The P4 Screener: Evaluation of a Brief Measure for Assessing Potential Suicide Risk in 2 Randomized Effectiveness Trials of Primary Care and Oncology Patients
Background: Depression is the most common mental disorder, and suicide is its most serious consequence. The primary objective of this study was to evaluate preliminary evidence for the P4 screener as a brief measure to assess potential suicide risk.
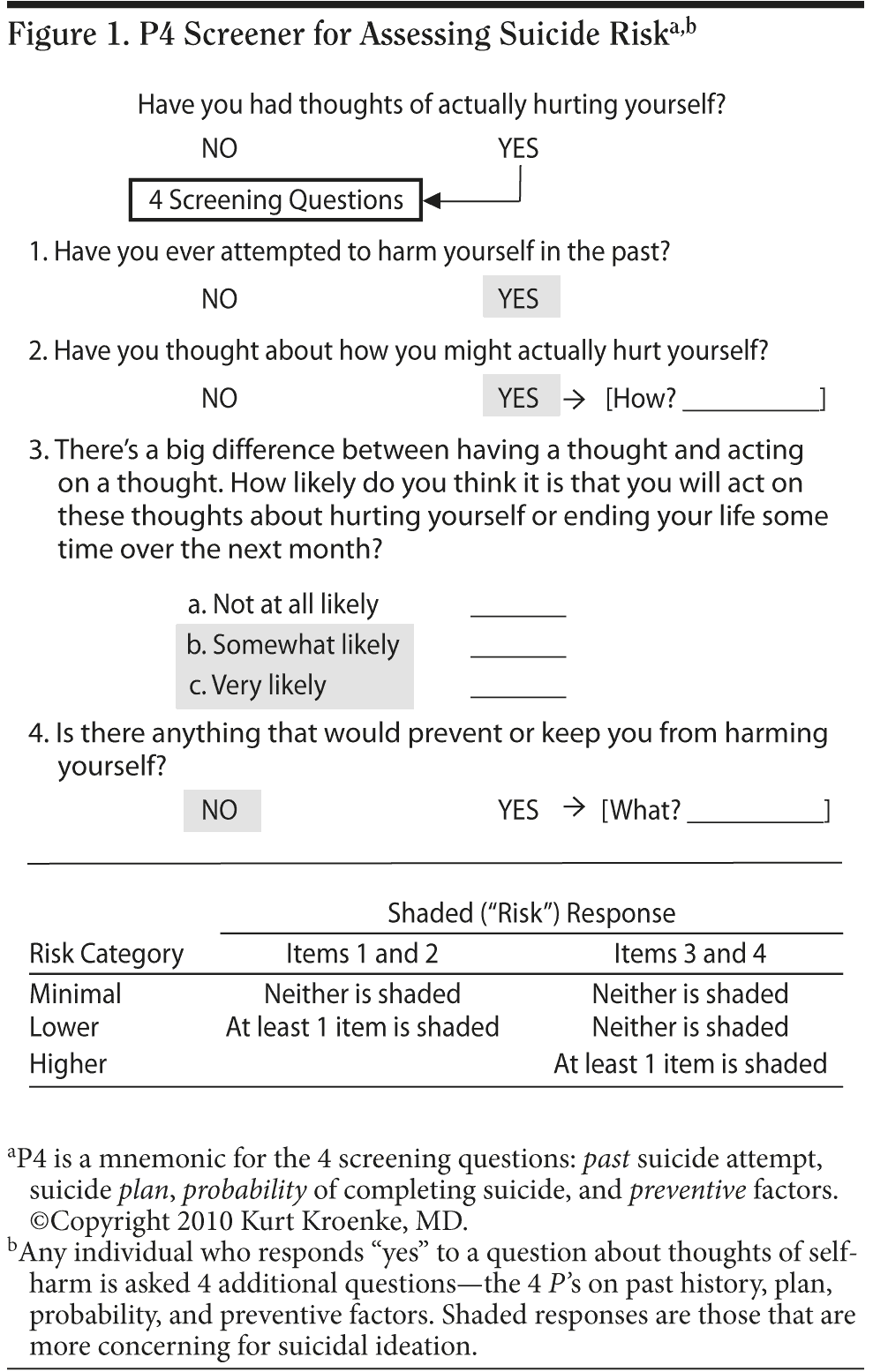
Method: The P4 screener was prospectively evaluated in 2 randomized effectiveness trials of primary care (January 2005-June 2008; N = 250) and oncology patients (March 2006-August 2009; N = 309). Potential suicide ideation was assessed at 5 time points in both trials: baseline and 1, 3, 6, and 12 months. The P4 screener asks about the “4 P’ s”: past suicide attempts, suicide plan, probability of completing suicide, and preventive factors. Patients were classified as minimal, lower, and higher risk based upon responses to these 4 items.
