Resource Utilization and Costs Associated With Insomnia Treatment in Patients With Major Depressive Disorder

ABSTRACT
Objective: To estimate resource utilization and costs associated with insomnia treatment among newly treated patients with major depressive disorder (MDD).
Method: Data from the MarketScan® Research Databases (Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Health and Productivity Management) were analyzed. Patients aged ≥ 18 years with a first prescription claim for an antidepressant between January 1, 2006, and December 31, 2007 (index date), were included in the analysis if they had ≥ 1 MDD diagnosis (ICD–9-CM criteria) in the 12 months prior to the claim and 24 months of continuous insurance coverage. Patients were categorized into 2 groups on the basis of the presence or absence of insomnia medication during the 12 months following the index date. Multivariate analyses were conducted to compare all-cause and MDD-related hospitalization and emergency room (ER) visits and costs in the year following the index date between patients with and without insomnia medication. Covariates for adjustment included age, gender, region, health plan type, baseline comorbidities, and health care utilization.
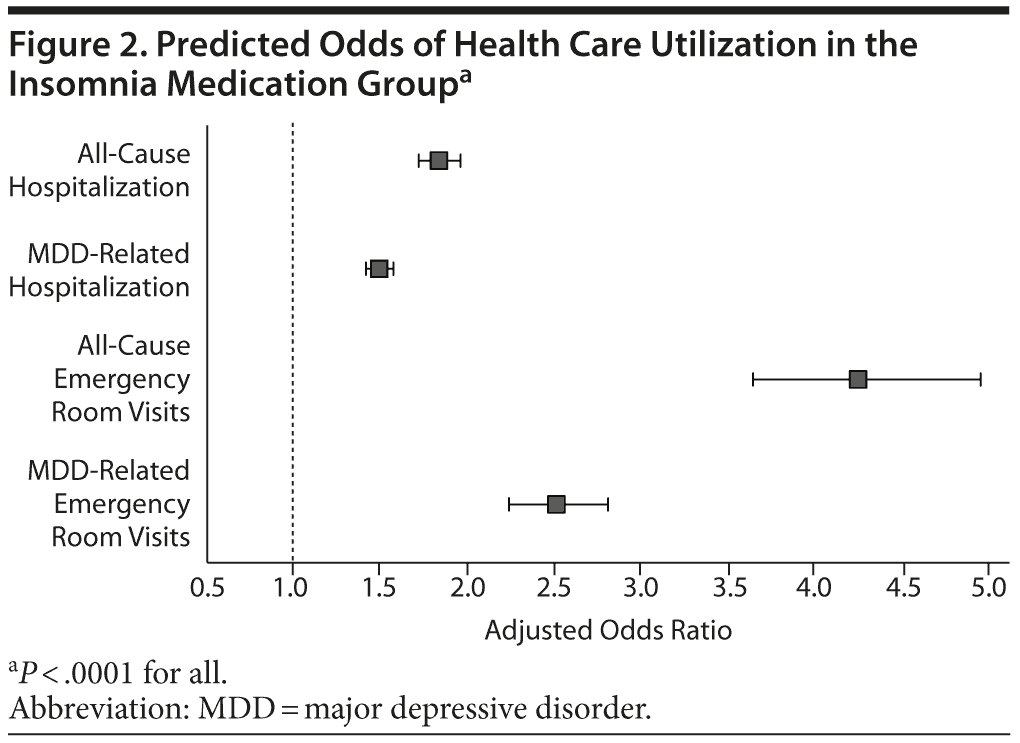
Results: The total sample size was 87,461 newly treated MDD patients with a mean (SD) age of 43.5 (15.1) years; 67% were women. Among newly treated patients, 10,339 (11.8%) took insomnia medication. Patients taking insomnia medication were significantly more likely to be hospitalized (OR = 1.84, 95% CI = 1.73-1.96 for all cause; OR = 1.50, 95% CI = 1.43-1.57 for MDD related; P < .0001) and to have ER visits (OR = 4.25, 95% CI = 3.65-4.95 for all cause; OR = 2.51, 95% CI = 2.24-2.81 for MDD related; P < .0001) than the patients not taking insomnia medication. Adjusted all-cause health care costs were $3,918 (95% CI = $3,599-$4,290) higher and MDD-related health care costs were $537 (95% CI = $492-$586) higher in the insomnia medication cohort compared with controls in the 12-months following the index date. Patients taking insomnia medication had $1,162 more indirect costs for short-term disability compared to the control group (95% CI = $746-$1,684).
