See Article by Kane et al.
Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Experts and clinical guidelines recommend using long-acting injectable (LAI) antipsychotics to prevent relapse among people diagnosed with schizophrenia who do not adhere to oral antipsychotic regimens. These recommendations belie a continued lack of strong evidence that LAIs have advantages over oral medications for this purpose.3 Ostensibly, the failure of LAIs to demonstrate the hypothesized advantages in improving adherence and related outcomes is due to methodological problems.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

What Is the Role of Long-Acting Injectable Antipsychotics in the Treatment of Schizophrenia?
Experts and clinical guidelines recommend using long-acting injectable (LAI) antipsychotics to prevent relapse among people diagnosed with schizophrenia who do not adhere to oral antipsychotic regimens.1,2 These recommendations belie a continued lack of strong evidence that LAIs have advantages over oral medications for this purpose.3 Ostensibly, the failure of LAIs to demonstrate the hypothesized advantages in improving adherence and related outcomes is due to methodological problems.3,4 Specifically, the requirement for research subjects to consent to participate in a study that is testing antipsychotic medications may preclude enrolling the nonadherent individuals who are most likely to benefit from long-acting preparations.
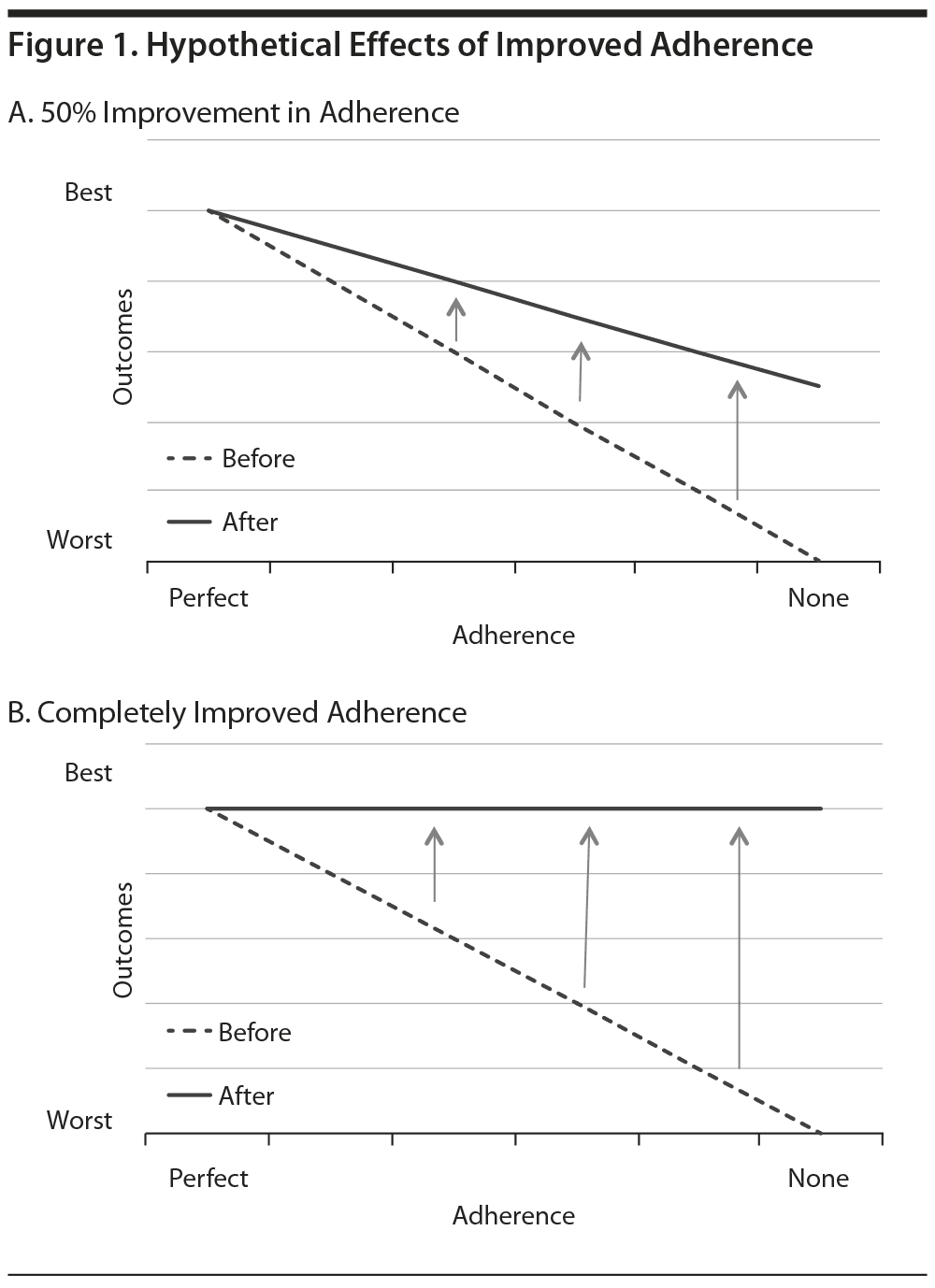
One issue for LAIs is the appropriate target population. Pharmaceutical companies that make and sell LAIs would prefer a broad market and do not want to restrict LAI use to the most nonadherent, dangerous, or severely ill patients. Instead, industry-sponsored trials have sought to establish that "partial adherence" should also be addressed and that patients who take oral antipsychotics inconsistently can benefit from LAIs. A recent study of adherence-enhancing strategies provides insight into this issue. Velligan and colleagues5 were able to improve adherence rates to 90%-92% with active interventions, while adherence was only 73% with the comparison treatment-as-usual condition. In spite of this significant improvement in adherence, the intervention groups had no significant advantages over the comparison group in the main outcomes, which were symptoms and global functioning. These results suggest that adherence interventions may not have much impact on the outcomes of people who already take most of their medications. Figure 1, which assumes a linear relationship between medication adherence and outcomes, demonstrates how the benefits of improved adherence on outcomes might vary according to the baseline level of adherence. Figure 1A shows how a hypothetical 50% reduction in nonadherence would be expected to have the greatest impact on individuals with the lowest levels of adherence at baseline. Improved medication adherence among those who already receive the medications most of the time might have marginal or undetectable benefits. Even if LAIs completely resolve adherence problems, any benefits might be difficult to perceive among those with high adherence at baseline (Figure 1B).