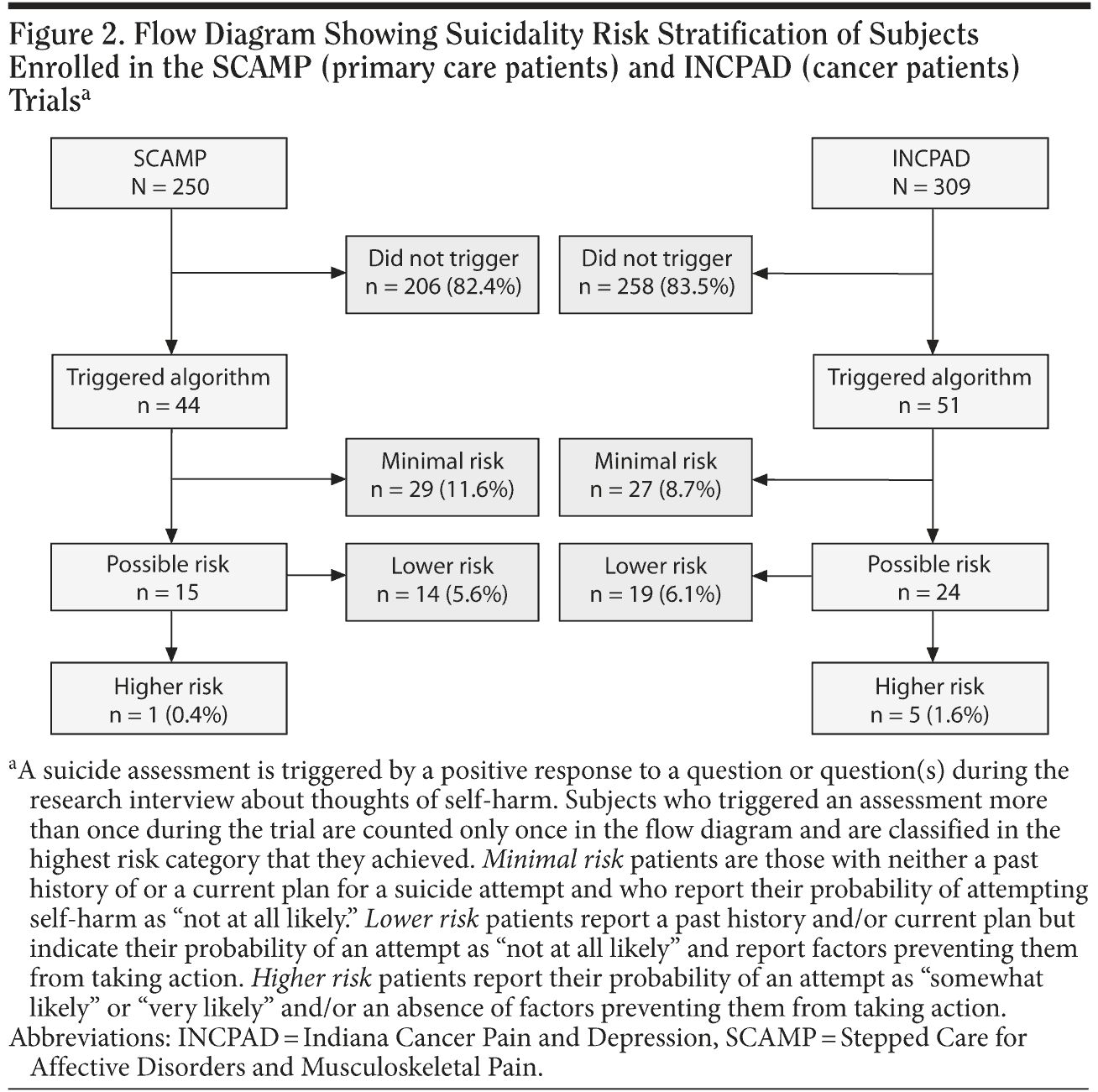
Results: A suicide assessment was triggered 1 or more times by 17.6% (44 of 250) of Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) participants and 16.5% (51 of 309) of Indiana Cancer Pain and Depression (INCPAD) participants at some point in the trial. Of the patients who triggered a suicide assessment, the majority (29 of 44 in SCAMP and 27 of 51 in INCPAD) were classified as minimal risk by the algorithm. Only 1 (0.4%) of the SCAMP participants and 5 (1.6%) of the INCPAD participants were classified as higher risk. Among the latter, the most common factors preventing patients from attempting suicide were the “4 F’ s”: faith, family, future hope, and fear of failing in their attempt.
Conclusions: Preliminary findings suggest that the P4 screener may be useful in assessing potential suicide risk in the clinical care of depressed patients as well as in clinical research.
Trial Registration: clinicaltrials.gov Identifier: NCT00118430 (SCAMP) and NCT00313573 (INCPAD)
Prim Care Companion J Clin Psychiatry 2010;12(6):e1-e8
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: March 6, 2010; accepted May 27, 2010.
Published online: December 23, 2010 (doi:10.4088/PCC.10m00978blu).
Corresponding author: Kurt Kroenke, MD, Regenstrief Institute, 6th Fl, 1050 Wishard Blvd, Indianapolis, IN 46202 ([email protected]).
Suicide is the eleventh leading cause of death in the United States, with roughly 30,000 lives lost per year.1 Globally, there were over 1 million deaths to suicide in 2000, or approximately 16 suicides per 100,000 individuals. Depression is the most prevalent and disabling mental health disorder and the leading risk factor for suicide. Other risk factors include alcohol and substance abuse, older age, male gender, social isolation, family history of suicide, past attempts, access to lethal arms, hopelessness, and chronic medical and neurologic disorders.
A number of studies have focused on suicidality among adults in primary care,2-8 adolescents,9,10 the elderly,11-14 and cancer patients.15-19 However, these studies have principally focused on the prevalence of and risk factors for suicidal ideation rather than an explicit assessment strategy.
Clinical Points
- The P4 screener assesses suicide risk by asking about the “4 P’ s”: past suicide attempts, a plan, probability of completing suicide, and preventive factors.
- Most participants in clinical trials of depressed medical patients who acknowledge thoughts of self-harm are ultimately classified as low risk by the P4 screener.
To date, there is no single recommended method to screen for suicidality.1 There have been single studies that have used 1 or a few questions inquiring directly about suicidal ideation.4,7,10 There are several longer scales such as the Beck Scale for Suicidal Ideation (21 items),20 the Columbia Suicide Severity Rating Scale (18 items),21 the Sheehan Suicide Tracking Scale (8 items),22 and the Nurses’ Global Assessment of Suicide Risk (15 items).23 In addition to the length of the scales, their scoring is more complicated, and they have often been tested in psychiatric rather than in general medical populations. A few studies have used more complex algorithms to assess suicidality.11,24 A simpler algorithm that helped inform the P4 screener was developed by Cole and colleagues.25
Suicidal ideation is often unrecognized in primary care or medical specialty settings due to an inability of patients to articulate their feelings to their health care providers and a discomfort among non-mental health clinicians to ask about such feelings.26,27 The additional concern that asking about suicidal thoughts may actually trigger suicidal ideation and behavior is unfounded.9 Although asking about suicide when identifying and treating depressed patients is considered standard of care, competing demands in medical practice create particular barriers to interview techniques that require prolonged probing.28 The sensitivity and discomfort surrounding suicide assessment and overestimates of how often urgent mental health referral may be required further accentuate these barriers. Surprisingly, there is even inconsistency in the degree to which psychiatrists ask about and document suicidal ideation in routine clinical practice, an omission which can be improved by the use of brief assessment measures.29,30
To address the need for an efficient yet valid means of assessing suicide risk in medical populations, this article describes the findings from 2 randomized controlled trials regarding a brief algorithm that has evolved over the course of 6 randomized effectiveness depression trials conducted by our research group in the past decade. Core items emerged from the first 4 trials leading to a standard algorithm that was prospectively evaluated in more detail in our 2 most recent trials.
