A large number of antidepressant drugs are available across the world. All have been compared against placebo, and many have been compared with some but not all other antidepressants. There is therefore little information about a hierarchy of the efficacy and acceptability of these drugs. About 9 years ago, a network meta-analysis attempted to rank the efficacy and acceptability of 12 newer antidepressant drugs in adults with major depressive disorder. Very recently, this network meta-analysis was updated to include 21 antidepressant drugs, most of which were introduced during the 1980s and afterward. The present article explains what meta-analysis and network meta-analysis do, summarizes the important findings of the 21-antidepressant network meta-analysis, and offers comments on the findings. In general, it appears that antidepressant drugs are associated with clinically significant superiority over placebo with regard to response and remission rates; that almost all antidepressants do not differ from placebo with regard to all-cause discontinuation; that escitalopram, mirtazapine, amitriptyline, venlafaxine, and paroxetine are associated with better response rates than certain other antidepressants; that reboxetine, trazodone, and fluoxetine are associated with poorer response rates than certain other antidepressants; that agomelatine, escitalopram, and vortioxetine are associated with lower all-cause discontinuation than certain other antidepressants; and that clomipramine, reboxetine, and duloxetine are associated with higher all-cause discontinuation than certain other antidepressants. Whereas this conclusion is necessarily subjective, escitalopram could be a first choice in the balance of efficacy and acceptability, and reboxetine, the last choice. The strengths and limitations of the network meta-analysis are examined, and some comments on the findings are offered.


ABSTRACT
A large number of antidepressant drugs are available across the world. All have been compared against placebo, and many have been compared with some but not all other antidepressants. There is therefore little information about a hierarchy of the efficacy and acceptability of these drugs. About 9 years ago, a network meta-analysis attempted to rank the efficacy and acceptability of 12 newer antidepressant drugs in adults with major depressive disorder. Very recently, this network meta-analysis was updated to include 21 antidepressant drugs, most of which were introduced during the 1980s and afterward. The present article explains what meta-analysis and network meta-analysis do, summarizes the important findings of the 21-antidepressant network meta-analysis, and offers comments on the findings. In general, it appears that antidepressant drugs are associated with clinically significant superiority over placebo with regard to response and remission rates; that almost all antidepressants do not differ from placebo with regard to all-cause discontinuation; that escitalopram, mirtazapine, amitriptyline, venlafaxine, and paroxetine are associated with better response rates than certain other antidepressants; that reboxetine, trazodone, and fluoxetine are associated with poorer response rates than certain other antidepressants; that agomelatine, escitalopram, and vortioxetine are associated with lower all-cause discontinuation than certain other antidepressants; and that clomipramine, reboxetine, and duloxetine are associated with higher all-cause discontinuation than certain other antidepressants. Whereas this conclusion is necessarily subjective, escitalopram could be a first choice in the balance of efficacy and acceptability, and reboxetine, the last choice. The strengths and limitations of the network meta-analysis are examined, and some comments on the findings are offered.
J Clin Psychiatry 2018;79(2):18f12254
To cite: Andrade C. Relative efficacy and acceptability of antidepressant drugs in adults with major depressive disorder: commentary on a network meta-analysis. J Clin Psychiatry. 2018;79(2):18f12254.
To share: https://doi.org/10.4088/JCP.18f12254
© Copyright 2018 Physicians Postgraduate Press, Inc.
There are more than 3 dozen antidepressant drugs currently available in different parts of the world (Table 1), of which at least 2 dozen are in reasonably regular use for the treatment of adults with major depressive disorder (MDD). With such a large choice, it becomes important to know how the antidepressants compare with each other with regard to efficacy and adverse effect outcomes so that evidence-based choices can be made in antidepressant selection. Whereas all antidepressants have been compared with placebo in randomized controlled trials (RCTs) and whereas many antidepressants have been compared with other antidepressants in head-to-head studies, no antidepressant has been directly compared with all other antidepressants. There is therefore no information available about a hierarchy of antidepressants with regard to efficacy and adverse effect outcomes.
Network meta-analysis offers a way to compare treatments with each other when all possible head-to-head studies are unavailable. A simple explanation about meta-analysis and network meta-analysis follows for the benefit of the reader who is unacquainted with the concepts.