Conclusions: The use of insomnia medications in newly treated patients with MDD appears to be associated with increased health care resource utilization and higher total and depression-related direct and indirect medical costs.
Prim Care Companion CNS Disord 2012;14(5):doi:10.4088/PCC.12m01374
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: March 5, 2012; accepted May 29, 2012.
Published online: September 27, 2012.
Corresponding author: Edward Kim, MD, MBA, Health Economics and Outcomes Research, East Hanover, NJ 07936-1080 ([email protected]).
Major depressive disorder (MDD) is associated with mood, cognitive, and physical symptoms that impair functioning.1 The lifetime prevalence of MDD among US adults is 13.2%, with an annual prevalence of 5.3%, making MDD and associated comorbidities one of the nation’s most compelling public health problems.2 Although no recent assessment exists to quantify the direct and indirect economic impact of depressive disorders, the total US economic burden of depression in 2000 was estimated at $83.1 billion, with direct medical costs accounting for only 31% of the total cost.3 Attributable workplace-related financial losses have been estimated at $44 billion to $51.5 billion annually.3,4 Treatment goals are aimed at inducing remission of the major depressive episode and achieving a full return to the patient’s level of functioning5; however, ineffective response to treatment in patients with depression is widespread.6,7
Sleep disturbances are a core symptom of depression and are often comorbid with depression.8,9 In addition, insomnia is a common residual symptom of depression, with incomplete treatment response.10,11 Previous studies indicate that 35% to 60% of individuals with chronic insomnia have comorbid depression and/or anxiety.12 Patients with depression and severe insomnia have higher rates of relapse and other negative outcomes such as suicide,13 worse quality of life,14 and increased medical morbidity.15 In addition, treatment with certain antidepressant medications may cause or worsen insomnia. Both of these considerations may further complicate treatment decision-making.16,17
In the general population, insomnia is a common condition, with a prevalence of 10% or affecting approximately 25 million people in the United States.18 Direct and indirect costs associated with insomnia have been estimated in the tens of billions annually,18 and claims analyses confirm a significant per-patient impact of insomnia on health care expenditures and productivity losses, including impaired work performance.19,20 Research conducted to compare work-related disability rates among patients with insomnia and depression found that, compared with depression, insomnia was an equally or more potent driver of disability contributing to retirement.12,13
While the costs associated with MDD and insomnia have been studied extensively as separate entities,3,4,7,12,16,19-24 scant research exists to examine the combined roles of MDD and insomnia in health care expenditures and impaired work function.2,6 The purpose of this study was to evaluate health care utilization, costs, and work productivity loss associated with insomnia in patients newly diagnosed with MDD.
METHOD
Health care utilization and cost data were analyzed from the MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits Databases. These databases represent the health care experience of enrollees in commercial health insurance plans sponsored by more than 100 large-sized and medium-sized employers in the United States, which includes monthly enrollment data, hospitalization and outpatient medical claims, outpatient prescription drug claims, and eligibility information. Work productivity loss data were obtained from the MarketScan® Health and Productivity Management Database. The study did not require informed consent or institutional review board approval because all study data were accessed using techniques compliant with the Health Insurance Portability and Accountability Act of 1996. Thus, no identifiable protected health information was extracted during the course of the study.