METHOD
Developmental History of the P4 Screener
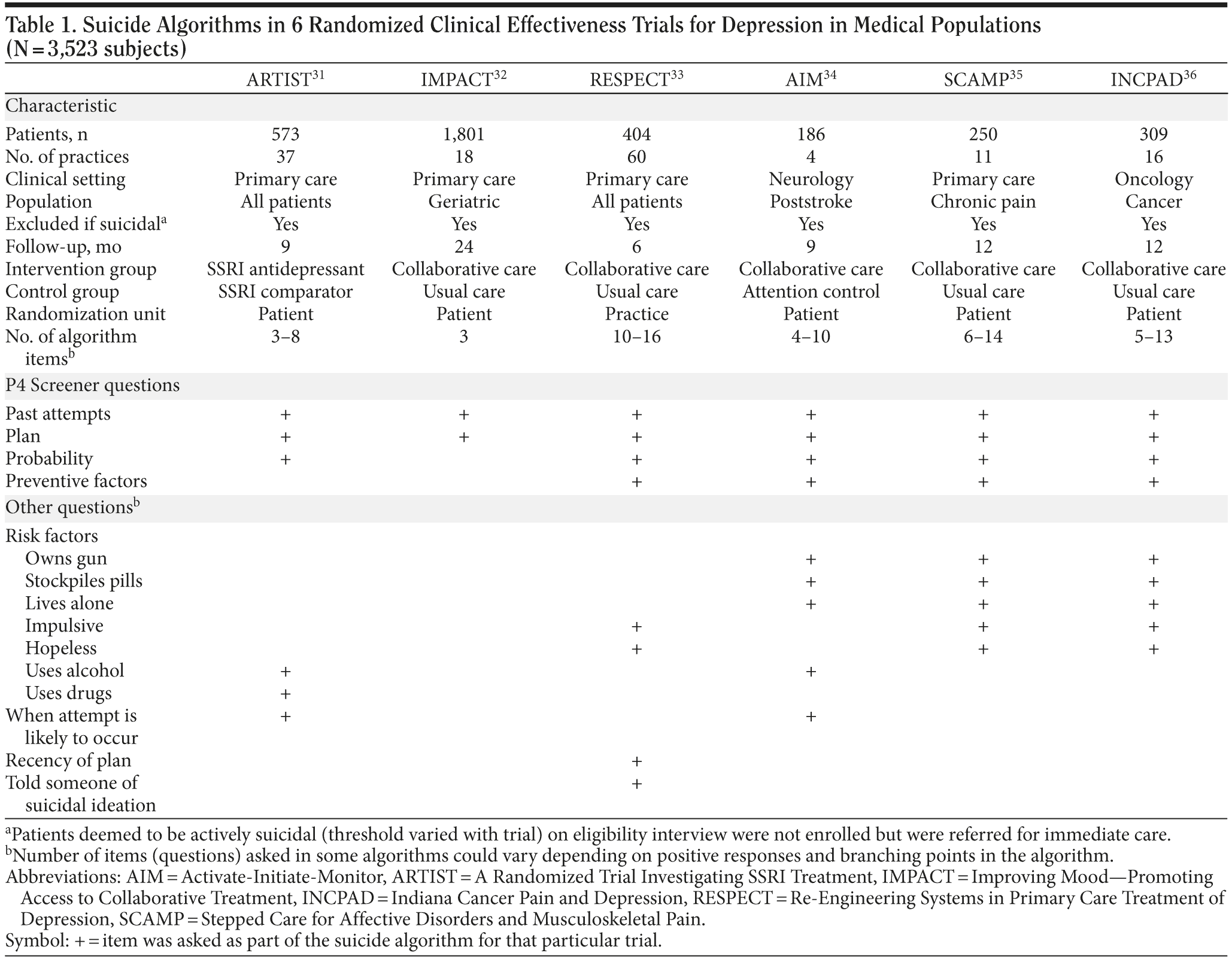
The P4 screener (Figure 1) was initially developed and refined in 4 earlier trials31-34 and subsequently tested in its final form in the 2 most recent trials.35,36 Some of the P4 items were originally developed by Cole and colleagues for use by primary care physicians.25 As summarized in Table 1, all 6 trials were effectiveness trials conducted in medical populations, of which 4 were situated in primary care and 2 in specialty settings. A total of 3,523 participants were enrolled from 146 practices and followed for 6 to 24 months. The intervention was a collaborative care approach to enhance depression treatment (typically with a nurse care manager supervised by a physician specialist) in 5 trials and an open-label active comparator design in 1 trial. The control group received usual care in 4 trials, nonspecific attention in 1 trial, and an active comparator in 1 trial. Only 2 of the trials—IMPACT (Improving Mood-Promoting Access to Collaborative Treatment)32 and RESPECT (Re-Engineering Systems in Primary Care Treatment of Depression)33—have published data related to suicidality among their study participants, and these trials have focused principally on risk stratification and predictors as well as the effects of depression treatment rather than on the algorithm itself.6,12,13
Each of the 6 trials had a structured assessment for assessing suicide risk that was triggered when patients endorsed potential thoughts of self-harm during baseline or follow-up interviews, typically by a positive response to the question about self-harm on the Hopkins Symptom Checklist 20-item depression scale37 (HSCL-20, item 13), the Patient Health Questionnaire 9-item depression scale38 (PHQ-9, item 9), or the Structured Clinical Interview for DSM-IV-TR Axis I Disorders39 (item 9). As shown in Table 1, the length of algorithms varied considerably among the trials, ranging from quite brief (3 items in the IMPACT algorithm) to lengthier (10-16 items in the RESPECT algorithm). However, 4 questions emerged as core items, all of which were included in algorithms used in the 4 most recent trials. These 4 core items (the “4 P’ s”) include questions about past attempts, suicide plans, probability of completing suicide, and preventive factors.