More information on target populations comes from 2 recent LAI vs oral antipsychotic studies, one that included patients at increased at risk of relapse6 and another in a broader population.4 Neither of these studies focused specifically on nonadherence, and the results showed no significant advantage of LAIs over oral antipsychotics for prevention of relapse or rehospitalization. In addition, although some have suggested that LAIs are better tolerated than oral preparations because of plasma levels that are relatively stable compared to those of oral agents,7 these 2 effectiveness studies found no tolerability advantage of LAIs.4,6 In both of these large, well-designed, and well-conducted studies, LAI risperidone was not significantly better tolerated than oral antipsychotics in terms of specific adverse effects or treatment discontinuations due to adverse effects.
The article in this issue by Kane and colleagues8 reports that LAI aripiprazole is efficacious for individuals diagnosed with schizophrenia who are acutely ill. Because relapse with subsequent hospitalization often occurs in the context of having stopped taking medications, this is exactly the situation in which LAIs might be most useful and have the greatest impact. The study provides specific new evidence that LAI aripiprazole, like other LAI antipsychotics, is an option for treating acute episodes of schizophrenia. Similar to LAI risperidone, there is a clear recommendation that LAI aripiprazole should be given along with oral antipsychotics initially to ensure that patients do not have a period without therapeutic levels of medicine. In contrast, LAI haloperidol and paliperidone can be effectively initiated using injected loading doses without need for oral supplementation.9 For patients who will not take oral medications, there may be advantages to medications that can achieve therapeutic levels with injections alone.
The study in this issue also provides useful information about the clinical effects of LAI aripiprazole. The authors found that, compared to placebo, aripiprazole was associated with more weight gain, adverse changes in fasting glucose levels, and akathisia. Although aripiprazole has a relatively benign profile of metabolic effects compared to many other antipsychotics,10,11 some patients gain weight while taking aripiprazole. In addition, the adverse effect of aripiprazole on glucose metabolism found by Kane et al8 has also been reported in another study.12 The take-home point is that all patients taking any antipsychotic should be monitored for adverse metabolic and neurologic effects, and if they occur these problems should be actively managed.