Meta-Analysis
Imagine that there are 10 studies that compare antidepressant A with antidepressant B. In some studies, A outperforms B; in others, B outperforms A; and in the rest, outcomes with the 2 drugs do not differ to a statistically significant extent. What conclusion might one draw?
Reviewing the literature, an expert might conclude that A is better because more RCTs found A superior to B than vice versa. Another expert might conclude that B is better because the studies that found B superior were larger, better designed, and better conducted or because the margins of superiority, favoring B, were larger. Clearly, expert judgements and the reasons leading to the conclusions may differ.
Meta-analysis offers a way out of this situation. Meta-analysis is a statistical method for combining the results of published and unpublished (where available) studies and presenting these results in the form of summary statistics.1 So, a meta-analysis of the A vs B RCTs discussed in this section could conclude, for example, that A is superior to B. The superiority could be expressed in terms of absolute or standardized difference between A and B on a depression rating scale or in terms of how much more likely a patient is to respond to or remit with A as compared with B, and so on. Likewise, meta-analysis could summarize adverse outcomes such as drug discontinuation rates and the rates of occurrence of different adverse effects. Summary statistics provided in meta-analyses include mean difference, standardized mean difference (SMD), relative risk, odds ratio, and numbers needed to treat or harm; 95% confidence intervals (CIs) accompany each of these.1-4
Whereas meta-analysis reduces the need to draw subjective conclusions from the research literature, the procedure itself requires subjective judgments at several steps. Meta-analysis is also associated with other limitations. How to interpret a meta-analysis and evaluate its limitations is well discussed elsewhere, and the reader is referred to these resources.1-6
Network Meta-Analysis
Problems arise when there are several treatments to be compared, such as antidepressants A through F. Antidepressant A may have been compared with B and C; B may have been compared with A, D, and F; and C may have been compared with A, D, E, and F. Thus, there is a network of pairwise comparisons; there may even have been 1 or more RCTs with 3 arms. Using this network of comparisons, can a statistical hierarchy be generated for efficacy and adverse effect outcomes? The answer is yes, through network meta-analysis.
Network meta-analysis is also known as multiple treatment comparison meta-analysis, mixed treatment comparison meta-analysis, or indirect meta-analysis. Network meta-analysis permits the estimation of summary statistics for all possible pairwise comparisons in the same model using direct and indirect evidence. Additionally, for each drug, a rank can be derived for the outcome under analysis. Thus, a hierarchy can be established for the treatments under comparison. Separate hierarchies are estimated for each efficacy and adverse effect outcome.
Network meta-analysis, how to read a network meta-analysis, and limitations of network meta-analysis have been well discussed elsewhere.7-10
Comparing 21 Antidepressants: A Network Meta-Analysis
About a decade ago, Cipriani et al11 described a network meta-analysis of 117 RCTs (pooled N = 25,928) of 12 newer antidepressants. They concluded that mirtazapine, escitalopram, venlafaxine, and sertraline were significantly more efficacious than duloxetine, fluoxetine, fluvoxamine, paroxetine, and reboxetine and that reboxetine was significantly less efficacious than all the other antidepressants. Escitalopram and sertraline were associated with significantly lower dropout rates relative to duloxetine, fluvoxamine, paroxetine, reboxetine, and venlafaxine.