Testing of the P4 Screener in the SCAMP and INCPAD Trials
A structured algorithm was used in the SCAMP (Stepped Care for Affective Disorders and Musculoskeletal Pain) and INCPAD (Indiana Cancer Pain and Depression) trials on the basis of experience in the first 4 trials. Both trials are registered in clinicaltrials.gov (identifier: NCT00118430 [SCAMP] and NCT00313573 [INCPAD]). SCAMP enrolled primary care patients with clinically significant depression and comorbid chronic musculoskeletal pain from January 2005 to June 2008. Of 756 eligible patients, 250 consented and were randomized to a collaborative care intervention or usual care. The intervention was administered by a nurse-physician team, which delivered optimized antidepressant therapy and a pain self-management program. The mean age of the SCAMP participants was 55.5 years; 52.8% were women; 60.4% were white, 36.4% were black, and 3.2% were other.
INCPAD enrolled patients with cancer-related pain or depression who were receiving care in community-based oncology practices from March 2006 to August 2009. Of 616 patients in which eligibility could be ascertained, 405 were randomized to a telecare-based collaborative care intervention or usual care; 309 of the 405 patients were depressed and are the focus of this article. The intervention was administered by a nurse-psychiatrist team, which optimized antidepressant and analgesic management. The mean age of the INCPAD participants was 55.5 years; 52.8% were women; 79.5% were white, 18.0% were black, and 2.5% were other.
As shown in Table 1, the algorithm guided interviewers in both SCAMP and INCPAD to ask about the 4 P’ s—past history, plan, probability, and preventive factors—and several clarifying questions; the latter were asked at the interviewer’s discretion. An algorithm form was completed if a suicide assessment was triggered at any of the 5 scheduled research interviews (baseline or 1, 3, 6, or 12 months) or during a scheduled clinical assessment by a nurse care manager (SCAMP) or by automated depression monitoring (INCPAD). In addition to the closed-ended questions, there were 2 open-ended questions that asked patients to describe their plan of action and any protective factors that prevented them from carrying it out.
Patients in whom the suicide algorithm was triggered were classified into 3 risk categories based upon responses to the P4 screener questions. Minimal risk patients were those who had neither a past history nor a suicide plan and also responded “not at all likely” to the question about probability of an attempt. Lower risk patients were those who indicated they had a plan and/or past history but responded “not at all likely” to the question about probability and noted there were factors preventing them from taking action. Higher risk patients were those who reported the probability of a suicide attempt as either “somewhat likely” or “very likely” and/or reported there were no factors preventing them from taking action.
Analysis
We tabulated the number of times the suicide assessment algorithm was triggered in the SCAMP and INCPAD trials. Since some patients triggered the suicide assessment multiple times over the 12-month trials, we also determined the number of patients who triggered the suicide assessment at least once and the proportion of patients classified as minimal, lower, and higher risk. Responses to the 2 open-ended questions about suicide plans and preventive factors were independently coded by 2 investigators trained in qualitative research. This coding resulted in 5 categories each for suicide plans (overdose, firearms, vehicular accidents, cutting self, other) and preventive factors (family, future hope, religious faith, fear of failure, other).
RESULTS
Frequency of Suicide Assessments
There were a total of 1,144 interviews at the 5 time points in SCAMP and 1,667 in INCPAD. A suicide assessment was triggered in 69 (6%) of the interviews in SCAMP and 83 (5%) in INCPAD. The likelihood of triggering a suicide assessment declined slightly after the first month and then remained stable. For example, the proportion of interviews triggering a suicide assessment in INCPAD at baseline and 1, 3, 6, and 12 months was 8.1% (25/309), 8.2% (22/269), 5.3% (13/246), 5.4% (12/223), and 5.5% (11/202), respectively. A similar temporal pattern was observed in SCAMP.
Suicidal Risk Stratification
Figure 2 outlines the risk stratification of patients from both trials who triggered the suicide algorithm. Since some patients triggered the algorithm more than 1 time during their 5 assessments in the trial, the total number of unique patients triggering the assessment in Figure 2 is less than the total number of interviews in which an assessment was triggered (Table 2). A suicide assessment was triggered 1 or more times by 17.6% (44 of 250) of SCAMP participants and 16.5% (51 of 309) of INCPAD participants. Thus, about 1 in 6 patients in both trials (1 enrolling depressed primary care patients with chronic pain and the other enrolling depressed patients with cancer of whom 56% had comorbid pain) triggered a suicide assessment when interviewed up to 5 times over a 12-month period.
Of the patients who triggered a suicide assessment, the majority (29 of 44 in SCAMP and 27 of 51 in INCPAD) were classified as minimal risk by our algorithm, meaning they had no past attempt or current plan and reported the probability of hurting themselves as “not at all likely.” Most of the remaining patients were classified as lower risk, meaning that although they reported a past history and/or plan, they considered the probability of hurting themselves as “not at all likely” and reported factors preventing them from taking action. Only 1 (0.4%) of the SCAMP patients and 5 (1.6%) of the INCPAD patients were classified as higher risk, defined as a self-assessed probability of self-harm as “somewhat likely” or “very likely” and/or an absence of factors preventing the patient from taking action.
