A Psychosomatic Perspective on Takotsubo Cardiomyopathy: A Case Report
Takotsubo cardiomyopathy is a reversible heart condition initially described in the Japanese literature in the 1990s. The typical presentation mimics an acute coronary syndrome. It is frequently found in elderly women in the context of emotional or physical stress in the absence of significant obstructive coronary disease. The prognosis of the disease is favorable just with supportive care, but little is known about recurrence. The association between onset of Takotsubo cardiomyopathy and emotional triggers is striking but has received little attention in the psychiatric literature. On the basis of current evidence, we suggest consideration of β-blockers for recurrence prevention.
Prim Care Companion for CNS Disorders 2011;13(2):e1-e4
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: March 13, 2010; accepted March 19, 2010.
Published online: March 24, 2011 (doi:10.4088/PCC.10br00980).
Corresponding author: George Costin, MD, Southern Illinois University School of Medicine, PO Box 19636, Springfield, IL 62794-9636 ([email protected]).
Takotsubo cardiomyopathy, also known as apical ballooning, stress cardiomyopathy, or “broken heart syndrome,” is a reversible heart condition that was initially described in the Japanese literature in the 1990s. Many cases have now been reported worldwide. The typical presentation may mimic acute coronary syndrome. Frequently, Takotsubo cardiomyopathy occurs in elderly women in the context of emotional or physical stress. The electrocardiogram (ECG) reveals abnormalities, and there may be elevation of cardiac enzymes. The echocardiogram and heart catheterization show regional wall motion abnormalities extending beyond a single epicardial vascular distribution. There is no evidence of obstructive coronary disease. Although there are no treatment guidelines, the symptoms usually resolve in a few days, and the echocardiogram normalizes in a few weeks.
Case Report
Ms A, a 78-year-old white woman with no history of coronary artery disease and no significant past psychiatric history, was seen in psychiatric consultation 2 days after she was admitted to the hospital in 2007 for chest pain. The chest pain started suddenly at rest at the hospital after her husband was admitted for an acute coronary syndrome. The pain was midsternal, dull, moderate, and persistent and responded to nitroglycerin drip after several hours. The patient denied shortness of breath, palpitations, diaphoresis, or fear of dying. She said she was worried about her husband and she felt “exasperated” that her husband would not come to the emergency department one day prior to that when his chest pain started.
Ms A’s past medical history was significant for atrial fibrillation status post pacemaker placement for bradycardia, hypertension, and carotid disease status post endarterectomy.
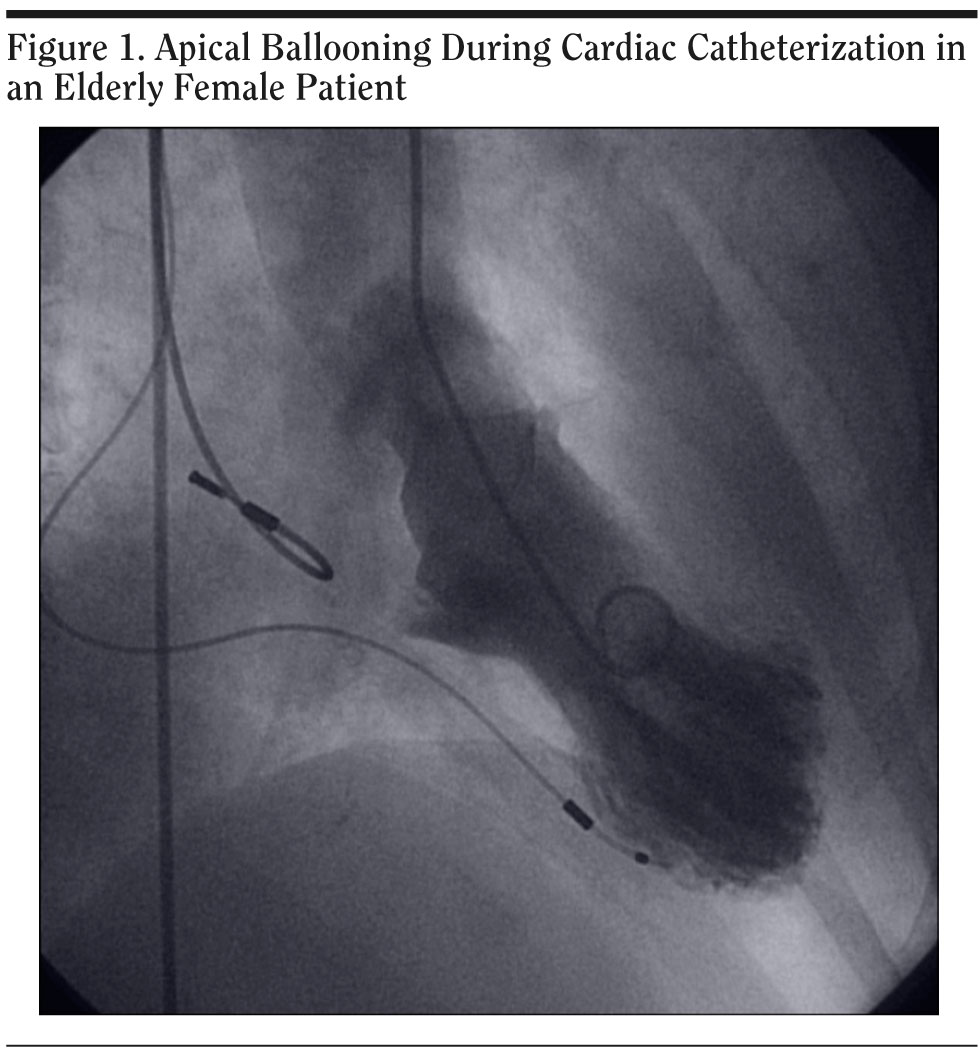
Ms A had a normal heart catheterization several years ago. She did not have a formal psychiatric diagnosis, but years ago she was treated with paroxetine by her primary care physician for symptoms of depression. Her medications at the time of admission included warfarin, metoprolol, and digoxin. The family history and social history were noncontributory. There was no history of abuse and no other stressor was identified. On the physical examination, a pacemaker was noticed in the left shoulder area and an endarterectomy scar was seen, but otherwise the examination revealed no abnormalities. At the time of the psychiatric consult, 2 days after the admission, the patient’s mental status examination was noncontributory. Her laboratory tests showed mildly elevated cardiac enzymes, and ECG showed atrial fibrillation with ventricular paced rhythm. Cardiac catheterization was done, which showed normal coronary arteries and apical ballooning (Figure 1). Left ventricular ejection fraction was 40%.
