Schizoaffective Disorder With Missed Diagnosis of Acute Porphyria: A Case Report and Overview

ABSTRACT
Acute porphyrias are often misdiagnosed and most commonly present as atypical neuropsychiatric symptoms or acute abdominal pain. Clinicians should suspect acute porphyrias in patients presenting with variable neuropsychiatric symptoms and unexplained pain. Proper identification can lead to less iatrogenicity associated with porphyrinogenic agents, appropriate management, and a better patient outcome. The case of a patient with hereditary coproporphyria, one of the acute porphyrias, is presented to illustrate the broad manifestations, unsuspected diagnosis, and difficulties in management.
Prim Care Companion CNS Disord
2011;13(6):doi:10.4088/PCC.11br01234
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: June 14, 2011; accepted June 22, 2011.
Published online: December 1, 2011.
Corresponding author: Gaurav Jain, MD, Southern Illinois University School of Medicine, 901 W Jefferson, PO Box 19642, Springfield, IL 62794-9642 ([email protected]).
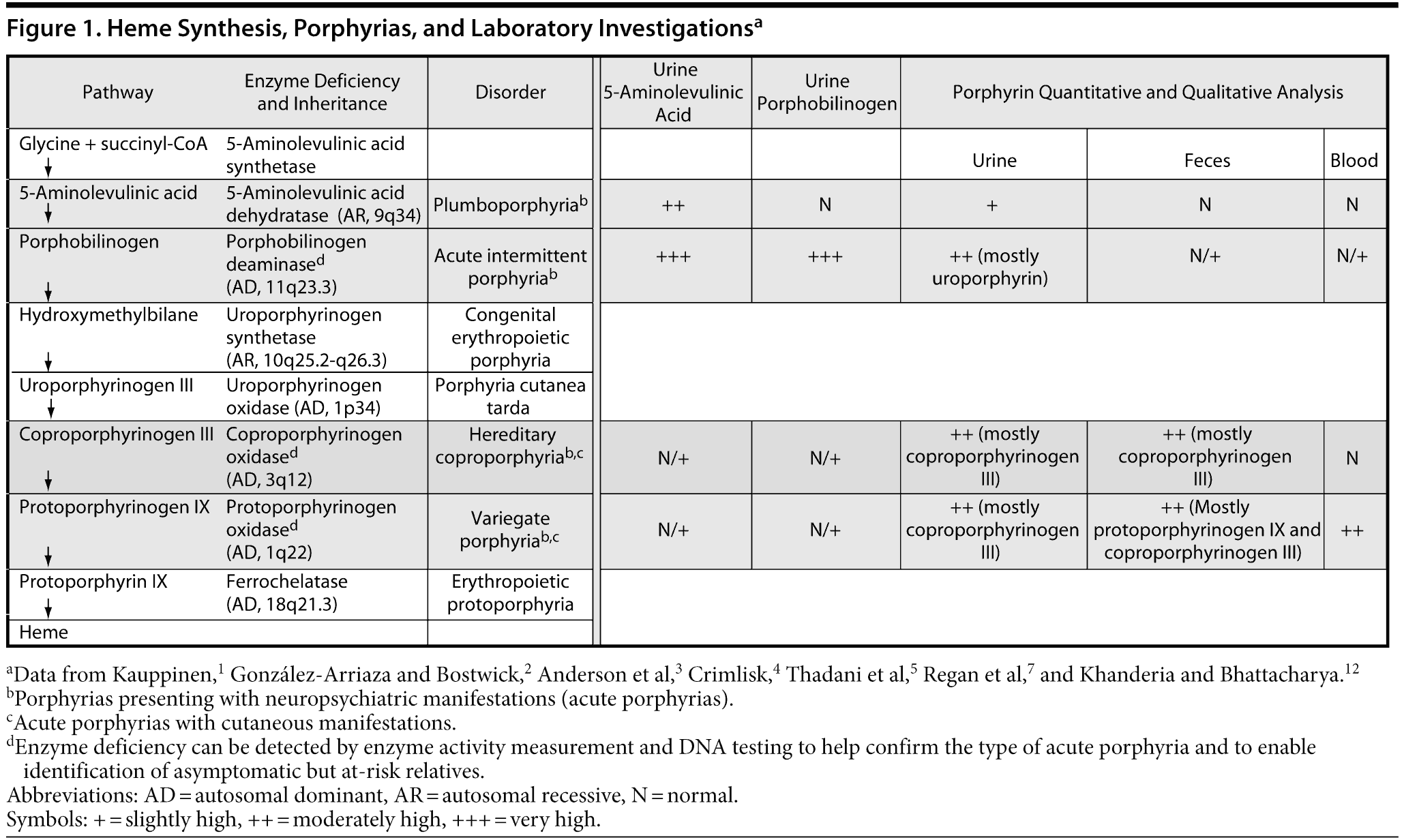
Porphyrias form a group of rare inherited metabolic disorders, each resulting from a partial deficiency of a specific enzyme in the heme biosynthesis pathway (Figure 1).1-3 Of the 7 types of porphyria, 4 present with neuropsychiatric manifestations (2 neuroporphyrias: acute intermittent porphyria and plumboporphyria and 2 neurocutaneous porphyrias: hereditary coproporphyria and variegate porphyria).1,2 These 4 types of porphyria are often termed “acute porphyrias.”2,4,5
Among patients with acute porphyrias, 20% to 58% have neuropsychiatric symptoms during acute exacerbations.2,6,7 Psychiatric manifestations include hysteria, anxiety, depression, phobias, psychosis, agitation, delirium, and restlessness.2,4 Some patients develop psychosis similar to schizophrenia.4 Neurovisceral manifestations include seizures, neuropathy, hyporeflexia, autonomic instability, abdominal pain, constipation, nausea, and vomiting.1,4 Severe abdominal pain is the most commonly reported presenting symptom during acute attacks.1,2,5 Recovery of mental function often lags behind physical recovery, and some patients report functional disturbances indefinitely.2
