from the inception of each database through March 2019 using keywords such as antidepressants, pregnancy, preconception, discontinuation, stop, recurrence, reintroduction, and relapse.
Study Selection: Original studies that involved pregnant women who discontinued antidepressants during preconception (ie, 3 months prior to pregnancy) or pregnancy and examined the relapse of depression during pregnancy (ie, the reemergence of depression or reintroduction of medication) and published in English were included. A total of 2,172 records were identified, and the full texts of 37 articles were reviewed. Eight studies met the inclusion criteria, 6 of which fulfilled the quality criteria, with 4 studies providing data for the meta-analysis.
Data Extraction: Data were extracted using a data extraction form developed for the purpose of this study. The Cochrane Collaboration Review Manager software version 5.3 was used to conduct the meta-analysis.
Results: Pooled data did not show higher risk of relapse of depression during pregnancy for women who discontinued antidepressants than for those who continued antidepressants (risk ratio [RR] = 1.74; 95% CI, 0.97 to 3.10; P = .06). In the subanalysis based on the severity and recurrence of depression in the study populations, the risk of relapse was significantly higher for populations suggestive of severe or recurrent depression (RR = 2.30; 95% CI, 1.58 to 3.35) but not for populations suggestive of mild or moderate depression severity (RR = 1.59; 95% CI, 0.83 to 3.04).
Conclusions: Women with severe or recurrent depression should be informed about the increased risk of relapse following antidepressant discontinuation, and those who discontinue antidepressants should be monitored for relapse.
J Clin Psychiatry 2020;81(4):19r13134
To cite: Bayrampour H, Kapoor A, Bunka M, et al. The risk of relapse of depression during pregnancy after discontinuation of antidepressants: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(4):19r13134.
To share: https://doi.org/10.4088/JCP.19r13134
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Family Practice, University of British Columbia, Vancouver, British Columbia, Canada
bReproductive Mental Health Program, BC Children’s Hospital, Vancouver, British Columbia, Canada
*Corresponding author: Hamideh Bayrampour, MSc, PhD, Department of Family Practice, University of British Columbia, Vancouver, BC, V6T 1Z3, Canada ([email protected]).
Depression is common during pregnancy with an overall prevalence rate of 12%.1 The rate of antidepressant use among pregnant women varies between 1.8% and 8% in different countries and settings.2-4 Pregnancy is a major factor for antidepressant discontinuation,5 as pregnant recipients are 5 times more likely to discontinue use than nonpregnant recipients.2,5 Only 1 in 4 women undergoing antidepressant treatment before conception reports taking antidepressants in the third trimester.6 Avoiding fetal exposure is the main reason women discontinue antidepressants.5
There are increased risks of adverse pregnancy and child outcomes associated with untreated depression.7-9 Prenatal exposure to antidepressants might also increase the risk of the adverse outcomes.10,11 However, it is challenging to disentangle the risk of exposure to antidepressants from underlying clinical and social factors associated with the indication of use.12 While numerous studies have been conducted to examine the risks associated with prenatal exposure to antidepressants, the effects of antidepressant discontinuation, including the risk of relapse, remain unclear. In 2006, in a prospective cohort study of 201 women, Cohen et al13 reported an overall relapse rate of 43% during pregnancy. They found that women who discontinued medication were more likely to experience a relapse of major depression than those who maintained their medication. However, another prospective study conducted in 201114 did not replicate such findings. It found that the risk for onset of a major depressive episode was similar between women who continued and those who discontinued antidepressants. In the general population, a 2015 meta-analysis15 showed a 2-fold increased risk of relapse after antidepressant discontinuation. Another systematic review16 among the general population showed that antidepressant discontinuation is associated with an increased risk of suicide attempts. Similar evidence during the perinatal period is scarce. A recent international review17 of the practice guidelines for the treatment of depression and the use of antidepressants during pregnancy revealed that 4 guidelines advised continuing antidepressants while 5 other guidelines did not explicitly advise or discourage continuation.
The aim of this systematic review and meta-analysis was to identify the current evidence in this area and determine the pooled effect of antidepressant discontinuation on the risk of relapse of depression during pregnancy. We also synthesized the identified evidence to determine factors contributing to antidepressant discontinuation and relapse of depression and the risk of suicidal ideation or suicide attempts after antidepressant discontinuation. This knowledge can aid decision-making regarding antidepressant use during pregnancy.
METHODS
Sources
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.18 An electronic literature search that included keywords such as antidepressants, pregnancy, preconception, discontinuation, stop, recurrence, reintroduction, and relapse was conducted using MEDLINE, EMBASE, CINAHL, and PsycINFO for studies dating from the inception of each database to March 2019 (see Supplementary Appendix 1 for the complete list of search strategies and results). The reference lists of the included articles and trial registries in ClinicalTrials.gov were reviewed for additional citations. Gray literature and unpublished studies were not retrieved. Since this was a systematic review, an institutional ethics board approval was not required.
Study Selection
We included original studies that (1) involved pregnant women who discontinued antidepressants during preconception (ie, 3 months prior to pregnancy)19 or pregnancy, (2) assessed relapse of depression during pregnancy, and (3) were published in English. If a study met the inclusion criteria but did not report relapse rates, the authors of the study were contacted. The presence of a comparison group was not an inclusion criterion; however, only studies with a comparison group were included in the meta-analysis. The exclusion criteria were case reports and studies that used other interventions for depression treatment or medication adherence. The main outcome was the rate of relapse of depression during pregnancy after antidepressant discontinuation. Relapse was defined as the reemergence of depression (ascertained by a clinical diagnosis or valid tools) or reintroduction of medication after discontinuation. Secondary outcomes included factors contributing to antidepressant discontinuation and relapse of depression and the risk of suicide ideation/attempts after discontinuation. Three authors (H.B., A.K., M.B.) independently reviewed the abstracts and full text of articles and extracted the data. Disagreements were resolved by discussion.