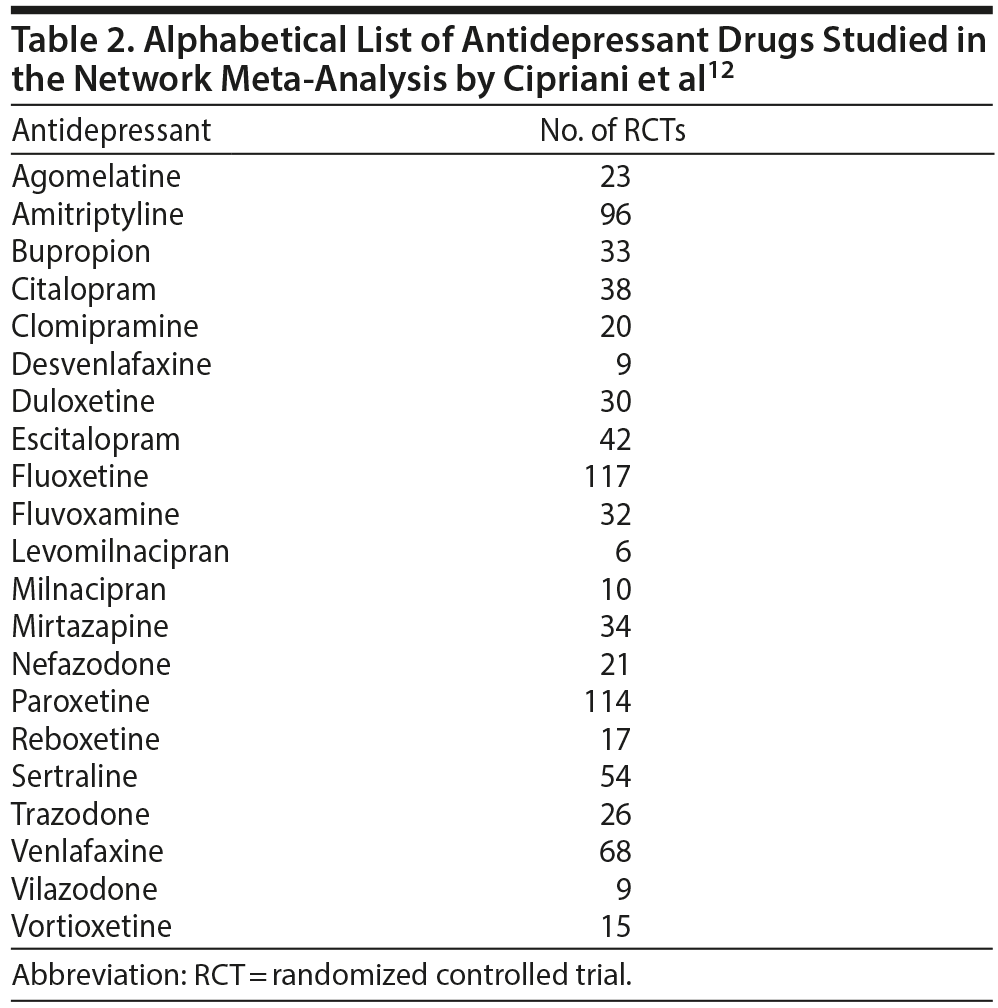
These authors12 have now published an extension of their previous network meta-analysis, examining 21 antidepressants (Table 2) in the new meta-analysis. They searched MEDLINE, the Cochrane database, clinical trial registries, drug company websites, reference lists, and other sources and identified 522 RCTs (pooled N = 116,477), completed or published between 1979 and 2016, in which drugs had been dosed within the therapeutic range. No trial included > 20% of patients with bipolar depression, psychotic depression, treatment-resistant depression, or depression with serious medical comorbidity. The primary efficacy outcome was the response rate, with response defined as at least 50% improvement in depression ratings. The primary acceptability outcome was the all-cause discontinuation rate, which is a composite of efficacy and tolerability. Outcomes were assessed as close to 8 (range, 4-12) weeks as possible, based on the available data.
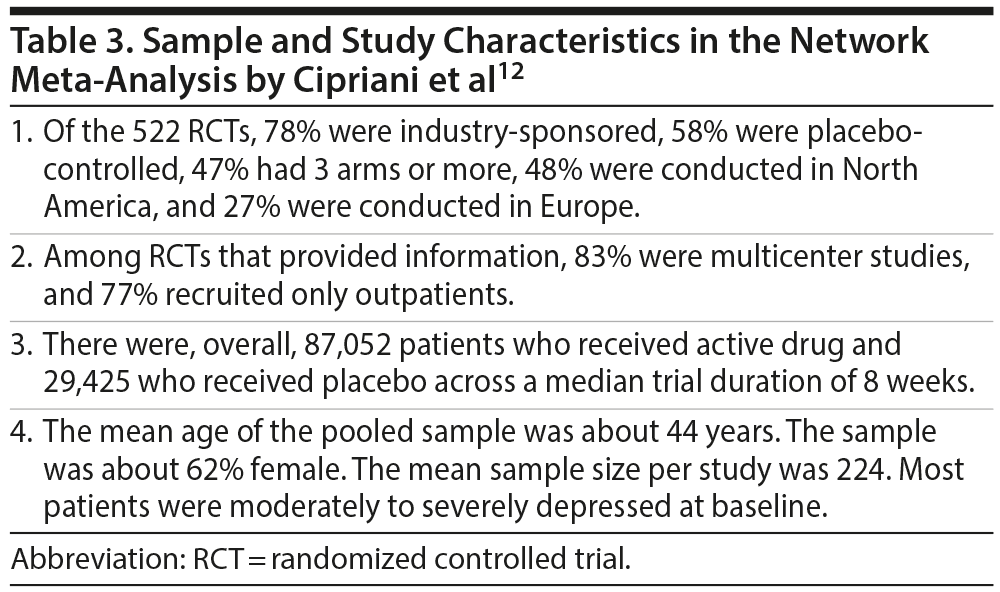
The primary analysis included 474 RCTs (pooled N = 106,966). Sample and study characteristics are summarized in Table 3. It is important for readers to be aware of these details because, as with studies based on other research designs, the findings of meta-analysis are best generalized to the samples characterized by the studies in the meta-analysis. As a general note, risk of bias was rated as low in 18% of the RCTs, moderate in 73%, and high in 9%.