Porphyrias are mainly inherited in an autosomal dominant manner with incomplete penetrance, but autosomal recessive and more complex patterns of inheritance are also possible (see Figure 1).1 Because the disease has variable expression, the estimated prevalence of gene carriers for acute porphyria in the general population of the United States is 1 to 2 per 10,000 people, with clinical disease manifesting in approximately 10% of these carriers.2,4 Tishler et al8 and Burgoyne et al6 reported prevalence rates of 0.21% and 0.48%, respectively, for acute porphyria in psychiatric inpatients, which were considerably higher than rates in the general population. Clinical manifestations arise after puberty, with a peak age presentation in the early thirties.1,5 Women are 4 to 5 times more likely to have clinical manifestations than men.5 In some patients with acute porphyrias, the neuropsychiatric manifestations overshadow the visceral symptoms such that the diagnosis of porphyria is not considered.2,4 We present a case of hereditary coproporphyria, one of the acute porphyrias, to illustrate broad manifestations, unsuspected diagnosis, and difficulties in management.
CASE PRESENTATION
Ms A, a 34-year-old woman with a history of seizure disorder, had been admitted to the hospital every 2 to 3 months over the last 4 years with sadness, excessive fatigue, paranoid delusions, auditory hallucinations, suicidal ideations/attempt, poor appetite, and sleep disturbances. In addition, Ms A would complain of vague moderate to severe abdominal pain, intermittent nausea, vomiting, and severe low back pain. More than 75% of her exacerbations were also accompanied by multiple seizures.

- Acute porphyrias are frequently misdiagnosed.
- Clinicians should suspect acute porphyrias in patients presenting with variable neuropsychiatric symptoms and unexplained intermittent pain.