The following data were extracted from each included study: study design, participant eligibility criteria, sample size and characteristics (eg, age at the first onset of depression, number of depressive episodes, medication types), and outcomes. Extracted data were synthesized by two reviewers independently (H.B., A.K.) to identify the rates of relapse, factors contributing to antidepressant discontinuation and relapse, and the risk of suicidal ideation or suicide attempts after antidepressant discontinuation. To quantify and determine the risk of relapse of depression after antidepressant discontinuation, we conducted a meta-analysis using the Cochrane Collaboration Review Manager software version 5.3.20 The rates of relapse of depression for the discontinued and maintained antidepressant groups were compared, and the risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using random-effects models. Statistical heterogeneity was examined using I2 values.

- In the literature, the risk of relapse of depression during pregnancy after antidepressant discontinuation is unclear.
- Populations with mild and moderate depression severity or recurrence did not appear to experience significant relapse during pregnancy following antidepressant discontinuation.
- Populations with severe or recurrent depression were at increased risk of relapse during pregnancy following antidepressant discontinuation.
We also performed a predefined subgroup analysis by stratifying the results based on the depression severity and recurrence of the study population. In previous research,21 the number of prior episodes of illness has been used to classify depression severity as mild or severe. In this review, in addition to the number of prior episodes, we used the age at the first onset and recruitment setting as extra indicators of illness severity and recurrence. Depression severity and recurrence were determined post hoc based on the following indicators: recruitment setting (regular prenatal clinics vs specialized psychiatric clinics), age at the first onset of depression (> 18 years), and the number of recurrent episodes. Using these characteristics when available, two reviewers (H.B. and A.K.) independently categorized the studies into two groups: studies whose populations had mild or moderate depression and studies whose populations had high depression severity or recurrence of depression (ie, severe/recurrent depression). Quality appraisal was performed using the Scottish Intercollegiate Guidelines Network (SIGN) methodology checklist.22 This checklist includes 14 items, 12 of which evaluate various biases, including selection, performance, attrition, and detection. Two reviewers (H.B. and A.K.) independently determined the risk of each type of bias by evaluating the corresponding items in the SIGN methodology checklist. The Cochrane Collaboration Review Manager risk-of-bias graph was adopted to present this information (Supplementary Figure 1).
RESULTS
A total of 2,172 records were identified. After removal of duplicates and screening of titles and abstracts, the full text of 37 articles was retrieved and reviewed. Using a PRISMA flow diagram, Figure 1 illustrates the search and selection processes and reasons for exclusion at each review stage. Ten studies met the inclusion criteria, 3 of which did not provide relapse data,14,23,24 and the authors were contacted. Deidentified data from the study by Yonkers et al14 were obtained through an agreement between the University-Industry Liaison Office at the University of British Columbia and the Office of Sponsored Projects at Yale University. Relapse rates in the study by Molenaar et al24 were confirmed through e-mail correspondence with the first author. However, discontinuation of antidepressants in this study took place anytime during pregnancy and 3 months postpartum; thus, this study was excluded. The authors of the third study23 did not respond. Overall, 8 studies2,13,14,21,25-28 were considered for quality appraisal and the risk-of-bias assessment. All studies were observational studies, and we used the SIGN methodology checklist. Based on the criteria listed in this checklist, the quality of 6 studies13,14,21,25,26,28 was rated high or acceptable, and the quality of 2 studies2,27 was rated low. The risk of bias was low in 1 study.13 Five studies14,21,25,26,28 had a moderate/unclear risk of bias, 3 of which25,26,28 did not provide sufficient methodological details; thus, several areas were coded as unclear. Two studies2,27 had a high risk of performance and detection biases with unclear selection and attrition biases and were excluded.