LAIs are considered a good option for people who are experiencing a first episode of psychosis and are likely to be highly ambivalent about the need for and benefits of antipsychotic treatment.13,14 Using LAIs to help prevent relapse and clinical deterioration in this group may help prevent disability. On the other hand, to promote engagement in treatment, a first episode of psychosis may be a time to be particularly sensitive to the preferences of patients. In this regard, short-acting medications that allow quick dosage adjustments may be preferable to LAIs for people who are developing their first impressions of medications that may be needed for years to come.
There is no evidence that newer versions of LAIs are significantly more effective than older versions. A recent report by McEvoy and colleagues9 described an NIMH-sponsored trial that found no significant advantages for LAI paliperidone over LAI haloperidol in overall effectiveness. Similarly, a study by Covell et al15 failed to find hypothesized advantages of switching to LAI risperidone from LAI versions of fluphenazine and haloperidol.
In summary, LAIs are not a simple solution to improved medication adherence and better outcomes for individuals with schizophrenia. The article in this issue demonstrates that LAI antipsychotics are efficacious for patients who are acutely ill and thus are a useful option in this situation to help prevent future relapses. To maximize the chance that this strategy will succeed, when starting a LAI on an inpatient unit it is essential to make sure that the outpatient providers can and will continue the LAI treatment. In addition, in this age of restricted formularies, it is important to select a medication that will be available to the patient given his or her insurance plan and resources. Further, improved outcomes will most likely require multifaceted approaches that do not simply focus on medications and medication adherence.
Author affiliations: Columbia University, Department of Psychiatry, New York, New York.
Potential conflicts of interest: Dr Stroup has received grant/research support from National Institute of Mental Health, Agency for Healthcare Research and Quality, and Auspex and has received support for CME from Genentech.
Funding/support: None reported.
REFERENCES
1. Kane JM, Leucht S, Carpenter D, et al; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The Expert Consensus Guideline Series: optimizing pharmacologic treatment of psychotic disorders. introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5-19. PubMed
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice Guideline for the Treatment of Patients With Schizophrenia, Second Edition. Am J Psychiatry. 2004;161(suppl):1-56. PubMed
3. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213. PubMed doi:10.1093/schbul/sbs150
4. Buckley PF, Schooler NR, Goff DC, et al; the PROACTIVE Study. Comparison of SGA oral medications and a long-acting injectable SGA: The PROACTIVE Study [published online ahead of print May 27, 2014]. Schizophr Bull. PubMed
5. Velligan D, Mintz J, Maples N, et al. A randomized trial comparing in person and electronic interventions for improving adherence to oral medications in schizophrenia. Schizophr Bull. 2013;39(5):999-1007. PubMed doi:10.1093/schbul/sbs116
6. Rosenheck RA, Krystal JH, Lew R, et al; CSP555 Research Group. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011;364(9):842-851. PubMed doi:10.1056/NEJMoa1005987
7. Nasrallah HA. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand. 2007;115(4):260-267. PubMed doi:10.1111/j.1600-0447.2006.00982.x
8. Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254-1260. doi:10.4088/JCP.14m09168
9. McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014;311(19):1978-1987. PubMed doi:10.1001/jama.2014.4310
10. Stroup TS, McEvoy JP, Ring KD, et al. Comparison of Antipsychotics for Metabolic Problems (CAMP): A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk. Am J Psychiatry. 2011;168:947-956. PubMed doi:10.1176/appi.ajp.2011.10111609
11. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
12. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12. PubMed doi:10.1016/j.schres.2008.10.011
13. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386. PubMed doi:10.1016/j.clinthera.2008.12.020
14. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry suppl. 2009;52:S63-S67. PubMed doi:10.1192/bjp.195.52.s63
15. Covell NH, McEvoy JP, Schooler NR, et al; Schizophrenia Trials Network. Effectiveness of switching from long-acting injectable fluphenazine or haloperidol decanoate to long-acting injectable risperidone microspheres: an open-label, randomized controlled trial. J Clin Psychiatry. 2012;73(5):669-675. PubMed doi:10.4088/JCP.11m07074
Submitted: September 16, 2014; accepted September 17, 2014.
Corresponding author: T. Scott Stroup, MD, MPH, Columbia University, Department of Psychiatry, 1051 Riverside Dr, New York, NY 10032 ([email protected]).
J Clin Psychiatry 2014;75(11):1261-1262 (doi:10.4088/JCP.14com09518).
© Copyright 2014 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!