- Avoidance of porphyrinogenic agents improves outcome.
Ms A’s history was negative for cutaneous or photosensitivity reactions, liver or thyroid dysfunction, and substance use disorders. Ms A was diagnosed with schizoaffective disorder (depressed type) per DSM-IV-TR criteria. Her repeated admissions to the hospital were attributed to poor compliance, resistance to treatment, or poor psychosocial conditions. A complex partial seizure disorder was diagnosed by the neurologist, and Ms A was maintained on phenytoin. She would improve within 7 to 10 days of hospitalization with various interventions that included removal from a stressful environment, treatment of dehydration, improved oral intake, and opioid medications for pain. The most common psychotropics used were sertraline 100-150 mg/d, risperidone 2-4 mg/d, diazepam 5-10 mg/d, and trazodone 50-150 mg/d. Multiple workups (including complete metabolic profile; complete blood count; amylase, lipase, and thyroid-stimulating hormone levels; computed tomography of the abdomen; and magnetic resonance imaging of the head and spine) were done due to her complaints of abdominal pain, nausea, vomiting, and back pain. No cause was identified. Hence, Ms A was diagnosed with an undifferentiated somatoform pain disorder.
Four years into the course of Ms A’s illness, porphyria was suspected due to unexplained abdominal pain. Tests for spot and 24-hour urinary porphobilinogen and erythrocyte porphobilinogen deaminase activity, as well as urine and fecal quantitative/qualitative porphyrin levels, were ordered. Urinary porphobilinogen and erythrocyte porphobilinogen deaminase activity was normal. Her urinary coproporphyrin I and III levels were mildly elevated at 8 μmol/mol (normal < 6) and 27 μmol/mol (normal < 14), respectively. Urinary uroporphyrin levels were normal. Fecal coproporphyrin and protoporphyrin levels were normal, as were liver function tests. The above tests were consistent with hereditary coproporphyria, although this diagnosis was not definitive due to normal fecal coproporphyrin levels (see Figure 1). However, Ms A’s clinical response and subsequent treatment were consistent with hereditary coproporphyria.