Synthesis of the Review Findings
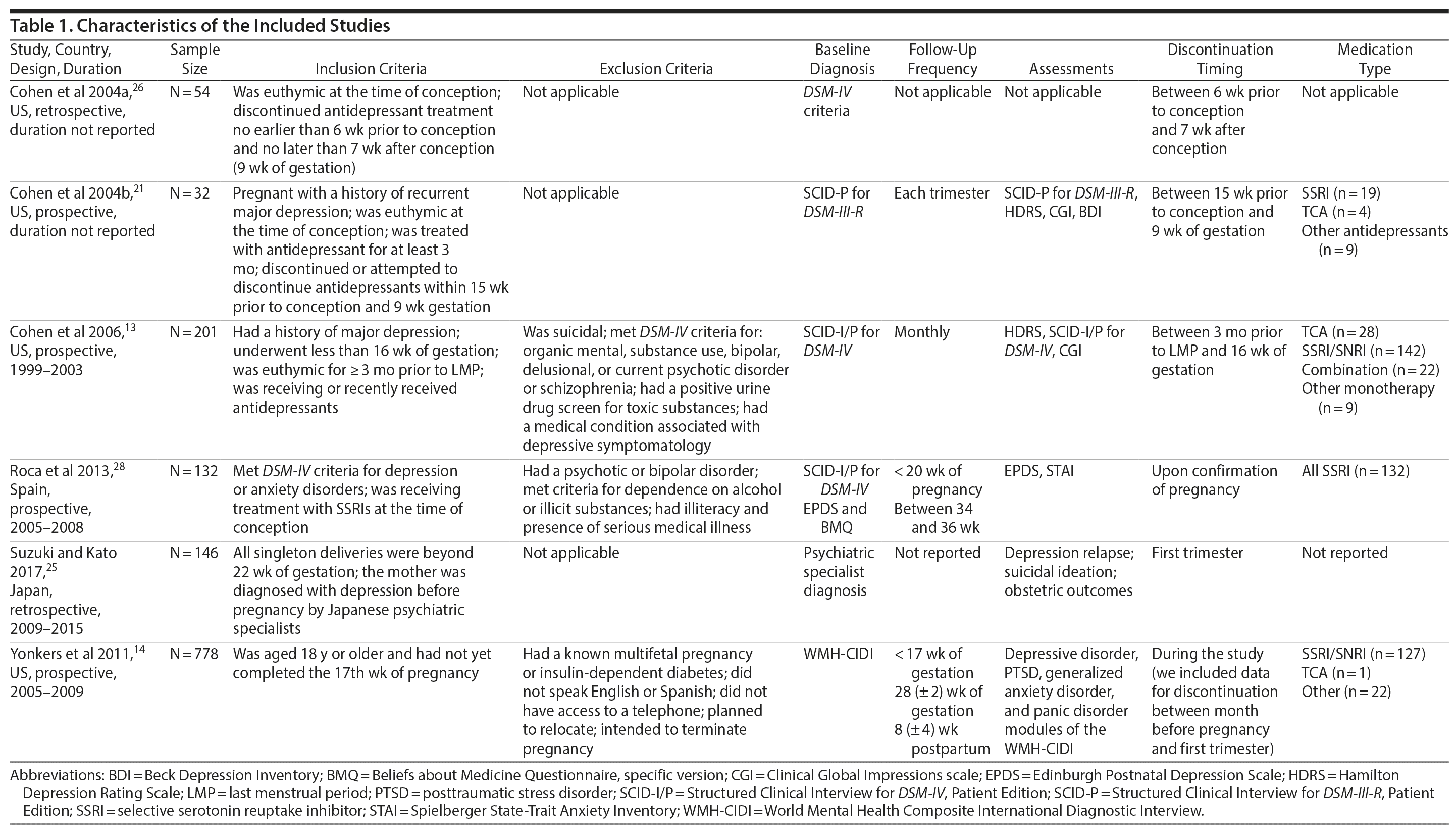
All 6 included studies were observational cohort studies; 4 were prospective studies,13,14,21,28 and 2 were retrospective studies.25,26 Four studies were conducted in the United States,13,14,21,26 1 in Spain,28 and 1 in Japan.25 At baseline, the presence or absence of depression was examined using DSM-III-R21 or DSM-IV criteria,13,26,28 the World Mental Health Composite International Diagnostic Interview,14 or a psychiatric specialist’s diagnosis.25 All prospective studies13,14,21,28 included at least 2 study visits; 1 study21 required a visit every trimester, and 1 study13 required monthly study visits. The study characteristics are presented in Table 1.
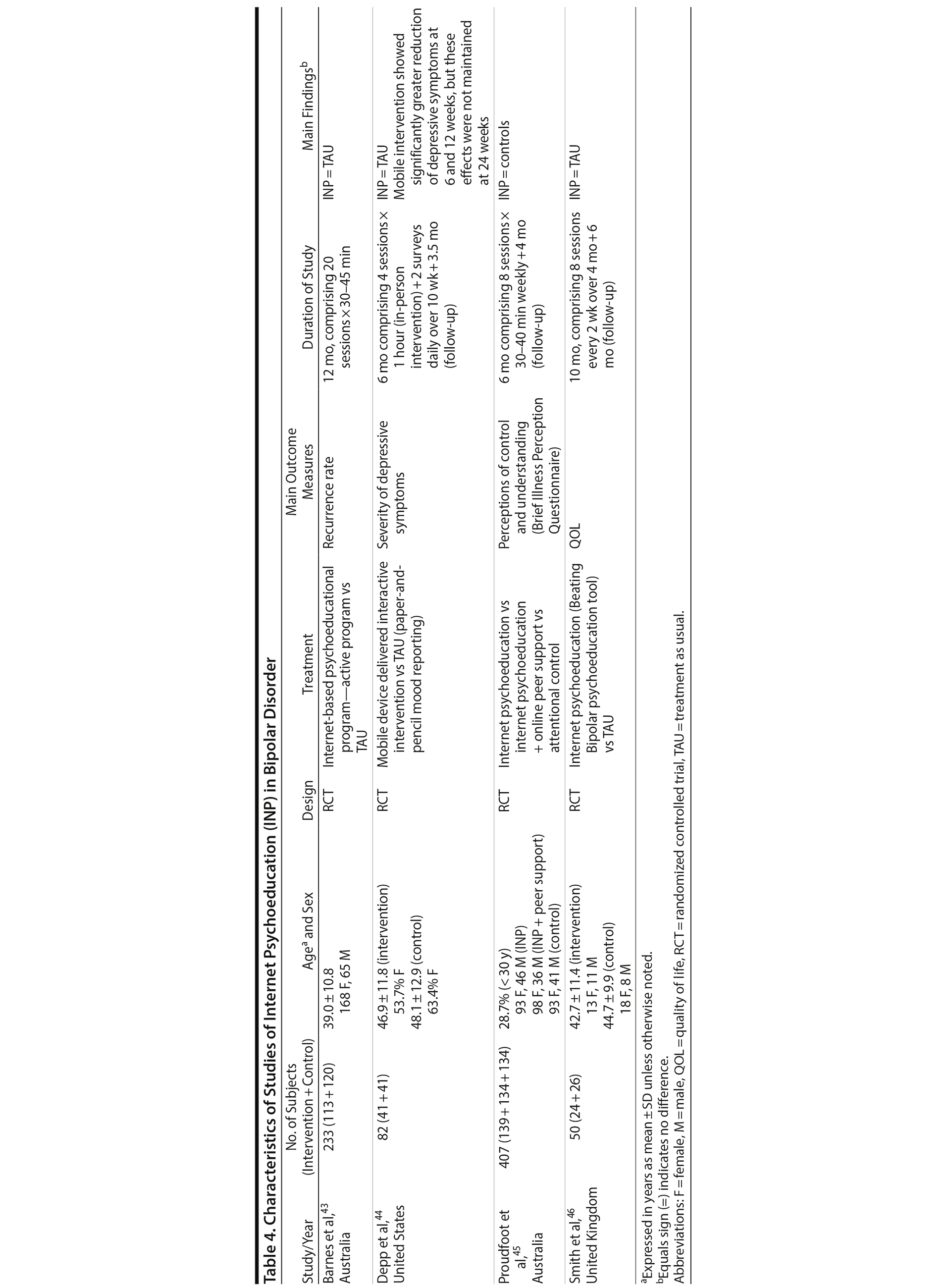
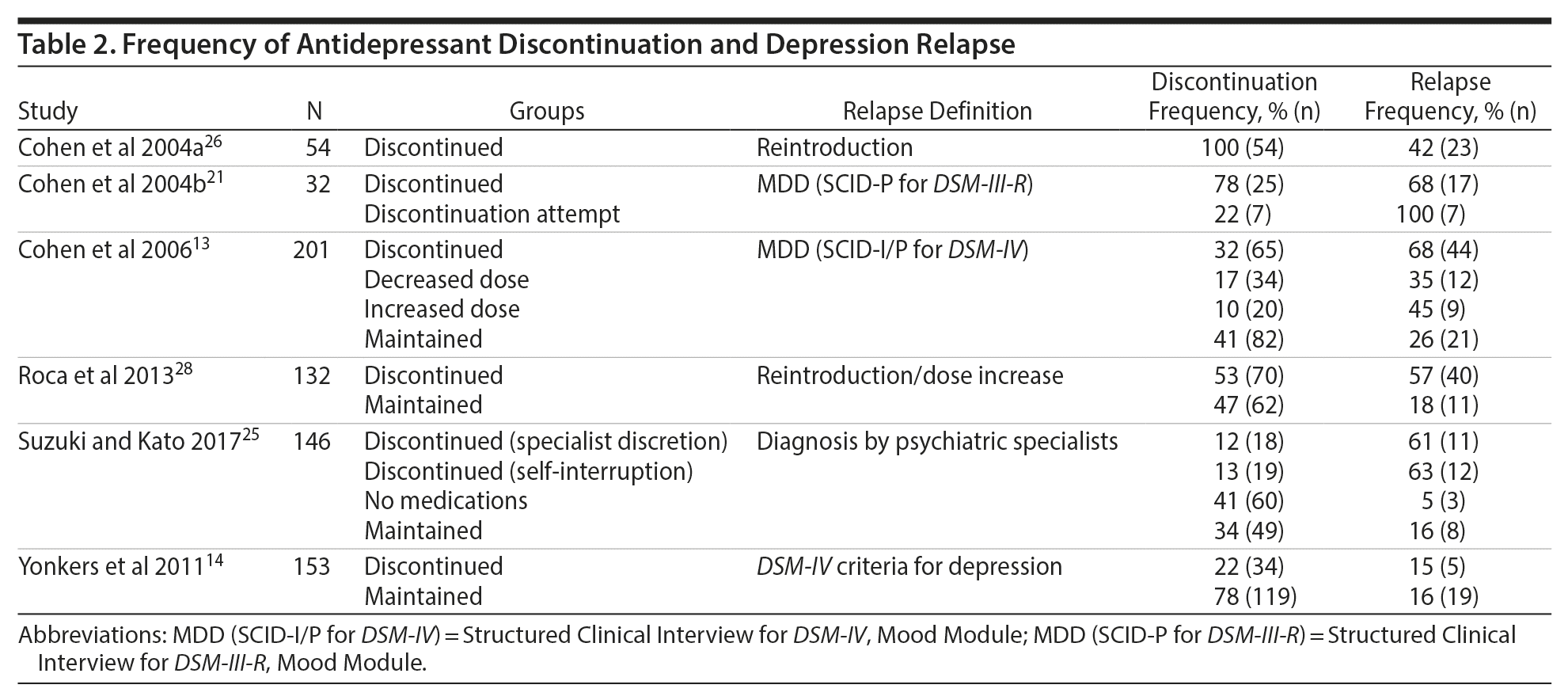
All studies except 126 included at least 1 comparison group. The control group in 1 of these studies21 consisted of women who decreased the antidepressant dose. Three studies included additional comparison groups consisting of patients who intended to taper use or attempted discontinuation,21 increased or decreased the medication dose,13 or interrupted medication based on the discretion of psychiatric specialists or by self-interruption.25 The frequency of discontinuation (Table 2) varied from 22%14 to 78%.21 A fear of the influence of antidepressant medications on the fetus was the main reason for interrupted medication use as reported in 1 included study.25 The types of medications were reported in 4 studies.13,14,21,28 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) were the most common types of medications, constituting between 59%21 and 71%13 of the prescribed medications. For example, in the 2006 study by Cohen et al,13 142 of 201 women reported taking SSRIs or SNRIs. Women who maintained their medication were more likely to be receiving an SSRI regimen than women who chose to alter their therapy regimen.13 Single pregnant women13 or those with an unplanned pregnancy28 were more likely to discontinue treatment. Other factors did not differ significantly between the maintained and discontinued antidepressant groups.13,28