The patient did not have recurrence of her chest pain during the hospitalization and had no other complaints. She was discharged home after 2 days without any change in medication. From a psychiatric perspective, her Axis I diagnosis was “emotional stress affecting a general medical condition” (per DSM-IV criteria) and Axis III diagnosis was Takotsubo cardiomyopathy. At 6-week follow-up with the cardiologist, she reported no other symptoms. Echocardiogram showed a left ventricular ejection fraction of 45%-50% and no other abnormalities.
Literature Review
Takotsubo cardiomyopathy is a form of reversible left ventricular systolic dysfunction that mimics an acute coronary syndrome in the absence of coronary artery disease. Although initially reported in Japan in the 1990s, many case reports have been published in Europe and North America more recently.1 As noted, Takotsubo cardiomyopathy is also known as apical ballooning syndrome, stress cardiomyopathy, ampula cardiomyopathy, and broken heart syndrome.2 The shape of the left ventricle in systole is similar to a pot Japanese fisherman use to catch octopuses, called a takotsubo.
Takotsubo cardiomyopathy is encountered mainly in women in their seventh decade (88.8%) and has a 1.7%-2.2% prevalence among patients with suspected acute coronary syndrome.3 The clinical presentation is that of an acute myocardial infarction usually with ST elevation. The most common symptoms are acute substernal chest pain (67.8%) and dyspnea (17.8%).3 The chest pain occurs at rest in 33%-71% of the patients.4 Takotsubo cardiomyopathy can present with or lead to complications including arrhythmias, cardiogenic shock, and death.3
An emotional stress trigger is identified in 26.8% (range, 14%-38%) and a physical stress trigger is identified in 37.8% (17%-77%).3,4 One-third of the patients do not have any trigger.3 A number of different emotional stressors have been identified in the literature: death, severe illness, or injury of a family member, friend, or pet; severe argument; legal proceedings; receiving bad news such as daughter’s divorce; spouse leaving for war; diagnosis of a major illness; car accident; move to a new residence; public speaking; or a surprise party.1 Also, Takotsubo cardiomyopathy has been reported with electroconvulsive therapy (ECT) and postpartum depression.5-8
Most common ECG changes are ST segment elevation in anterior precordial leads in 81.6%, while T wave abnormalities are seen in 64.3% and Q waves in 31.8%. Cardiac markers are usually mildly elevated: troponin in 86.2% and CK-MB in 73.9%.3 The kinetics of the enzymes do not appear to follow the slow rise and fall observed in myocardial infarction, as several studies show that the peak is at the initial presentation.4 Coronary angiography shows normal coronary arteries in 80.6% of the cases or mild (< 50%) luminal stenosis. On echocardiography, left ventricular ejection fraction is decreased (ranging from 20% to 49%), improving in a matter of days to weeks to 60%-76%.3 The most common abnormality on imaging studies is apical ballooning, but akinesis or dyskinesis of other regions of the left or right ventricle have been described.2
The pathogenesis of Takotsubo cardiomyopathy is not well understood. Although it mimics acute coronary syndrome, coronary arteriography does not show lesions that could account for the wall motion abnormalities.9 Diffuse multivessel vasospasm was mostly reported in Japan,3 but overall it has been reported in less than 11% of the cases published in the literature.9 Microvascular ischemia in the absence of obstructive coronary artery disease has also been proposed. Abnormal myocardial perfusion of the apical and midventricular segments was seen, but cardiac imaging (scintigraphic or magnetic resonance) did not show myocardial necrosis.9
The “metabolic” myocardial stunning secondary to catecholamine stimulation seems to be the favorite hypothesis. Support comes from nuclear imaging techniques utilizing metabolic tracers and studies comparing catecholamine levels in Takotsubo cardiomyopathy and acute myocardial infarction with similar degree of clinical heart failure. Also, in a rat model, a similar cardiomyopathy was prevented by pretreatment with α- and β-adrenergic antagonists, and myocardial biopsies in patients with Takotsubo cardiomyopathy demonstrated findings consistent with catecholamine-mediated toxicity.9