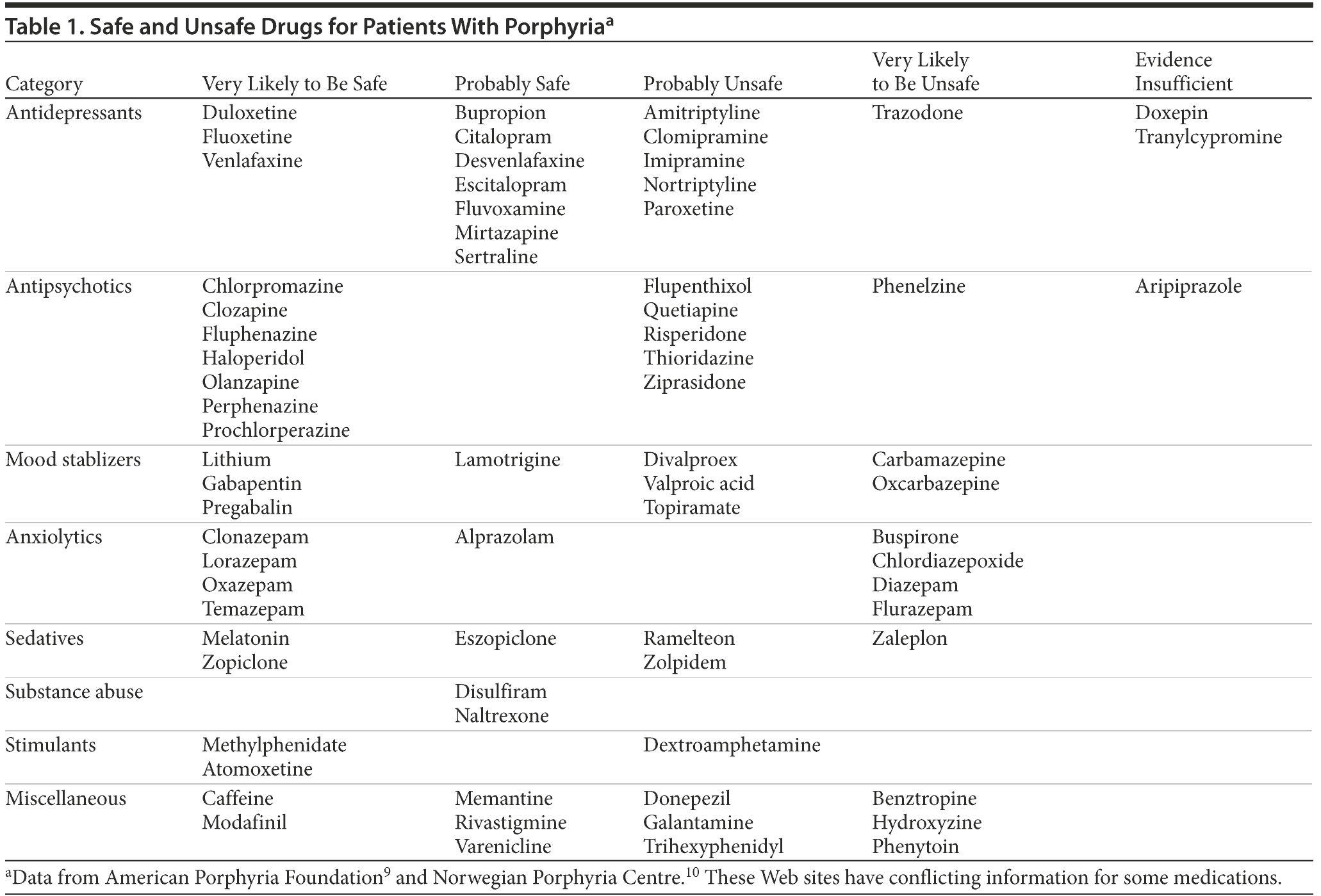
Ms A’s management was modified to treat and prevent acute porphyria. Her diagnosis per DSM-IV-TR criteria was revised to major depressive disorder. She was given dextrose 10% at 100 mL/h for 36 hours. Safe medications (nonporphyrinogenic) were determined by searching the American Porphyria Foundation Drug Database9 and changes were made. Risperidone was discontinued. Diazepam and trazodone were switched to clonazepam for anxiety control and insomnia. Ms A responded favorably within 48 hours, with resolution of psychotic symptoms, abdominal pain, nausea, vomiting, and back pain. Her depression was also significantly improved. She was educated about the precipitating factors including medications to avoid. Ms A was encouraged to seek help for smoking cessation. She has been stable on sertraline 150 mg/d without hospitalization for 1 year, which is the longest period without exacerbation of her psychiatric illness in her 5-year clinical presentation.
DISCUSSION
Physicians should have a high index of suspicion for acute porphyrias in patients frequently presenting with variable neuropsychiatric symptoms associated with unexplained pain problems, especially abdominal pain. The recognition of acute porphyrias is important in view of widespread use of porphyrinogenic agents and iatrogenic attacks complicating the clinical course and misleading the management of the syndrome.1,3,9,10 In the present case, the diagnosis was entertained because of the following: (1) the uncommon course of psychotic symptoms, (2) unexplained abdominal pain symptoms, (3) indeterminate seizures and their temporal correlation with psychiatric symptoms, (4) back pain of unknown cause, and (5) a confusing constellation of symptoms with intermittent exacerbations.