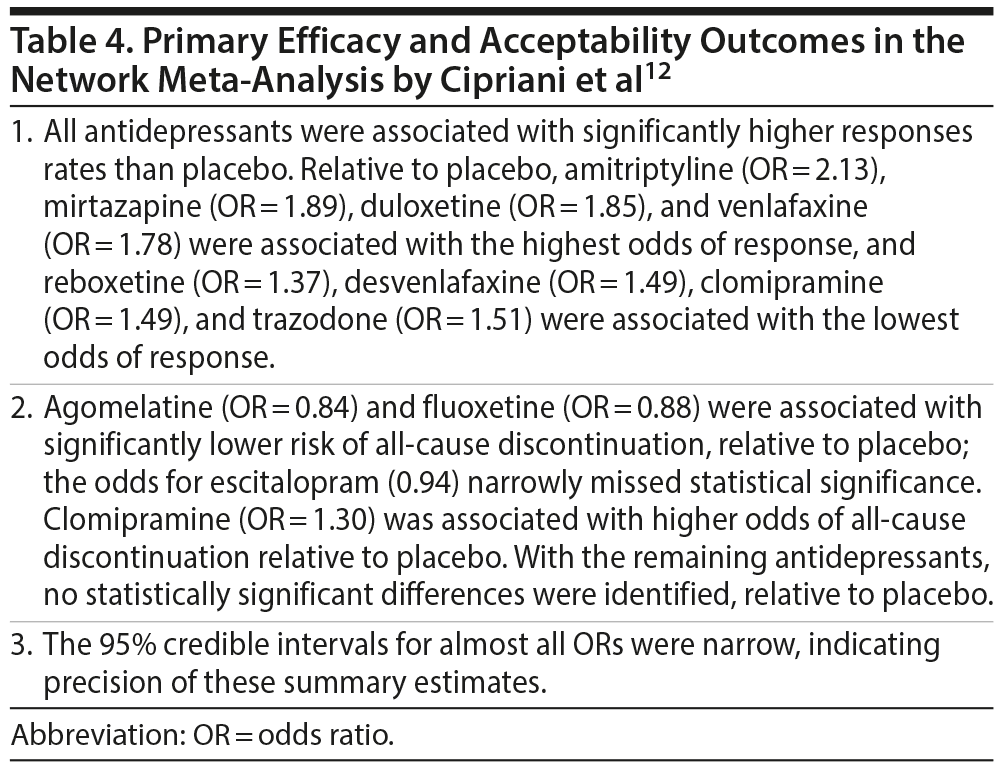
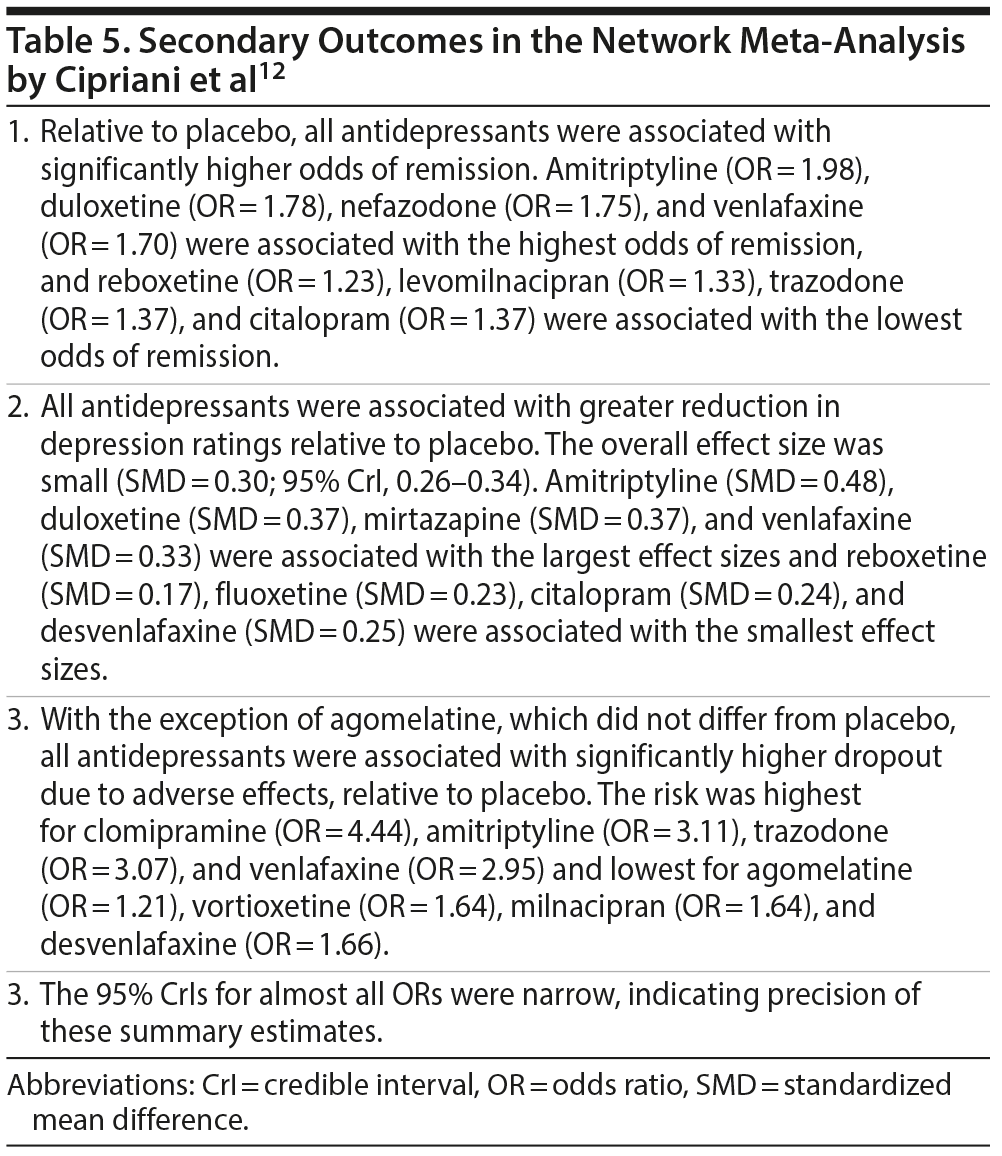
Findings from the primary efficacy (432 RCTs; pooled N = 103,443) and acceptability (422 RCTs; pooled N = 99,787) analyses for antidepressants vs placebo are presented in Table 4. Interestingly, smaller and older studies found larger effects for drug vs placebo. Secondary outcomes—remission, attenuation of depression ratings, and dropout due to adverse events for antidepressant vs placebo—are presented in Table 5.
The results of head-to-head comparisons, based on 194 RCTs (pooled N = 34,196), are presented in Tables 6 and 7. Levomilnacipran, vilazodone, and desvenlafaxine were not included in the head-to-head comparisons because there were too few RCTs for valid meta-analysis for these drugs. There were 153 pairwise comparisons between 18 antidepressants with 24 pairs showing significant differences in response rates and 34 pairs showing significant differences in all-cause discontinuation rates (Tables 6 and 7).
When surface under the cumulative ranking curve results were examined from head-to-head comparisons, the antidepressants that ranked highest for response, remission, and improvement in depression rating outcomes included escitalopram, vortioxetine, amitriptyline, and bupropion; the antidepressants that ranked lowest were reboxetine, trazodone, fluoxetine, and citalopram. Agomelatine, escitalopram, and vortioxetine were associated with the highest ranks for tolerability when discontinuation due to adverse events was examined, and clomipramine, reboxetine, duloxetine, amitriptyline, and venlafaxine were associated with the lowest ranks for tolerability.