Studies defined relapse of depression as a fulfillment of DSM criteria,13,14,21 a diagnosis ascertained by a psychiatric specialist,25 an increase in the antidepressant dose,28 or a reintroduction of antidepressant medication.26 The frequency of relapse ranged from 15%14 to 68%,13,21 with 4 studies13,21,25,28 reporting a relapse frequency of approximately 60% or greater. All studies reported that the highest relapse rates occurred in the first trimester. Younger (< 32 years of age)13 nulliparous women25 were at greater risk of relapse. Cohen et al (2006)13 also observed that single women tended to have a higher risk of relapse than married women. No significant associations between ethnicity, educational level, baseline antidepressant treatment, and the risk of relapse were reported.13 The chronicity of illness was the most commonly reported predictor of relapse.13,21,26 Women with a duration of depressive illness of more than 5 years had nearly a 3-fold increased risk of relapse.13 Women with a history of severe depression also tended to relapse more quickly than women with mild depression (80% vs 38%).21 Other predictors of relapse included the number of recurrent episodes,13,14 high Edinburgh Postnatal Depression Scale scores during early pregnancy,28 and history of suicide attempts.26 Two studies13,21 examined the effect of tapering medication or decreasing dose on relapse of depression. In a study of 32 participants,21 all women who decreased the antidepressant dose from the dosage used to maintain euthymia experienced relapse. In another study with 201 participants,13 the women who decreased their medication had a 35% rate of relapse, which was between the rate of those who maintained (26%) and those who discontinued (68%) antidepressants.
Only 1 study25 examined the effect of relapse on obstetric outcomes, and the effect was null. Among the women who experienced relapse after discontinuing antidepressants, depression was improved by the resumption of medications.25 Medication reintroduction also reduced the risk of relapse; however, the risk remained substantially greater than that for women who retained their medication regimen throughout pregnancy.13 Suzuki and Kato25 reported that 3 in 37 women in the discontinued antidepressant group had suicidal ideations, while 1 in 49 women in the continued antidepressant group had suicidal ideations. The difference was not statistically significant. Cohen et al (2006)13reported no suicidal attempts among the participants during the course of the study.
Meta-Analysis