Sample Selection and Patient Cohorts
Major depressive disorder was identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD–9-CM) codes 296.2 (MDD, single episode), 296.3 (MDD, recurrent episode), or 311.xx0 (depressive disorder, not elsewhere classified). Patients aged ≥ 18 years with a first prescription claim for an antidepressant between January 1, 2006, and December 31, 2007 (index date), were included in the analysis if they had ≥ 1 MDD diagnosis in the 12 months prior to the claim. The antidepressants considered were selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, norepinephrine-dopamine reuptake inhibitors, and/or noradrenergic and specific serotonin antidepressants (Appendix 1 provides specific antidepressant names). Patients were required to be continuously enrolled for medical and pharmacy benefits in the 12 months before and after the index date. Patients were excluded if they had antidepressant medications in the 12 months prior to the index date or if they had any diagnosis of Alzheimer’s disease, other dementia, schizophrenia, or bipolar disorder and/or a prescription claim for antipsychotic or bipolar disorder medications in the 12 months prior to the index date. Outcomes were assessed in the 12 months following the first claim for an antidepressant.
Patient cohorts were identified on the basis of the presence or absence of a claim for an insomnia medication on the day of the first claim for an antidepressant or during the 12-month follow-up period. The control cohort consisted of patients with no prescription claim for an insomnia medication, while the insomnia cohort consisted of patients with ≥ 1 prescription claims for an insomnia medication during the same period. Insomnia medications considered were zolpidem tartrate, butisol sodium, pentobarbital, carbromal, flurazepam hydrochloride, quazepam, triazolam, eszopiclone, ethchlorvynol, estazolam, temazepam, ramelteon, secobarbital sodium, and zaleplon.25
Outcome Measures
The primary outcomes were health care resource utilization, health care costs, and work productivity loss.26-29 Resource utilization was defined as the number of hospitalizations and emergency room (ER) and outpatient visits.27-29 All-cause and MDD-related resource utilization was assessed. Total heath care costs consisted of total medical amounts, the sum of out-of-pocket costs (ie, prescription drug costs) and payer/medical costs, and were adjusted to year 2009 $US values using the index for medical care in the Consumer Price Index (http://www.bls.gov/cpi/home.htm#data). Medical costs also equaled ER, hospitalization, and outpatient costs.26 Both MDD-related resource use and MDD-related health care costs required the presence of a primary MDD diagnosis for hospitalizations and ER visits leading to hospitalization and either a primary or secondary MDD diagnosis for ER and outpatient visits. MDD-related outpatient prescription drug costs were costs for antidepressants. Work productivity loss was characterized as the number of paid time off and short-term disability (STD) days. Costs associated with productivity loss were calculated for paid time off (number of paid time off absence days ×— 8 hours ×— a mean hourly wage) and STD (STD days ×— 8 hours ×— a mean hourly wage). Data from the US Bureau of Labor Statistics for 2010 were used to obtain the mean hourly wage for all industries.30
Statistical Analysis
Differences in baseline characteristics including gender, age, region, health plan type, number of hospitalizations and ER visits, health care costs, Charlson Comorbidity Index (CCI), medication burden (defined as the number of distinct therapeutic classes of prescription drugs), and all of the outcomes in the follow-up year between the 2 groups were evaluated using χ2 (categorical variables) and t tests (continuous variables). In this study, the CCI was used to control for comorbidities and medical claims (inpatient and outpatient records) from 12 months before the index hospitalizations were reviewed. The adaptation by Deyo et al31 of the CCI measurement method was used, which includes 17 categories of comorbid conditions identified using ICD-9-CM diagnosis codes. These conditions were weighted to create a single comorbidity score.32
Multivariate logistic regression was used to assess the relationship between the presence of insomnia medication and all-cause as well as MDD-related hospitalizations and ER visits in the follow-up period.33 Multivariate linear γ regression with log link or 2-part models was used to predict all-cause as well as MDD-related health care costs, and indirect costs with paid time off as well as STD in the follow-up period, on the basis of distribution of cost variables.34 In the 2-part models, the first part was for the probability of nonzero costs using logistic regression and the second part was for the cost-level conditional on nonzero costs using linear γ regression with log link. Covariates for the above models included age, gender, region, and health plan type and baseline health care costs, hospitalizations, ER visits, CCI scores, and medication burden. As a sensitivity analysis, all outcomes were also assessed in the second year after the index date (24-month follow-up period).