As final notes, results of various predefined sensitivity analyses did not much change the overall results, and there was no evidence of bias arising from unidentified studies.
Comments: Strengths and Limitations
This is the single largest meta-analysis in world literature on the safety and efficacy of antidepressant medication in adults with nonpsychotic, nonrefractory, medically stable MDD.12 It is especially notable because of the sourcing and inclusion of 86 unpublished RCTs and hence the reduction of bias resulting from a "file drawer" effect. It is also notable because, by writing to the authors of the RCTs, additional, unpublished information was obtained for 274 RCTs. Thus, the authors left no stone unturned to make their meta-analysis as complete, comprehensive, and unbiased as possible.
The first limitation that needs to be considered is that the RCTs in the meta-analysis12 were conducted on patients with (mostly) MDD (so bipolar depression is largely excluded) and that patients (mostly) did not have psychotic depression, did not have significantly medical comorbidity, and were not treatment-resistant. Thus, the findings of the meta-analysis can be generalized only to patients with these characteristics. So, the findings cannot guide, for example, the choice of antidepressant in patients who have failed to respond to 1 or more antidepressants. Likewise, the findings cannot help the reader draw conclusions about safety and efficacy of antidepressant drugs during continuation and maintenance therapy; all that the meta-analysis summarizes are outcomes in RCTs that are approximately 8 weeks in duration.
A related limitation is that the findings of the meta-analysis12 can only be generalized to the treatment of depression as conducted in RCTs. Because patients in real life are diagnosed, assessed, treated, and followed up rather differently from those in clinical trials, the findings of this meta-analysis may not necessarily accurately predict results in real life. These caveats apply to results of all clinical trials and meta-analyses of such trials, and not to this meta-analysis12 alone.
On a related matter, confidence intervals for most estimates were narrow, indicating precision of the estimates. Here, precision is a consequence of the very large number of studies included in the meta-analysis. Precision of an estimate tells the reader nothing about the external validity of the findings, that is, the applicability of the findings in real-life settings.
Regrettably, the authors12 did not study many older antidepressants that are still popular in some parts of the world; important among these are monoamine oxidase inhibitors, imipramine, dothiepin, and nortriptyline. Amitriptyline and clomipramine were included only because these are on the World Health Organization list of essential medicines.
There were 153 head-to-head comparisons for response outcomes and 153 head-to-head comparisons for discontinuation outcomes. There were 24 and 34 significant results, respectively, in these pairwise comparisons. It is possible that some of these significant results were chance findings (type 1 or false-positive errors) arising from the testing of a large number of hypotheses. Prudence therefore dictates that only consistent findings should be accepted, such as the efficacy advantage apparent for escitalopram over many other antidepressants, or the fewer discontinuations associated with agomelatine relative to many other antidepressants.
General Comments
About 10 years ago, Kirsch et al13 published a meta-analysis of 35 placebo-controlled RCTs of fluoxetine, venlafaxine, nefazodone, and paroxetine that had been submitted to the US Food and Drug Administration. An important conclusion of this meta-analysis was that the advantage for antidepressants over placebo was clinically significant only in patients who were severely depressed at baseline. This meta-analysis13 was widely interpreted and cited to imply that antidepressant drugs are ineffective, or at least that antidepressant drugs are overrated as antidepressant interventions.
The network meta-analysis of Cipriani et al12 changes little or changes much, depending on where one looks. The overall effect size (SMD) for reduction in depression ratings for antidepressants vs placebo was small at 0.30, and the highest effect size, with amitriptyline, was a mere 0.48, which is still in the "small" effect size range. However, the effect sizes for response rates (relative risk [RR]) were more impressive, with almost all antidepressants being associated with response rates that were at least 50% greater than that with placebo and with well over half of the antidepressants being associated with remission rates that were at least 50% higher than that with placebo. Whether one is pleased by the high RRs for response and remission rates or disappointed by the low SMDs for reduction in depression ratings is a matter of choice. Why it is so difficult for antidepressant drugs to comprehensively outperform placebo is out of the scope of the present article; perhaps the reader may consider that there is more to placebo-related improvement than the placebo effect alone.14
An interesting though disquieting finding was that, in the head-to-head comparisons, when an antidepressant was the drug of interest in an RCT, it was significantly more effective than when it was a comparator drug and when another antidepressant was the drug of interest. This suggests investigator or sponsor bias in the design, conduct, analysis, and/or presentation of the study. This also undermines reader trust in antidepressant trials and in meta-analyses that include such trials. Happily, the magnitude of the bias was not large (OR = 1.18; 95% credible interval, 1.09-1.27).