Four studies13,14,25,28 had a comparison group consisting of women who maintained antidepressants and were included in the meta-analysis. The total sample included 518 women; 302 women in the maintained group and 206 women in the discontinued group. In the first analysis, we compared the risk of relapse among women who maintained antidepressants to that of women who discontinued antidepressants using random-effects models. Pooled data from all studies did not show higher risk of relapse for women who discontinued antidepressants than for those who continued antidepressants (RR = 1.74; 95% CI, 0.97 to 3.10; P = .06; Figure 2). For the subanalysis based on the severity or recurrence of depression, 3 studies14,25,28 met the criteria for populations suggestive of mild or moderate severity and 1 study13 included a population suggestive of severe/recurrent depression. In this analysis, the risk of relapse was significantly higher for population suggestive of severe/recurrent depression (RR = 2.30; 95% CI, 1.58 to 3.35) but not for those of mild or moderate severity (RR = 1.59; 95% CI, 0.83 to 3.04) (Figure 3). Tests for funnel plot asymmetry to determine publication bias were not performed due to the small number of included studies (ie, < 10).29
DISCUSSION
The findings of this meta-analysis showed that, overall, the discontinuation of antidepressants is not associated with a significant increased risk of relapse of depression during pregnancy. In this review, the main predictors of relapse included the illness chronicity,13,21,26 number of previous recurrent episodes,13,14 and history of suicide attempts,26 suggesting that greater depressive illness severity may contribute to an increased risk of relapse. In the subgroup analysis based on depression severity or recurrence, the risk of relapse among studies with populations suggestive of mild or moderate depression remained nonsignificant. However, for the population suggestive of severe/recurrent depression, the risk of relapse after discontinuation of antidepressants was more than 2 times higher than those who maintained treatment. A meta-analysis of 72 trials of major depressive disorders in the general population15 showed a greater risk of relapse or recurrence of a depressive disorder in participants who received a placebo than those who received medications (RR = 1.90; 95% CI, 1.73 to 2.08).