Although biochemical measurements of excreted porphyrins and porphyrin precursors reveal acute porphyrias in the majority of cases, none of these tests are sensitive or specific for acute porphyrias. When exposed to normal lighting, urine porphyrin concentration is reduced by 50% in 24 hours.4 False-negative results of urine porphyrin quantification may be caused by a time lag between sample collection and testing.2,3,11 In hereditary coproporphyria, the increase in aminolevulinic acid and porphobilinogen levels may be more transient than in acute intermittent porphyria and therefore may lead to normal test results.3 Thus, the laboratory results significantly depend on the phase of illness, methods used, and speed of evaluation of the sample.3,11 These facts make the diagnosis of acute porphyrias difficult. Repeated testing is advised in cases wherein the syndrome is suspected, such as in our case with the symptom constellation listed above.
Common precipitating factors of acute porphyrias are high stress situation (physiologic and psychological), endogenous hormones (progesterone, luteal phase), and crash dieting.1-5 Cigarette smoking, alcohol, and drugs through their interactions with cytochrome P450 enzymes, which lead to induction of heme synthesis, play an important role in acute porphyrias.1-5,9,10 Table 1 shows a list of common psychotropic drugs that are safe or unsafe in patients with porphyria.1,9,10 For a detailed list of unsafe drugs, please refer to the drug databases on the Web sites of the American Porphyria Foundation9 or Norwegian Porphyria Centre.10 Information from the above Web sites was conflicting for some drugs. Therefore, we advise readers to review more literature before choosing treatment. The pathogenesis of the neuropsychiatric manifestations remains unclear and poorly understood, but multiple hypotheses have been proposed, including central nervous system metabolic abnormalities, ischemia, demyelination, oxidative stress, free radical damage, aminolevulinic acid direct neurotoxicity, and nervous tissue heme deficiency.1-5,12
In hereditary coproporphyria, 35% of patients have acute attacks and 29% have skin symptoms,13 which may explain the absence of dermatologic findings in our case. In patients with hereditary coproporphyria and skin symptoms, high excretion of fecal coproporphyrin III, frequently accompanied by increased urinary coproporphyrin excretion and a plasma fluorescence peak at 619 nm, is the biochemical hallmark of the disease.12,14 Coproporphyrinogen oxidase activity is usually diminished, and mutation screening can be used to detect symptom-free patients in the family.1
Clinicians should suspect a porphyria in patients presenting with intermittent and variable neuropsychiatric symptoms and unexplained visceral pain and/or seizures. Results from quantitative tests for porphyrins in the urine, stool, and plasma may prove valuable in making a diagnosis sooner and could lead to a better patient outcome.
Drug names: alprazolam (Xanax, Niravam, and others), aripiprazole (Abilify), atomoxetine (Strattera), benztropine (Cogentin and others), bupropion (Wellbutrin, Aplenzin, and others), buspirone (BuSpar and others), carbamazepine (Carbatrol, Equetro, and others), chlordiazepoxide (Librium and others), citalopram (Celexa and others), clomipramine (Anafranil and others), clonazepam (Klonopin and others), clozapine (Clozaril, FazaClo, and others), desvenlafaxine (Pristiq), dextroamphetamine (Dexedrine and others), diazepam (Diastat, Valium, and others), disulfiram (Antabuse), divalproex (Depakote), donepezil (Aricept and others), doxepin (Zonalon, Silenor, and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), eszopiclone (Lunesta), fluoxetine (Prozac and others), flurazepam (Dalmane and others), fluvoxamine (Luvox and others), gabapentin (Neurontin and others), galantamine (Razadyne), haloperidol (Haldol and others), imipramine (Tofranil and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), lorazepam (Ativan and others), memantine (Namenda), methylphenidate (Focalin, Daytrana, and others), mirtazapine (Remeron and others), modafinil (Provigil), naltrexone (Vivitrol, ReVia, and others), nortriptyline (Pamelor, Aventyl, and others), olanzapine (Zyprexa), oxcarbazepine (Trileptal and others), paroxetine (Paxil, Pexeva, and others), phenelzine (Nardil), phenytoin (Dilantin, Phenytek, and others), pregabalin (Lyrica), prochlorperazine (Compro and others), quetiapine (Seroquel), ramelteon (Rozerem), risperidone (Risperdal and others), rivastigmine (Exelon and others), sertraline (Zoloft and others), temazepam (Restoril and others), topiramate (Topamax and others), tranylcypromine (Parnate and others), trazodone (Oleptro and others), valproic acid (Depakene, Stavzor, and others), varenicline (Chantix), venlafaxine (Effexor and others), zaleplon (Sonata and others), ziprasidone (Geodon), zolpidem (Ambien, Edluar, and others).