The diagnosis of Takotsubo cardiomyopathy is a clinical one. The presence of regional wall motion abnormalities beyond a single epicardial coronary contribution, the absence of obstructive coronary artery disease or plaque rupture that would explain the above-mentioned abnormalities, and new ECG abnormalities or troponin elevation establish diagnosis.4,9
There are no clear treatment guidelines for Takotsubo cardiomyopathy. Usually it is a transient disorder managed with supportive therapy. ECG monitoring, aspirin, heparin, and β-blockers should be considered since Takotsubo cardiomyopathy mimics an acute coronary syndrome.1 Monitoring in the hospital on telemetry, performing echocardiogram or magnetic resonance imaging of the heart, and evaluating for dynamic obstruction in the left ventricular outflow tract in selected cases are suggested.9 Standard therapy for left ventricular dysfunction as well as anticoagulation may be considered. An echocardiogram should be obtained prior to discharge, if necessary, to assess left ventricular function. Consider another echocardiogram in 1-3 months.9 γ-Aminobutyric acid (GABA) agonists, catecholamine receptor blockade, calcium channel blockers, free radical scavengers, and antioxidants are under investigation for different types of neurocardiac damage.10 Literature on the psychiatric aspects of treatment is lacking.
The prognosis is generally favorable with supportive care. The average in-hospital mortality is 1.1%.3 Recurrence of the disease is documented in 0%-8% of patients,4 but the true recurrence is unknown because of lack of good follow-up.
Discussion
Takotsubo cardiomyopathy is a recently described heart condition, and its association with emotional stressors is striking. In the psychiatric literature, it has been discussed in a letter to the editor11 and in case reports relating apical ballooning to ECT and postpartum depression.5-8 We present a case report of Takotsubo cardiomyopathy from a consultation liaison psychiatry perspective with an emphasis on the possible pharmacologic treatment options.
Stress has different types of adverse side effects on the cardiovascular system. Acute emotional stress can cause left ventricular dysfunction (stress cardiomyopathy or Takotsubo cardiomyopathy), myocardial ischemia, or arrhythmias.12 Chronic stress can contribute to hypertension, atherosclerosis, coronary artery disease, and other conditions.13 Stress is thought to affect the heart through the central and autonomic nervous system, and the relationship between the heart and the nervous system has been recently revisited.10 Takotsubo cardiomyopathy is part of stress-related cardiomyopathy syndromes that include left ventricular dysfunction associated with intracranial hemorrhage, ischemic stroke, and head trauma and transient left ventricular dysfunction in acute medical illness.9 Although they all share the catecholamine-mediated mechanism, Takotsubo cardiomyopathy is the only such syndrome that can manifest after an acute emotional stress.
Basic science research suggested that β-blockers may have an important role in fear memory consolidation.14 In clinical research, the role of β-blockers was investigated for the prevention of acute stress disorder and early posttraumatic stress disorder, but the number and the size of the studies on the topic are small and the only β-blocker studied was propranolol.15 Propranolol, a β-blocker that crosses the brain-blood barrier, may have a preventive effect on subsequent posttraumatic stress disorder, as was noted in a randomized control study,16 and was shown to achieve clinical remission of target symptoms after acute stress in an open-label study.17
In view of the above data, there may be an indication for the indefinite use of propranolol after a diagnosis of Takotsubo cardiomyopathy both for its peripheral effects on myocardial stunning and for central effects related to emotional hyperarousal. Our patient was already on metoprolol treatment at the time when she was diagnosed with Takotsubo cardiomyopathy. Although metoprolol and propranolol have similar lipophilicity, to the best of our knowledge, no study to date supports the use of metoprolol for prevention of acute stress.
