Epigenetics and Methylomics in Psychiatry
Stephen M. Stahl, MD, PhD
Issue: Not all inherited traits, including psychiatric disorders, are due to changes in the DNA sequence of genes. In fact, many characteristics of cells, especially neurons, are due instead to the selective activating and silencing of genes in a dynamic process known as epigenetics.
Psychiatric genetics is evolving rapidly. No longer are genes thought to directly and by themselves cause psychiatric illnesses. Instead, we now know genes can code for molecules that regulate information processing in behavior- producing neuronal circuits.1 When a critical number of molecular abnormalities are present in key brain circuits, individuals with those abnormal circuits are considered at risk for developing a psychiatric disorder if those circuits become sufficiently stressed by the environment.1 Thus, what appears to be inherited within the structure of genes is risk for mental illness rather than mental illness per se. Whether that risk becomes manifest as an overt psychiatric disorder or remains silent seems to depend on whether genes and the environment conspire, ie, necessary abnormalities in gene products of brain circuits must be combined with sufficient stress from the environment to arrive at a mental illness. This notion of environment/gene interaction causing a mental illness is sometimes called the stress diathesis hypothesis of mental illness,1 and epigenetics is a key mechanism whereby the environment is able to conspire with genes.2-7
How Do the Epigenetic Mechanisms and Methylomics of Gene Activation and Silencing Work?
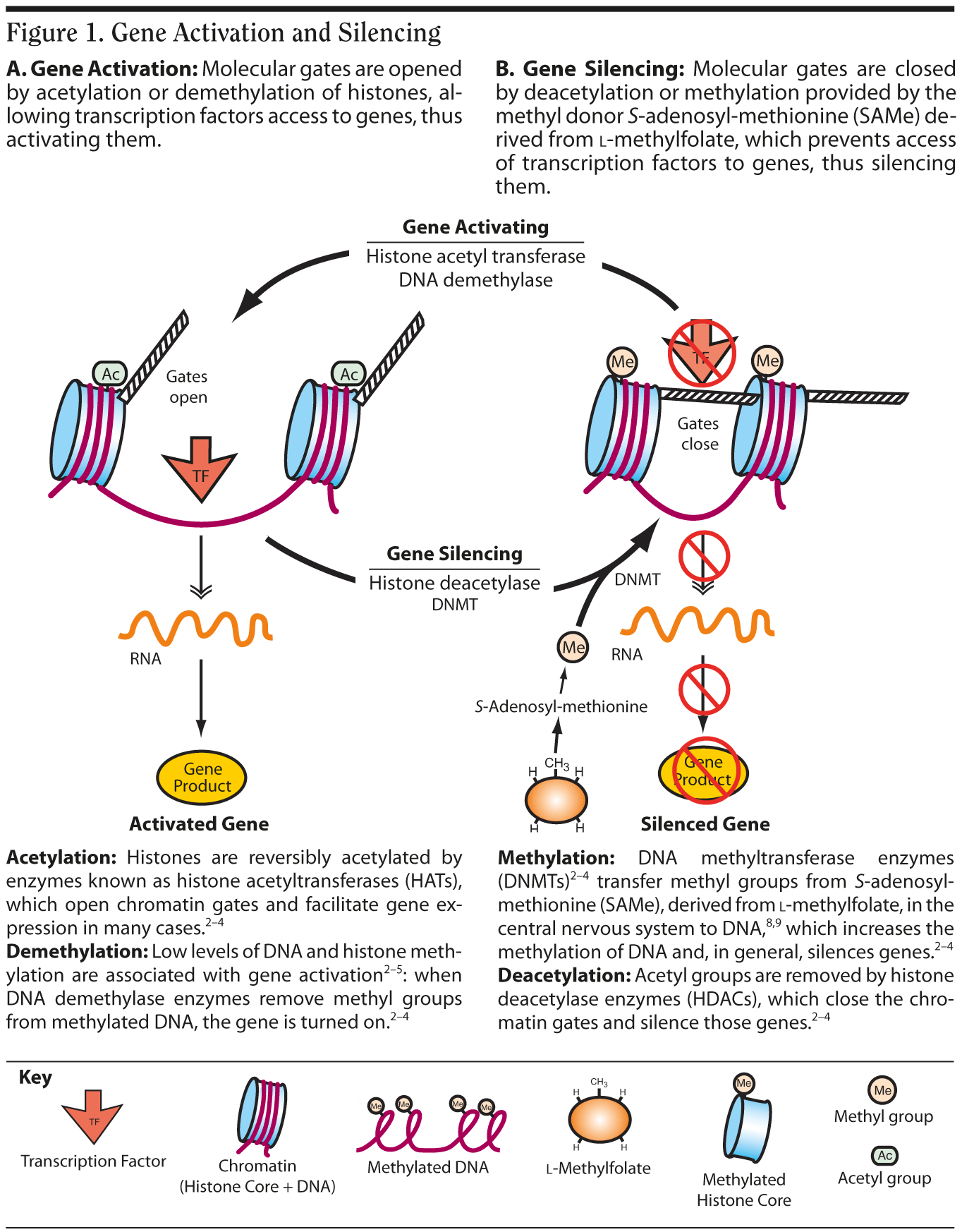
Epigenetics is the method by which cells control which of their DNA is transcribed then translated.2-4 Every cell manages this process by modifying either the genes themselves or the histone proteins that bind to their genes (Figure 1),2-5 which in turn either opens (Figure 1A) or closes (Figure 1B) molecular gates and regulates whether a gene is turned on or off.