A suicide assessment was triggered during only 1 interview by 65 patients, in 2 interviews by 18 patients, and in 3 or more interviews by 12 patients. In the 30 patients who triggered a suicide assessment more than once, the risk class remained constant in 19 patients and changed across interviews in 11. The risk class went down in 4 patients over time, went up in 5, and changed directions twice in 2.
Of note, there were an additional 250 nondepressed patients with chronic pain in SCAMP who were assessed at baseline and 3 and 12 months as part of a secondary cohort study, and there were 96 nondepressed cancer patients with pain in INCPAD who were assessed at baseline and 1, 3, 6, and 12 months. In both trials, all participants underwent an identical assessment battery including depression measures. The number of patients who triggered the suicide assessment in these nondepressed pain groups was only 7 (2.8%) in SCAMP and 5 (5.2%) in INCPAD, confirming the strong linkage between suicidal ideation and depression.
Severity of Depression and Suicidal Risk Class
Since some patients triggered a suicide assessment more than once over the 12 months of follow-up in each trial, we examined depression severity at the interview level. Of 2,391 evaluable interviews in the SCAMP and INCPAD trials, a suicide assessment was triggered in 144 (6.0%). The mean participant HSCL-20 score across all interviews was 1.42, compared to a mean HSCL-20 score of 2.21 for the 83 interviews classified as minimal risk and 2.37 for the 62 interviews classified as lower (n = 53) or higher risk (n = 9); the latter 2 categories were combined because of the small number of interviews in the higher risk category. Depression was therefore more severe (P < .0001) in patients who triggered a suicide assessment, but did not differ significantly among the different risk categories in those who triggered an assessment.
Suicide Plans and Preventive Factors
There were 58 suicide assessments in the 2 trials in which a specific plan was elicited from the patient. The most common plans involved medication overdose (n = 26), using a gun (n = 13), intentional vehicular accident (n = 8), and cutting oneself (n = 7). There were 75 suicide assessments in the 2 trials in which the patients reported factors that would prevent them from harming themselves. The most common preventive factors were the “4 F’ s”: family (n = 46), future hope (n = 17), faith (n = 13), and fear of failing in their attempt (n = 6).
Suicide Attempts
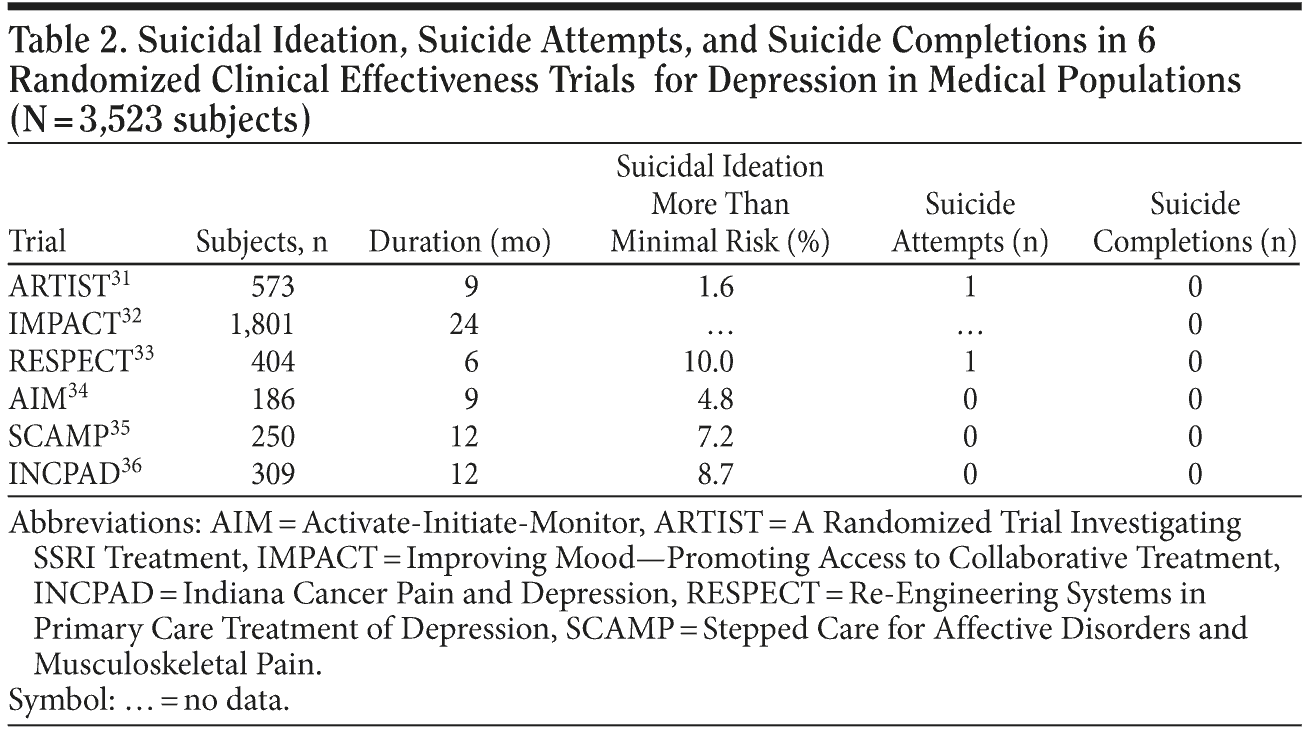
Table 2 reports data on suicide attempts and completed suicides for the 6 trials (1 trial only had data on suicide completions). In the 3,523 depressed patients enrolled in the 6 trials, there were only 2 known suicide attempts and no completed suicides. The 2 patients who attempted suicide did so by ingesting unknown quantities of medication and were in the earlier trials that led to the algorithm tested in SCAMP and INCPAD. Both had triggered a suicide assessment. The patient who attempted suicide in ARTIST (A Randomized Trial Investigating SSRI Treatment) did so shortly after study enrollment and had relatively severe depressive symptoms (PHQ-9 score of 20). The patient was determined to be low risk as a result of the assessment, primarily because he did not have a plan. The assessment was conducted approximately 5 days prior to the suicide attempt, and, therefore, the patient may not have been at high risk or suicidal at that time (K.K., MD, unpublished data, 2010). The patient who attempted suicide in RESPECT had been classified as intermediate risk on her baseline interview given her thoughts of self-harm but no active plan.6 Her primary care physician already was treating her with paroxetine. After the overdose, the patient’s primary care physician changed her pharmacotherapy to venlafaxine and referred her to a psychologist for psychotherapy as well.6
DISCUSSION
Our study has several important findings. First, the probability of serious suicidality as manifest by high-risk suicidal ideation, suicide attempts, or suicide completions is quite low in clinically depressed primary care or medical specialty patients enrolled in depression treatment effectiveness trials. Second, a simple algorithm inquiring about the 4 P’ s—past attempts, current plans, probability of an attempt, and preventive factors—can serve as a brief screen for risk stratification of patients identified as having potential thoughts of self-harm. Third, even in patients who have such thoughts, specific factors can often be identified that are currently preventing them from acting on their thoughts, the most common protective factors being the 4 F’ s: family, future hope, faith, and fear of failing in their attempt.