RESULTS
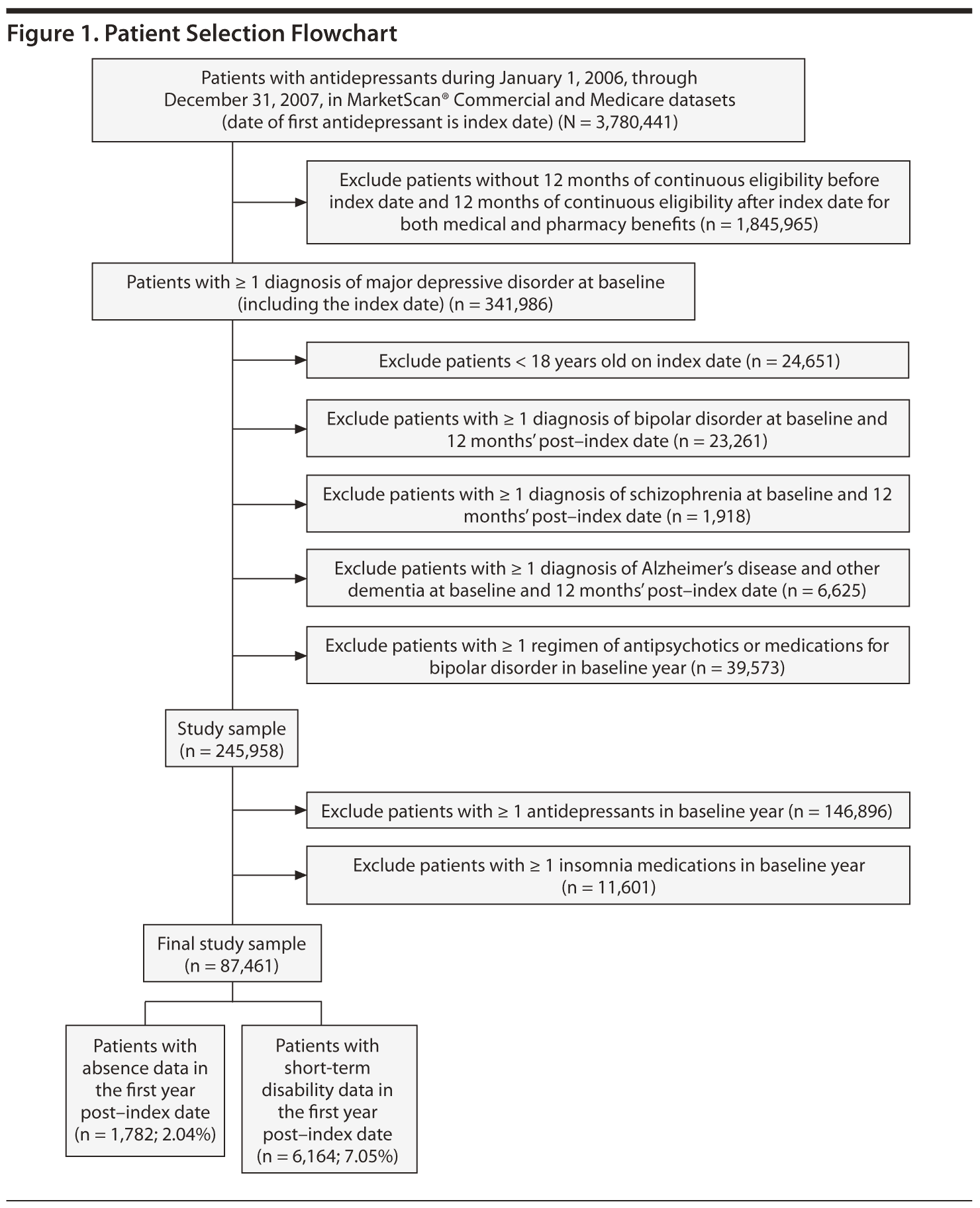
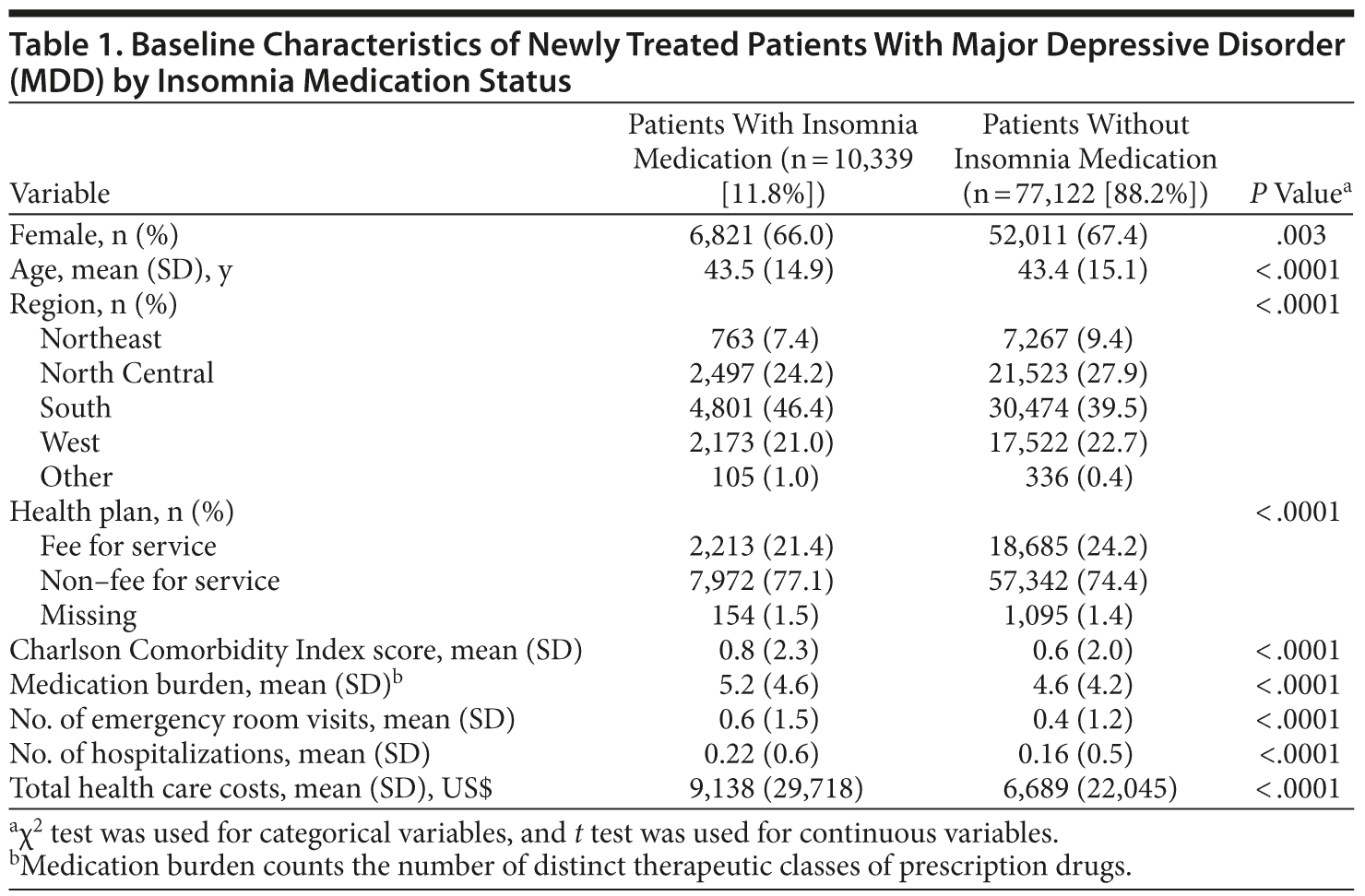
After inclusion and exclusion criteria were applied, the study sample consisted of 87,461 newly treated patients with MDD who had at least 12 months of follow-up data (Figure 1). Of this sample, 77,122 patients (88.2%) did not take insomnia medications (control cohort), and 10,339 (11.8%) did take insomnia medications (insomnia cohort). Patient baseline characteristics are summarized in Table 1. The mean (SD) age of the patients was 43.5 (15.1) years, and 67.3% were women. Compared to the control cohort, patients taking insomnia medication had a significantly lower proportion of women (P = .003) and higher comorbidity, medication burden, resource utilization, and health care costs (P < .0001).