The evidence on the risk of suicide following antidepressant discontinuation during pregnancy is scarce. A few case studies30,31 have reported suicide incidents following antenatal antidepressant discontinuation. In a small study of 36 women, Einarson et al32 reported suicidal ideation in almost one-third of the patients after antidepressant discontinuation. Among the general population, antidepressant discontinuation is associated with an increased risk of suicide attempts (odds ratio = 1.61; 95% CI, 1.34 to 1.92).16 In this review, only 2 studies13,25 reported data on suicidal ideation or attempts following antidepressant discontinuation, and both studies reported no significant differences. Suzuki and Kato25 observed that women who discontinued antidepressants tended to have a higher risk of suicidal ideation than those who continued (8% vs 2%). This finding is noteworthy given that suicide is a rare outcome and that women who discontinued antidepressants were more likely to experience severe depression25 or remain at higher risk for relapse despite the resumption of medication.13
Many women discontinue antidepressants during pregnancy, mostly to prevent fetal exposure.25 There are increased risks of adverse pregnancy and child outcomes associated with prenatal exposure to antidepressants.10,11,33 On the other hand, untreated depression can also increase the risk of poor pregnancy and child outcomes.7-9 Nonetheless, there is not strong evidence that the use of antidepressants to treat depression during pregnancy can improve these outcomes,34 prevent postpartum depression,35-37 or improve infant-mother attachment.38 A recent systematic review34 that examined the comparative effects of antidepressant medications and untreated major depression found that the risk of low birth weight (LBW) and related pregnancy outcomes did not differ between antidepressant-treated women and untreated depressed women. In a large population-based study, there were no significant differences in the risk of preterm birth (PTB) and LBW among women who received either SSRIs or other antidepressants during pregnancy and that of women who discontinued antidepressants before pregnancy. Both groups had a higher risk of PTB and LBW than those who never received antidepressants.39 Similarly, there is not strong evidence that the use of non-pharmacologic interventions for depression is effective in improving birth outcomes.40,41 In a randomized controlled trial of 226 pregnant women,40 no significant differences in the gestational age, birth weight, or Apgar scores were found when comparing the cognitive-behavioral therapy (CBT) group with the care-as-usual group. In this review, only 1 study25 examined pregnancy outcomes associated with antidepressant discontinuation, and the study reported no significant differences between the PTB and cesarean birth of women who discontinued antidepressants or relapsed and those of women who continued medications.
In this review, the rate of relapse despite maintaining antidepressants varied between 7% and 26%. A recent study42 reported that 20% of pregnant women receiving antidepressants remained depressed. Cohen et al13 reported that 10% of pregnant women in their study required an increase in their antidepressant dose. This group appeared distinct from those who maintained their antidepressant treatment, as this group had a higher risk of relapse of depression (45% vs 26%) and a more rapid time to relapse. In another study,43 two-thirds of women required an increase in their daily dose, mostly around 27 weeks of gestation, to maintain euthymia. No associations were detected between age, educational level, or personal and familial psychiatric history and dose adjustment. Antidepressant metabolism during pregnancy can vary among individuals based on the expression of certain genes.44 In a recent study,42 a significantly higher proportion of women in the fast metabolizer group had depressive symptoms in the first trimester than that of women in the slow metabolizer group. This evidence highlights the heterogeneity of pregnant women in terms of their response to antidepressants and suggests that each individual, depending on her unique genotypes and personal history, may require specific changes in antidepressant dosage to maintain therapeutic plasma levels. The practice of precision medicine has been encouraged, but genetic testing is currently not mandatory to initiate treatment.42,45 A further understanding of the interactions between phenotypes and genotypes can have important implications to inform appropriate treatment adjustments during pregnancy.
Two studies13,21 examined the relapse risk after tapering antidepressants. Among women who decreased their medication dose from a previous optimal dose or discontinued 1 antidepressant from a combination regimen, the relapse risk was slightly higher than that of women who maintained consistent antidepressant therapy throughout pregnancy, but the relapse risk was lower than that of women who discontinued antidepressants (35%, 26%, and 68%, respectively).13 In a smaller study,21 all women who decreased the antidepressant dose experienced relapse. Recent trials among the general population have demonstrated that the tapering of antidepressants combined with preventive CBT can be as effective as the continuation of the same dose.46 Similar evidence during the perinatal period is scarce but emerging; in an open preliminary study of CBT for the prevention of depression recurrence among pregnant women who discontinued antidepressant treatment,47 75% of the women did not relapse after the completion of 12 CBT sessions. In a recent trial on the treatment of postpartum depression,48 a combination of psychotherapeutic interventions (ie, detached mindfulness and stress management training) with a standard SSRI was shown to be superior to and have a longer-lasting improvement than the use of only antidepressants.
The use of clinical diagnostic interviews to ascertain relapse in the majority of included studies is one of the strengths of our findings. Other strengths include the application of strict criteria for quality appraisal and risk-of-bias assessment and the involvement of 3 reviewers in most stages of the review. Several limitations of this review should be considered when interpreting the findings. The number of included studies and their sample sizes were relatively small. There was significant heterogeneity among the included studies. Only 1 study13 had a low risk of bias, and it was the only study in the subanalysis that met the criteria for the category of populations with severe/recurrent depression. The included studies did not specifically report the severity of prior episodes of depression. Thus, we used the number of prior episodes, age at the first onset of depression, and recruitment setting as proxy to estimate depression severity or recurrence. Being euthymic at the time of recruitment was an inclusion criterion in 3 studies. Two studies used reintroduction as an indicator for relapse.21,28 However, the sensitivity analysis did not change the direction of the total effect (results not shown). Only 113 of the included studies in the meta-analysis reported relapse information for subgroups of maintained group based on medication dosage (ie, no change, decrease, increase).