The low rate of serious suicidal behavior manifest in our 6 trials was only slightly higher in the PROSPECT trial of 598 patients with late-life depression, which was also conducted in a general medical population with an explicit aim of reducing suicide40; the PROSPECT trial had 3 suicide attempts and 1 completed suicide. A few cautionary points should be noted. All 6 of our effectiveness trials excluded patients considered to be seriously suicidal at baseline (of note, suicidal ideation was not included in eligibility criteria in PROSPECT). Although this exclusion applied to very few potential study subjects, it means such patients would not have been enrolled in our effectiveness trials. While it is likely that such patients would be identified by the P4 screener, the rare occurrence of suicide attempts and completions in these clinical trials may underestimate true rates among the entire population of depressed patients in clinical practice. Also, all patients were enrolled in a clinical trial, which tends to enhance treatment beyond that which may occur in clinical practice settings. Clearly, screening for suicidal risk should be done in parallel with optimizing depression treatment.
A second limitation of our study was the rarity of actual suicide attempts. As noted, such events are uncommon in clinical practice and even rarer in clinical trials. Because of this, our findings must be considered largely descriptive until further validation occurs. Such efforts could include administration of the P4 screener to a sample of more seriously depressed patients (eg, psychiatric inpatients) or testing with a case-control design in which P4 responses are compared between patients with and without suicide attempts or suicidal behaviors. Also, comparing the performance of the P4 screener with longer suicidality scales is desirable.
Suicidal ideation is clearly an imperfect proxy for suicidal behavior. Certainly, some patients who are serious about committing suicide hide their plans from their family, friends, and clinicians or proceed to make an attempt even if their ideation is disclosed to or detected by the clinician. The fact that suicide remains an uncommon event relative to the prevalence of depression, particularly in patients receiving care in medical rather than psychiatric referral settings, makes it exceptionally difficult to gather sufficient data regarding the sensitivity and specificity of any measure in detecting actual suicide attempts. Even the data from multiple large trials that prompted the US Food and Drug Administration (FDA) to issue a black box warning about antidepressants and suicide risk were based much more on suicidal ideation than suicidal behavior.
Our study has several strengths. Although based on patients eligible and willing to enroll in clinical trials, the effectiveness design of the trials increases the generalizability of our findings to clinical practice. In contrast to efficacy trials, effectiveness trials are more pragmatic with fewer exclusion criteria, are less tightly controlled “real world” administration of treatments, and include a wider spectrum of patients in terms of age, medical comorbidity, socioeconomic disparities, and other characteristics. A second strength is both the large number of patients studied (> 3,500) and the diversity of clinical settings, including both primary care and medical specialty populations. Third, the structured assessment algorithm and prospective data collection in the SCAMP and INCPAD trials allowed us to gather detailed quantitative and qualitative data that further inform us about the nature of suicidal ideation in depressed medical patients.
The availability of a brief measure for assessing suicidality is especially timely for several reasons. Depression screening has been recommended by the US Preventive Services Task Force as long as there are systems in place to enhance appropriate treatment.41,42 Brief and even ultrabrief measures (2-4 items) have been found to have excellent operating characteristics for depression screening.43,44 However, no comparable evidence-based brief screening measure to assess suicidal ideation has been proposed. In evaluating chest pain, physicians learn to risk-stratify patients with a few specific questions to identify the urgency of the situation as well as those patients needing referral and/or hospitalization. Similarly, clinicians would benefit considerably by having an efficient way for assessing suicide risk in depressed medical patients that can distinguish the rare patient that needs urgent “same-day” psychiatric evaluation from the majority of patients who can have either initial treatment and follow-up in the medical setting or referral to (or collaboration with) mental health specialists.
A second benefit to having a brief assessment tool relates to recent concerns by the FDA and others that initiating antidepressant therapy may be associated with a slight increased risk of suicidal ideation, particularly in adolescents and young adults.45,46 The algorithm in Figure 1 provides a simple yet structured means of documenting suicide assessment, both for clinical and medicolegal purposes. Third, our work may inform current suicide prevention efforts in health care systems such as the Department of Defense and Veterans Health Administration. Military personnel and veterans, especially those returning from Iraq and Afghanistan, have been found to have higher suicide rates than the general population. For example, the Veterans Health Administration has implemented a new performance measure requiring a screen for suicidal ideation when veterans screen positive for depression.