Finally, whereas this is not the forum to pit evidence-based psychiatry against experience-based psychiatry, what does one make of clinical experience that some antidepressants are next to useless because of problems related to efficacy or tolerability? Agomelatine, for example, has been spectacularly effective in European antidepressant trials but has been a complete marketing failure in India and possibly certain other countries, as well, because of concerns about efficacy; in contrast, despite general appreciation of efficacy, amitriptyline and even imipramine are infrequently prescribed as antidepressants because of the unacceptably high adverse effect burden. Insights of this nature do not emerge from meta-analyses.
Take-Home Messages
This network meta-analysis12 was represented by 10 pages of published text and 290 pages of supplementary materials; can the information be boiled down to a few simple take-home messages? The answer, straightaway, is no. If the answer had been yes, perhaps the main paper and the supplementary materials could have been condensed, and this very commentary, summarizing a monumental effort, would have been unnecessary. So, if the most important take-home messages were to be listed, perhaps these would be the messages derived from Tables 4, 5, 6, and 7:
- In adults with nonpsychotic, nonrefractory, medically stable MDD, all antidepressant drugs, dosed within the therapeutic range, were associated with significantly higher response and remission rates than placebo after approximately 8 weeks of treatment. The response and remission advantage for drugs was also clinically significant for most antidepressants.
- Only 1 antidepressant, clomipramine, was associated with a significantly higher risk of all-cause discontinuation relative to placebo.
- In head-to-head comparisons of efficacy, operationalized as response rates, escitalopram, mirtazapine, amitriptyline, venlafaxine, and paroxetine were most often associated with superiority over certain other antidepressants.
- In head-to-head comparisons of response rates, reboxetine, trazodone, and fluoxetine were most often associated with inferiority relative to certain other antidepressants.
- In head-to-head comparisons of acceptability, operationalized as all-cause discontinuations, agomelatine, escitalopram, and vortioxetine were most often associated with superiority over certain other antidepressants.
- In head-to-head comparisons of acceptability, clomipramine, reboxetine, and duloxetine were most often associated with inferiority relative to certain other antidepressants.
Take-Home Messages: Summary
Here is a wry summary of the take-home messages that summarize this summary of and commentary on an enormous and information-packed network meta-analysis12: In clinical practice that resembles antidepressant RCTs in study selection criteria, dosing, assessments, follow-up, and other clinical actions, escitalopram could be the best choice and reboxetine, the worst choice.
Although the results of meta-analysis are expected to remove the need for subjective interpretations of RCTs, when the quantity of information made available by the meta-analysis is large, subjective interpretations of the results are unavoidable.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Streiner DL. Using meta-analysis in psychiatric research. Can J Psychiatry. 1991;36(5):357-362. PubMed CrossRef
2. Lam RW, Kennedy SH. Using meta-analysis to evaluate evidence: practical tips and traps. Can J Psychiatry. 2005;50(3):167-174. PubMed CrossRef
3. Russo MW. How to review a meta-analysis. Gastroenterol Hepatol (N Y). 2007;3(8):637-642. PubMed
4. Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312(2):171-179. PubMed CrossRef
5. Streiner DL. I have the answer, now what’s the question? why meta-analyses do not provide definitive solutions. Can J Psychiatry. 2005;50(13):829-831. PubMed CrossRef
6. Huf W, Kalcher K, Pail G, et al. Meta-analysis: fact or fiction? how to interpret meta-analyses. World J Biol Psychiatry. 2011;12(3):188-200. PubMed CrossRef
7. Mills EJ, Ioannidis JP, Thorlund K, et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308(12):1246-1253. PubMed CrossRef
8. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. PubMed CrossRef
9. Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130-137. PubMed CrossRef
10. Tonin FS, Rotta I, Mendes AM, et al. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017;15(1):943. PubMed CrossRef
11. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. PubMed CrossRef
12. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis [published online ahead of print February 20, 2018]. Lancet. PubMed CrossRef
13. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. PubMed CrossRef
14. Andrade C. There’s more to placebo-related improvement than the placebo effect alone. J Clin Psychiatry. 2012;73(10):1322-1325. PubMed CrossRef
This PDF is free for all visitors!