Author affiliations: Department of Internal Medicine and Psychiatry (Drs Jain and Resch), Department of Psychiatry (Dr Bennett), and Department of Internal Medicine, Division of Hematology and Oncology, Simmons Cancer Institute (Dr Godwin), Southern Illinois University School of Medicine, Springfield.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Previous presentations: This case was presented in the clinical vignette competition at the Association of Medicine and Psychiatry Conference; September 24-25, 2010; Chicago, Illinois. A portion of this report was also presented as a poster presentation at the 13th Annual American Society of Interventional Pain Physicians meeting; June 25-29, 2011; Arlington, Virginia.
Acknowledgment: The authors thank Kristina Dzara, PhD, Department of Psychiatry, Southern Illinois University School of Medicine, Springfield, for valuable feedback during the preparation of this manuscript. Dr Dzara reports no conflicts of interest related to the subject of this article.
REFERENCES
1. Kauppinen R. Porphyrias. Lancet. 2005;365(9455):241-252. PubMed
2. Gonzסlez-Arriaza HL, Bostwick JM. Acute porphyrias: a case report and review. Am J Psychiatry. 2003;160(3):450-459. PubMed doi:10.1176/appi.ajp.160.3.450
3. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439-450. PubMed
4. Crimlisk HL. The little imitator—porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry. 1997;62(4):319-328. PubMed doi:10.1136/jnnp.62.4.319
5. Thadani H, Deacon A, Peters T. Diagnosis and management of porphyria. BMJ. 2000;320(7250):1647-1651. PubMed doi:10.1136/bmj.320.7250.1647
6. Burgoyne K, Swartz R, Ananth J. Porphyria: reexamination of psychiatric implications. Psychother Psychosom. 1995;64(3-4):121-130. PubMed doi:10.1159/000289001
7. Regan L, Gonsalves L, Tesar G. Acute intermittent porphyria. Psychosomatics. 1999;40(6):521-523. PubMed
8. Tishler PV, Woodward B, O’ Connor J, et al. High prevalence of intermittent acute porphyria in a psychiatric patient population. Am J Psychiatry. 1985;142(12):1430-1436. PubMed
9. American Porphyria Foundation. Drug Database. http://www.porphyriafoundation.com/drug-database. Accessed October 7, 2011.
10. The Norwegian Porphyria Centre (NAPOS). The Drug Database for Acute Porphyria . http://www.drugs-porphyria.org/. Accessed October 7, 2011.
11. American Porphyria Foundation. Health care professionals: AIP, HCP, VP & ADP. http://www.porphyriafoundation.com/for-healthcare-professionals/types-of-porphyria/AIP. Accessed October 7, 2011.
12. Khanderia U, Bhattacharya A. Acute intermittent porphyria: pathophysiology and treatment. Pharmacotherapy. 1984;4(3):144-150. PubMed
13. Brodie MJ, Thompson GG, Moore MR, et al. Hereditary coproporphyria: demonstration of the abnormalities in haem biosynthesis in peripheral blood. Q J Med. 1977;46(182):229-241. PubMed
14. K×¼hnel A, Gross U, Doss MO. Hereditary coproporphyria in Germany: clinical-biochemical studies in 53 patients. Clin Biochem. 2000;33(6):465-473. PubMed doi:10.1016/S0009-9120(00)00159-4