Despite its public health importance, the degree to which suicide is preventable remains uncertain.1,47-51 Nonetheless, there is general agreement that asking about suicidal ideation is an indicator of high-quality depression care and should be the standard of practice. While only a small fraction of patients expressing suicidal ideation actually attempt suicide, the group as a whole is more seriously depressed and warrants more aggressive treatment and closer follow-up. The fact that depression is common while completed suicide is rare means that an individual clinician will encounter hundreds of depressed patients in his or her lifetime, yet (fortunately) will experience few or no completed suicides. This necessitates a brief approach to assessing suicide risk that conforms to the realities of a busy clinical practice,27,52 wherein complex psychosocial issues can be especially burdensome.53,54 In addition to time constraints, a lack of perceived competence or comfort in assessing suicidal ideation may be a barrier for some non-mental health clinicians, a factor that could be addressed by training in a structured assessment approach. To this end, the brief algorithm developed over the course of 6 randomized effectiveness trials may be a useful tool for clinicians, educators, and researchers.
Drug names: paroxetine (Paxil, Pexeva, and others), venlafaxine (Effexor and others).
Author affiliations: Indiana University, Bloomington (Ms Dube); VA HSRD Center of Excellence on Implementation of Evidence-Based Practices, Roudebush VAMC, Indianapolis; Indiana University School of Medicine, Regenstrief Institute Inc, Indianapolis (Drs Kroenke, Bair, and Williams); and Community Home Health Hospice and Symptom Management Group, Indianapolis (Dr Theobald), Indiana.
Potential conflicts of interest: Dr Kroenke has served as a consultant to Eli Lilly and Forest, has received grant/research support from Eli Lilly and Pfizer, and has received honoraria from Eli Lilly, Forest, and Pfizer. Drs Bair, Theobald, and Williams and Ms Dube report no financial or other relationships relevant to the subject of this article.
Funding/support: This study was supported by grants from the National Institute of Mental Health (R01 MH-071268) and National Cancer Institute (R01 CA-115369), for which Dr Kroenke was principal investigator.
REFERENCES
1. Gaynes BN, West SL, Ford CA, et al; US Preventive Services Task Force. Screening for suicide risk in adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140(10):822-835. PubMed
2. Cooper-Patrick L, Crum RM, Ford DE. Identifying suicidal ideation in general medical patients. JAMA. 1994;272(22):1757-1762. PubMed doi:10.1001/jama.272.22.1757
3. Lish JD, Zimmerman M, Farber NJ, et al. Suicide screening in a primary care setting at a Veterans Affairs medical center. Psychosomatics. 1996;37(5):413-424. PubMed
4. Olfson M, Weissman MM, Leon AC, et al. Suicidal ideation in primary care. J Gen Intern Med. 1996;11(8):447-453. PubMed doi:10.1007/BF02599038
5. Schulberg HC, Bruce ML, Lee PW, et al. Preventing suicide in primary care patients: the primary care physician’s role. Gen Hosp Psychiatry. 2004;26(5):337-345. PubMed doi:10.1016/j.genhosppsych.2004.06.007
6. Schulberg HC, Lee PW, Bruce ML, et al. Suicidal ideation and risk levels among primary care patients with uncomplicated depression. Ann Fam Med. 2005;3(6):523-528. PubMed doi:10.1370/afm.377
7. Nutting PA, Dickinson LM, Rubenstein LV, et al. Improving detection of suicidal ideation among depressed patients in primary care. Ann Fam Med. 2005;3(6):529-536. PubMed doi:10.1370/afm.371
8. Verger P, Brabis PA, Kovess V, et al. Determinants of early identification of suicidal ideation in patients treated with antidepressants or anxiolytics in general practice: a multilevel analysis. J Affect Disord. 2007;99(1-3):253-257. PubMed doi:10.1016/j.jad.2006.09.012
9. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA. 2005;293(13):1635-1643. PubMed doi:10.1001/jama.293.13.1635
10. Joiner TE Jr, Pfaff JJ, Acres JG. A brief screening tool for suicidal symptoms in adolescents and young adults in general health settings: reliability and validity data from the Australian National General Practice Youth Suicide Prevention Project. Behav Res Ther. 2002;40(4):471-481. PubMed doi:10.1016/S0005-7967(01)00017-1
11. Oyama H, Koida J, Sakashita T, et al. Community-based prevention for suicide in elderly by depression screening and follow-up. Community Ment Health J. 2004;40(3):249-263. PubMed doi:10.1023/B:COMH.0000026998.29212.17
12. Un×¼tzer J, Tang L, Oishi S, et al; for the IMPACT Investigators. Reducing suicidal ideation in depressed older primary care patients. J Am Geriatr Soc. 2006;54(10):1550-1556. PubMed doi:10.1111/j.1532-5415.2006.00882.x
13. Vannoy SD, Duberstein P, Cukrowicz K, et al. The relationship between suicide ideation and late-life depression. Am J Geriatr Psychiatry. 2007;15(12):1024-1033. PubMed doi:10.1097/JGP.0b013e3180cc2bf1
14. Links PS, Heisel MJQA, Quastel A. Is suicide ideation a surrogate endpoint for geriatric suicide? Suicide Life Threat Behav. 2005;35(2):193-205. PubMed doi:10.1521/suli.35.2.193.62870
15. Emanuel EJ, Fairclough DL, Daniels ER, et al. Euthanasia and physician-assisted suicide: attitudes and experiences of oncology patients, oncologists, and the public. Lancet. 1996;347(9018):1805-1810. PubMed doi:10.1016/S0140-6736(96)91621-9
16. Henderson JM, Ord RA. Suicide in head and neck cancer patients. J Oral Maxillofac Surg. 1997;55(11):1217-1221, discussion 1221-1222. PubMed doi:10.1016/S0278-2391(97)90170-1
17. Miller M, Mogun H, Azrael D, et al. Cancer and the risk of suicide in older Americans. J Clin Oncol. 2008;26(29):4720-4724. PubMed doi:10.1200/JCO.2007.14.3990
18. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738. PubMed doi:10.1200/JCO.2007.13.8941