Conclusion
Takotsubo cardiomyopathy is a psychosomatic condition that has received little attention in the psychiatric literature. Empirical evidence favors long-term β-blocker therapy to address recurrence from a cardiologic perspective.1,9 From a psychiatric perspective, β-blocker therapy may be helpful for decreasing further emotional impact on the patient’s functional status. An important question for future research is whether there is any difference between different β-blockers in terms of acute stress prevention. Also, in acute stress, altering the autonomic activity of the heart using relaxation therapy, biofeedback, social support, meditation, hypnosis, slow breathing, or yoga may be beneficial.12 Further research on the psychiatric issues involved in this condition is necessary for a better understanding of this newly recognized important cardiac entity.
Drug names: digoxin (Lanoxin and others), metoprolol (Toprol, Lopressor, and others), paroxetine (Paxil, Pexeva, and others), propranolol (Inderal and others), warfarin (Coumadin, Jantoven, and others).
Author affiliations: Department of Internal Medicine and Psychiatry, Southern Illinois University School of Medicine, Springfield, Illinois.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Acknowledgment: Library research assistance provided by St John’s Hospital Health Sciences Library and Southern Illinois University School of Medicine Library staff (Springfield, Illinois).
References
1. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417. PubMed doi:10.1016/j.ahj.2007.11.008
2. Aurigemma G. Acute stress cardiomyopathy and reversible left ventricular dysfunction. Cardiology Rounds. 2006. www.cardiologyrounds.org. Accessed September 14, 2010.
3. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. PubMed doi:10.1093/eurheartj/ehl032
4. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865. PubMed
5. O’ Reardon JP, Lott JP, Akhtar UW, et al. Acute coronary syndrome (Takotsubo cardiomyopathy) following electroconvulsive therapy in the absence of significant coronary artery disease: case report and review of the literature. J ECT. 2008;24(4):277-280. PubMed doi:10.1097/YCT.0b013e31815fa4ab
6. Kent LK, Weston CA, Heyer EJ, et al. Successful retrial of ECT two months after ECT-induced takotsubo cardiomyopathy. Am J Psychiatry. 2009;166(8):857-862. PubMed doi:10.1176/appi.ajp.2008.08081278
7. Yaqub Y, Jenkins LA, Nugent KM, et al. Postpartum depression and apical ballooning syndrome (takotsubo syndrome). J Obstet Gynaecol Can. 2009;31(8):736-739. PubMed
8. Chandra PA, Golduber G, Chuprun D, et al. Tako-tsubo cardiomyopathy following electroconvulsive therapy. J Cardiovasc Med (Hagerstown). 2009;10(4):333-335. PubMed
9. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118(4):397-409. PubMed doi:10.1161/CIRCULATIONAHA.106.677625
10. Samuels MA. The brain-heart connection. Circulation. 2007;116(1):77-84. PubMed doi:10.1161/CIRCULATIONAHA.106.678995
11. Can MM, Tanboga IH, Turkyilmaz E, et al. Takotsubo cardiomyopathy in a patient with emotional stress. Am J Psychiatry. 2008;165(8):1052-1053. PubMed doi:10.1176/appi.ajp.2008.08010127
12. Ziegelstein RC. Acute emotional stress and cardiac arrhythmias. JAMA. 2007;298(3):324-329. PubMed doi:10.1001/jama.298.3.324
13. Esch T, Stefano GB, Fricchione GL, et al. Stress in cardiovascular diseases. Med Sci Monit. 2002;8(5):RA93-RA101. PubMed
14. Adamec R, Muir C, Grimes M, et al. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 2007;179(2):192-207. PubMed doi:10.1016/j.bbr.2007.02.001
15. Davidson JR. Pharmacologic treatment of acute and chronic stress following trauma: 2006. J Clin Psychiatry. 2006;67(suppl 2):34-39. PubMed
16. Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51(2):189-192. PubMed doi:10.1016/S0006-3223(01)01279-3
17. Pastrana Jiménez JI, Catalina Romero C, Garc×a Diéguez N, et al. Pharmacological treatment of acute stress disorder with propranolol and hypnotics. Actas Esp Psiquiatr. 2007;35(6):351-358. PubMed