In the cell nucleus, DNA (with the genes that make up DNA) is wound around a histone protein core into a compact structure called chromatin.2-5 One way gene transcription is effected is by biochemically modifying the histones via methylation, acetylation, phosphorylation, and ubiquitination2-4 that alters the compactness of the chromatin spool and allows the DNA to be loosened, resulting in activation, or tightened, resulting in silencing (Figure 1). Although genes themselves can be directly methylated, acetylation of histones and methylation of histones and DNA are the most extensively studied epigenetic mechanisms.4
Inhibitors of histone deacetylase enzymes (HDACs) are currently the major pharmacologic mechanism for experimentally manipulating epigenetic mechanisms. Novel and selective inhibitors of HDACs are in development to stop the removal of acetyl groups and keep genes activated. Valproic acid is known to be an HDAC inhibitor.4,5 Evidence suggests that HDAC inhibitors may enhance memory formation.4
Methylomics, a concept borrowed from cancer research and developmental neurobiology, is now being applied to neuroscience and psychiatry6,7 and may prove to be of vital importance in mediating gene-environment interactions.2-7 Methylation may be influenced by methyl donor molecules like l-methylfolate and S-adenosyl-methionine (SAMe),8,9 which could facilitate key gene silencing, or by inhibition of DNA methyltransferase enzymes, which could stop the methylation of DNA and thus keep key genes active. Both excessive "hyper" methylation and deficient "hypo" methylation have been associated with the functionality of critical genes in various psychiatric disorders.6,7 Both methylation and demethylation of DNA are implicated in long-term memory consolidation.4
Take-Home Points
- Genes are wrapped in proteins called histones that form a substance called chromatin.
- Histones serve as access gates to molecules that control whether genes are activated or silenced.
- Gates are opened or closed by chemical modifications to histones, including acetylation, phosphorylation, ubiquitination, and methylation. DNA itself can also be methylated.
- Methylomics is the regulation of gene function by methylation and holds promise as an explanation for how the environment interacts with genes in conditions as diverse as normal development, learning, cancer, and psychiatric disorders.
The Importance of Epigenetics and Methylomics
The importance of epigenetics to psychiatry is exploding.2-7 For example, epigenetics may resolve the dilemma of how identical twins with the exact same DNA can have one member with schizophrenia and the other not.6 Theoretically, the abnormal gene is silenced in the normal twin but activated in the twin with schizophrenia.
Epigenetics even solves the biologic puzzle in a person’s body of how different cells can have the exact same DNA but be so vastly different in form and function.4 Each cell seems to learn what to turn on and what to turn off during normal development. Until recently, it was thought that once in place, the epigenetic mechanisms of a given cell were hardwired into that cell and its descendents for life. Now, however, it seems that there are some important exceptions to the immutability of epigenetic mechanisms of tremendous relevance to psychiatry.2-7 Specifically, life experiences can recruit epigenetic mechanisms in neurons to activate or silence genes that regulate cognition, behavior, and even psychiatric disorders. The stress diathesis hypothesis suggests that if the environment ("stress") activates abnormal genes ("diathesis," ie, risk), abnormal gene products would be formed.1 By affecting molecules critical for brain circuit function, inefficient information processing in those brain circuits, eg, psychiatric symptoms, could result. Furthermore, it is possible that problems could occur even with normal genes if the environment leads to activation at the wrong time or place or inactivation resulting in the loss of its critical gene product.2-7 What is so exciting about this new understanding of epigenetic mechanisms in psychiatry is the possibility that the formation of cognitions, memories, behaviors, and psychiatric symptoms might be reversible by targeting epigenetic mechanisms with novel pharmacotherapies.4
References
1. Stahl SM. Stahl’s Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008.
2. Nestler EJ. Epigenetic mechanisms in psychiatry. Biol Psychiatry. 2009;65(3):189-190. PubMed doi:10.1016/j.biopsych.2008.10.030
3. Tsankova N, Renthal W, Kumar A, et al. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355-367. PubMed doi:10.1038/nrn2132
4. Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65(3):191-197. PubMed doi:10.1016/j.biopsych.2008.09.002
5. Guidotti A, Dong E, Kundakovic M, et al. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci. 2009;30(2):55-60. PubMed doi:10.1016/j.tips.2008.10.010
6. Abdolmaleky HM, Smith CL, Zhou RJ, et al. Epigenetic alterations of dopaminergic system in major psychiatric disorders. In: Yan Q, ed. Pharmacogenomics in Drug Discovery and Development. NY: Humana Press; 2008.
7. Abdolmaleky HM, Smith CL, Faraone SV, et al. Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet. 2004;127B(1):51-59. PubMed doi:10.1002/ajmg.b.20142
8. Stahl SM. l-Methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353. PubMed doi:10.4088/JCP.v69n0901
9. Stahl SM. Novel therapeutics for depression: l-methylfolate (6 (S)-5 methyltetrahydrofolate or MTHF) as a trimonoamine modulator and antidepressant augmenting agent. CNS Spectr. 2007;12(10):423-428. PubMed
Brainstorms is a section of The Journal of Clinical Psychiatry aimed at providing updates of novel concepts emerging from the neurosciences that have relevance to the practicing psychiatrist.
From the Neuroscience Education Institute in Carlsbad, California, and the Department of Psychiatry at the University of California San Diego.
For reprint and financial disclosure information, go to www.psychiatrist.com/brainstorms.
doi:10.4088/JCP.09bs05563
© Copyright 2009 Physicians Postgraduate Press, Inc.