Every person receiving antidepressants who is planning a pregnancy or becomes pregnant encounters a difficult dilemma. The absence of confirmative evidence regarding the effectiveness of depression treatments to improve birth outcomes intensifies this dilemma by necessitating the weighing of the maternal benefits of using antidepressants with the risk of prenatal exposure. The findings of our review showed that the discontinuation of antidepressants did not increase the risk of relapse during pregnancy; however, there was a trend toward the increased risk of relapse (P = .06). A significant number of pregnant women (up to 75%) discontinue antidepressants.6 Women may choose to discontinue antidepressants on their own or after consulting a health professional.32 While further studies are required to compare the relapse risk of these two groups, the limited available data show that the risk of relapse increases after discontinuation regardless of how the decision was made.25
The general consensus among clinical practice guidelines is that the potential harms and benefits of antidepressants should be discussed with pregnant women so they can make well-informed decisions.17 Beliefs about the necessity of medications and the perception that the benefits of antidepressants outweigh the risks are important factors in the continuation of and adherence to medications.23,24,49 As Miller50 noted when explaining the risks of antidepressants during pregnancy, maternity care providers should avoid errors of omission by explaining the risks of discontinuing treatment. While the effectiveness and safety of psychotherapy interventions as a complete alternate therapy for women who discontinue antidepressants remain to be further examined, there is evidence that a combination of psychotherapy and pharmacotherapy interventions is as effective as antidepressants when tapering off medications,46,51 is more effective than the use of antidepressants alone,46,48 and can increase medication adherence.32,52 Women with severe/recurrent depression should be informed about the increased risk of relapse following antidepressant discontinuation, and those who discontinue antidepressants should be monitored for relapse.
Submitted: October 24, 2019; accepted January 31, 2020.
Published online: June 9, 2020.
Author contributions: Dr Bayrampour conceptualized the study; acquired, analyzed, and interpreted the data; and drafted the manuscript. Dr Kapoor conducted the literature search; acquired, analyzed, and interpreted the data; and drafted parts of the Methods and Results sections of the manuscript. Ms Bunka acquired and interpreted the data and revised the manuscript for important intellectual content. Dr Ryan contributed to the study design and interpretation of data and revised the manuscript for important intellectual content. All authors have approved the final version of the manuscript.
Potential conflicts of interest: The authors declare no competing interests.
Funding/support: There was no funding source for this study.
Supplementary material: See accompanying pages.
REFERENCES
1.Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86-92. PubMed CrossRef
2.Bénard-Laribiרre A, Pambrun E, Sutter-Dallay AL, et al. Patterns of antidepressant use during pregnancy: a nationwide population-based cohort study. Br J Clin Pharmacol. 2018;84(8):1764-1775. PubMed CrossRef
3.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1-194.e5. PubMed CrossRef
4.Zoega H, Kieler H, N׸rgaard M, et al. Use of SSRI and SNRI antidepressants during pregnancy: a population-based study from Denmark, Iceland, Norway and Sweden. PLoS One. 2015;10(12):e0144474. PubMed CrossRef
5.Petersen I, Gilbert RE, Evans SJW, et al. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry. 2011;72(7):979-985. PubMed CrossRef
6.Ververs T, Kaasenbrood H, Visser G, et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863-870. PubMed CrossRef
7.Zou R, Tiemeier H, van der Ende J, et al. Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. Am J Psychiatry. 2019;176(9):702-710. PubMed CrossRef
8.El Marroun H, Zou R, Muetzel RL, et al. Prenatal exposure to maternal and paternal depressive symptoms and white matter microstructure in children. Depress Anxiety. 2018;35(4):321-329. PubMed CrossRef
9.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321-e341. PubMed CrossRef
10.Nielsen SW, Ljungdalh PM, Nielsen J, et al. Maternal use of selective serotonin reuptake inhibitors during pregnancy is associated with Hirschsprung’s disease in newborns—a nationwide cohort study. Neonatal Intensive Care. 2018;31(3):29-35.
11.Jordan S, Morris JK, Davies GI, et al. Selective serotonin reuptake inhibitor (SSRI) antidepressants in pregnancy and congenital anomalies: analysis of linked databases in Wales, Norway and Funen, Denmark. PLoS One. 2016;11(12):e0165122. PubMed CrossRef
12.Prady SL, Hanlon I, Fraser LK, et al. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring’s outcomes. Arch Women Ment Health. 2018;21(2):127-140. PubMed
13.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507. PubMed CrossRef
14.Yonkers KA, Gotman N, Smith MV, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848-854. PubMed
15.Sim K, Lau WK, Sim J, et al. Prevention of relapse and recurrence in adults with major depressive disorder: systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;19(2):pyv076. PubMed CrossRef
16.Valuck RJ, Orton HD, Libby AM. Antidepressant discontinuation and risk of suicide attempt: a retrospective, nested case-control study. J Clin Psychiatry. 2009;70(8):1069-1077. PubMed CrossRef
17.Molenaar NM, Kamperman AM, Boyce P, et al. Guidelines on treatment of perinatal depression with antidepressants: an international review. Aust N Z J Psychiatry. 2018;52(4):320-327. PubMed CrossRef
18.Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. PubMed CrossRef
19.Gnoth C, Godehardt D, Godehardt E, et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18(9):1959-1966. PubMed CrossRef
20.Review Manager (RevMan) Version 5.3 for Windows [computer program]. Copenhagen, Denmark: Cochrane Collaboration; 2014.
21.Cohen LS, Nonacs RM, Bailey JW, et al. Relapse of depression during pregnancy following antidepressant discontinuation: a preliminary prospective study. Arch Women Ment Health. 2004;7(4):217-221. PubMed CrossRef
22.Methodology Checklist 2: Controlled Trials. Scottish Intercollegiate Guidelines Network (SIGN) website. http://www.sign.ac.uk/checklists-and-notes.html. 2017.