19. Walker J, Waters RA, Murray G, et al. Better off dead: suicidal thoughts in cancer patients. J Clin Oncol. 2008;26(29):4725-4730. PubMed doi:10.1200/JCO.2007.11.8844
20. Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the scale for suicidal ideation. J Consult Clin Psychol. 1979;47(2):343-352. PubMed doi:10.1037/0022-006X.47.2.343
21. Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035-1043. PubMed doi:10.1176/appi.ajp.164.7.1035
22. Coric V, Stock EG, Pultz J, et al. Sheehan Suicidality Tracking Scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6(1):26-31. PubMed
23. Cutcliffe JR, Barker P. The Nurses’ Global Assessment of Suicide Risk (NGASR): developing a tool for clinical practice. J Psychiatr Ment Health Nurs. 2004;11(4):393-400. PubMed doi:10.1111/j.1365-2850.2003.00721.x
24. Brown GK, Bruce ML, Pearson JL. High-risk management guidelines for elderly suicidal patients in primary care settings. Int J Geriatr Psychiatry. 2001;16(6):593-601. PubMed doi:10.1002/gps.468
25. Cole S, Raju M, Barrett J, et al. The MacArthur Foundation Depression Education Program for Primary Care Physicians: background and rationale. Gen Hosp Psychiatry. 2000;22(5):299-358. PubMed doi:10.1016/S0163-8343(00)80007-9
26. Williams JW Jr, Rost K, Dietrich AJ, et al. Primary care physicians’ approach to depressive disorders: effects of physician specialty and practice structure. Arch Fam Med. 1999;8(1):58-67. PubMed doi:10.1001/archfami.8.1.58
27. Feldman MD, Franks P, Duberstein PR, et al. Let’s not talk about it: suicide inquiry in primary care. Ann Fam Med. 2007;5(5):412-418. PubMed doi:10.1370/afm.719
28. Williams JWJ Jr. Competing demands: does care for depression fit in primary care? J Gen Intern Med. 1998;13(2):137-139. PubMed doi:10.1046/j.1525-1497.1998.00032.x
29. Malone KM, Szanto K, Corbitt EM, et al. Clinical assessment versus research methods in the assessment of suicidal behavior. Am J Psychiatry. 1995;152(11):1601-1607. PubMed
30. Duffy FF, Chung H, Trivedi M, et al. Systematic use of patient-rated depression severity monitoring: is it helpful and feasible in clinical psychiatry? Psychiatr Serv. 2008;59(10):1148-1154. PubMed doi:10.1176/appi.ps.59.10.1148
31. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA. 2001;286(23):2947-2955. PubMed doi:10.1001/jama.286.23.2947
32. Un×¼tzer J, Katon W, Callahan CM, et al; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836-2845. PubMed doi:10.1001/jama.288.22.2836
33. Dietrich AJ, Oxman TE, Williams JW Jr, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329(7466):602-605. PubMed doi:10.1136/bmj.38219.481250.55
34. Williams LS, Kroenke K, Bakas T, et al. Care management of poststroke depression: a randomized, controlled trial. Stroke. 2007;38(3):998-1003. PubMed doi:10.1161/01.STR.0000257319.14023.61
35. Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301(20):2099-2110. PubMed doi:10.1001/jama.2009.723
36. Kroenke K, Theobald D, Norton K, et al. The Indiana Cancer Pain and Depression (INCPAD) trial: design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. Gen Hosp Psychiatry. 2009;31(3):240-253. PubMed doi:10.1016/j.genhosppsych.2009.01.007
37. Walker J, Sharpe M, Kroenke K, et al. The HSCL-20: one questionnaire, two versions. J Psychosom Res. 2010;68(3):313-314. PubMed doi:10.1016/j.jpsychores.2009.11.002
38. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509-521.
39. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2001.
40. Bruce ML, Ten Have TR, Reynolds CF 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091. PubMed doi:10.1001/jama.291.9.1081
41. US Preventive Services Task Force. Screening for depression: recommendations and rationale. Ann Intern Med. 2002;136(10):760-764. PubMed
42. US Preventive Services Task Force. Screening and treatment for major depressive disorder in children and adolescents: US Preventive Services Task Force Recommendation Statement. Pediatrics. 2009;123(4):1223-1228. PubMed doi:10.1542/peds.2008-2381
43. Williams JW Jr, Pignone M, Ramirez G, et al. Identifying depression in primary care: a literature synthesis of case-finding instruments. Gen Hosp Psychiatry. 2002;24(4):225-237. PubMed doi:10.1016/S0163-8343(02)00195-0
44. Mitchell AJ, Coyne JC. Do ultra-short screening instruments accurately detect depression in primary care? a pooled analysis and meta-analysis of 22 studies. Br J Gen Pract. 2007;57(535):144-151. PubMed
45. Gunnell D, Ashby D. Antidepressants and suicide: what is the balance of benefit and harm. BMJ. 2004;329(7456):34-38. PubMed doi:10.1136/bmj.329.7456.34
46. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696. PubMed doi:10.1001/jama.297.15.1683
47. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074. PubMed doi:10.1001/jama.294.16.2064
48. Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif. 2008;32(1):77-108. PubMed doi:10.1177/0145445507304728
49. Paris J. Predicting and preventing suicide: do we know enough to do either? Harv Rev Psychiatry. 2006;14(5):233-240. PubMed doi:10.1080/10673220600968662
50. Pitman A. Policy on the prevention of suicidal behaviour; one treatment for all may be an unrealistic expectation. J R Soc Med. 2007;100(10):461-464. PubMed doi:10.1258/jrsm.100.10.461
51. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24. PubMed doi:10.4088/JCP.07m03904
52. Klinkman MS. Competing demands in psychosocial care: a model for the identification and treatment of depressive disorders in primary care. Gen Hosp Psychiatry. 1997;19(2):98-111. PubMed doi:10.1016/S0163-8343(96)00145-4
53. Wetterneck TB, Linzer M, McMurray JE, et al; Society of General Internal Medicine Career Satisfaction Study Group. Worklife and satisfaction of general internists. Arch Intern Med. 2002;162(6):649-656. PubMed doi:10.1001/archinte.162.6.649
54. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169(4):333-334. PubMed doi:10.1001/archinternmed.2008.548