23.Misri S, Eng AB, Abizadeh J, et al. Factors impacting decisions to decline or adhere to antidepressant medication in perinatal women with mood and anxiety disorders. Depress Anxiety. 2013;30(11):1129-1136. PubMed CrossRef
24.Molenaar NM, Brouwer ME, Kamperman AM, et al. Recurrence of depression in the perinatal period: clinical features and associated vulnerability markers in an observational cohort. PLoS One. 2019;14(2):e0212964. PubMed CrossRef
25.Suzuki S, Kato M. Deterioration/relapse of depression during pregnancy in Japanese women associated with interruption of antidepressant medications. J Matern Fetal Neonatal Med. 2017;30(10):1129-1132. PubMed CrossRef
26.Cohen LS, Altshuler LL, Stowe ZN, et al. Reintroduction of antidepressant therapy across pregnancy in women who previously discontinued treatment: a preliminary retrospective study. Psychother Psychosom. 2004;73(4):255-258. PubMed CrossRef
27.Swanson SA, Hernandez-Diaz S, Palmsten K, et al. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol Drug Saf. 2015;24(9):934-942. PubMed CrossRef
28.Roca A, Imaz ML, Torres A, et al. Unplanned pregnancy and discontinuation of SSRIs in pregnant women with previously treated affective disorder. J Affect Disord. 2013;150(3):807-813. PubMed CrossRef
29.Deeks JJ, Higgins JPT, Altman DG. Prediction intervals from a random-effects meta-analysis. Cochrane Handbook for Systematic Reviews of Interventions website. https://training.cochrane.org/handbook/current/chapter-10#section-10-10-4-3. Retrieved May 13, 2020.
30.Dell DL, O’ Brien BW. Suicide in pregnancy. Obstet Gynecol. 2003;102(6):1306-1309. PubMed
31.Ejaz R, Leibson T, Koren G. Selective serotonin reuptake inhibitor discontinuation during pregnancy: at what risk? Can Fam Physician. 2014;60(12):1105-1106. PubMed
32.Einarson A, Selby P, Koren G. Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44-48. PubMed
33.Coughlin CG, Blackwell KA, Bartley C, et al. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. 2015;125(5):1224-1235. PubMed CrossRef
34.Mitchell J, Goodman J. Comparative effects of antidepressant medications and untreated major depression on pregnancy outcomes: a systematic review. Arch Women Ment Health. 2018;21(5):505-516. PubMed CrossRef
35.Howard LM, Hoffbrand S, Henshaw C, et al. Antidepressant prevention of postnatal depression (Cochrane review). Chinese Journal of Evidence-Based Medicine. 2005;5(10):735-742.
36.Wisner KL, Perel JM, Peindl KS, et al. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62(2):82-86. PubMed CrossRef
37.Wisner KL, Perel JM, Peindl KS, et al. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161(7):1290-1292. PubMed CrossRef
38.Troutman BR, Momany AM. Use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy and disorganized infant-mother attachment. J Reprod Infant Psychol. 2012;30(3):261-277. PubMed CrossRef
39.Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? a population-based investigation. PLoS One. 2016;11(12):e0168115. PubMed CrossRef
40.Burger H, Bockting CL, Beijers C, et al. Pregnancy Outcomes After a Maternity Intervention for Stressful Emotions (PROMISES): a randomised controlled trial. Adv Neurobiol. 2015;10:443-459. PubMed CrossRef
41.Snapper LA, Hart KL, Venkatesh KK, et al. Cohort study of the relationship between individual psychotherapy and pregnancy outcomes. J Affect Disord. 2018;239:253-257. PubMed CrossRef
42.Bérard A, Gaedigk A, Sheehy O, et al; OTIS (MotherToBaby) Collaborative Research Committee. Association between CYP2D6 genotypes and the risk of antidepressant discontinuation, dosage modification and the occurrence of maternal depression during pregnancy. Front Pharmacol. 2017;8:402. PubMed CrossRef
43.Hostetter A, Stowe ZN, Strader JR Jr, et al. Dose of selective serotonin uptake inhibitors across pregnancy: clinical implications. Depress Anxiety. 2000;11(2):51-57. PubMed CrossRef
44.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989-1008. PubMed CrossRef
45.Bérard A, Lacasse A. Validity of perinatal pharmacoepidemiologic studies using data from the RAMQ administrative database. Can J Clin Pharmacol. 2009;16(2):e360-e369. PubMed
46.Bockting CLH, Klein NS, Elgersma HJ, et al. Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised controlled trial. Lancet Psychiatry. 2018;5(5):401-410. PubMed CrossRef
47.Psaros C, Freeman M, Safren SA, et al. Discontinuation of antidepressants during attempts to conceive: a pilot trial of cognitive behavioral therapy for the prevention of recurrent depression. J Clin Psychopharmacol. 2014;34(4):455-460. PubMed CrossRef
48.Ahmadpanah M, Nazaribadie M, Aghaei E, et al. Influence of adjuvant detached mindfulness and stress management training compared to pharmacologic treatment in primiparae with postpartum depression. Arch Women Ment Health. 2018;21(1):65-73. PubMed CrossRef
49.Lupattelli A, Spigset O, Björnsdóttir I, et al. Patterns and factors associated with low adherence to psychotropic medications during pregnancy—a cross-sectional, multinational web-based study. Depress Anxiety. 2015;32(6):426-436. PubMed CrossRef
50.Miller LJ. Ethical issues in perinatal mental health. Psychiatr Clin North Am. 2009;32(2):259-270. PubMed CrossRef
51.Molenaar NM, Brouwer ME, Bockting CLH, et al. Stop or go? preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry. 2016;16(1):72. PubMed CrossRef
52.Sirey JA, Banerjee S, Marino P, et al. Adherence to depression treatment in primary care: a randomized clinical trial. JAMA Psychiatry. 2017;74(11):1129-1135. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at [email protected].
This PDF is free for all visitors!