Heath Care Resource Utilization and Costs
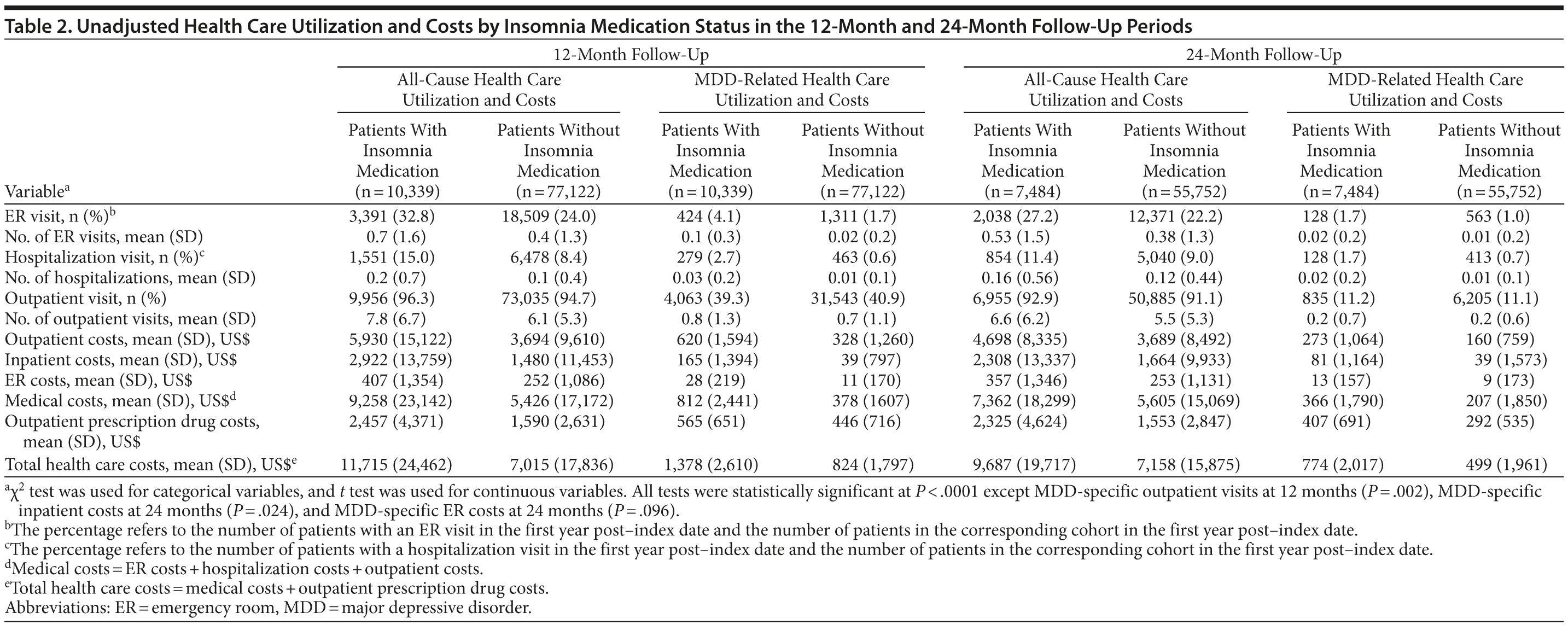
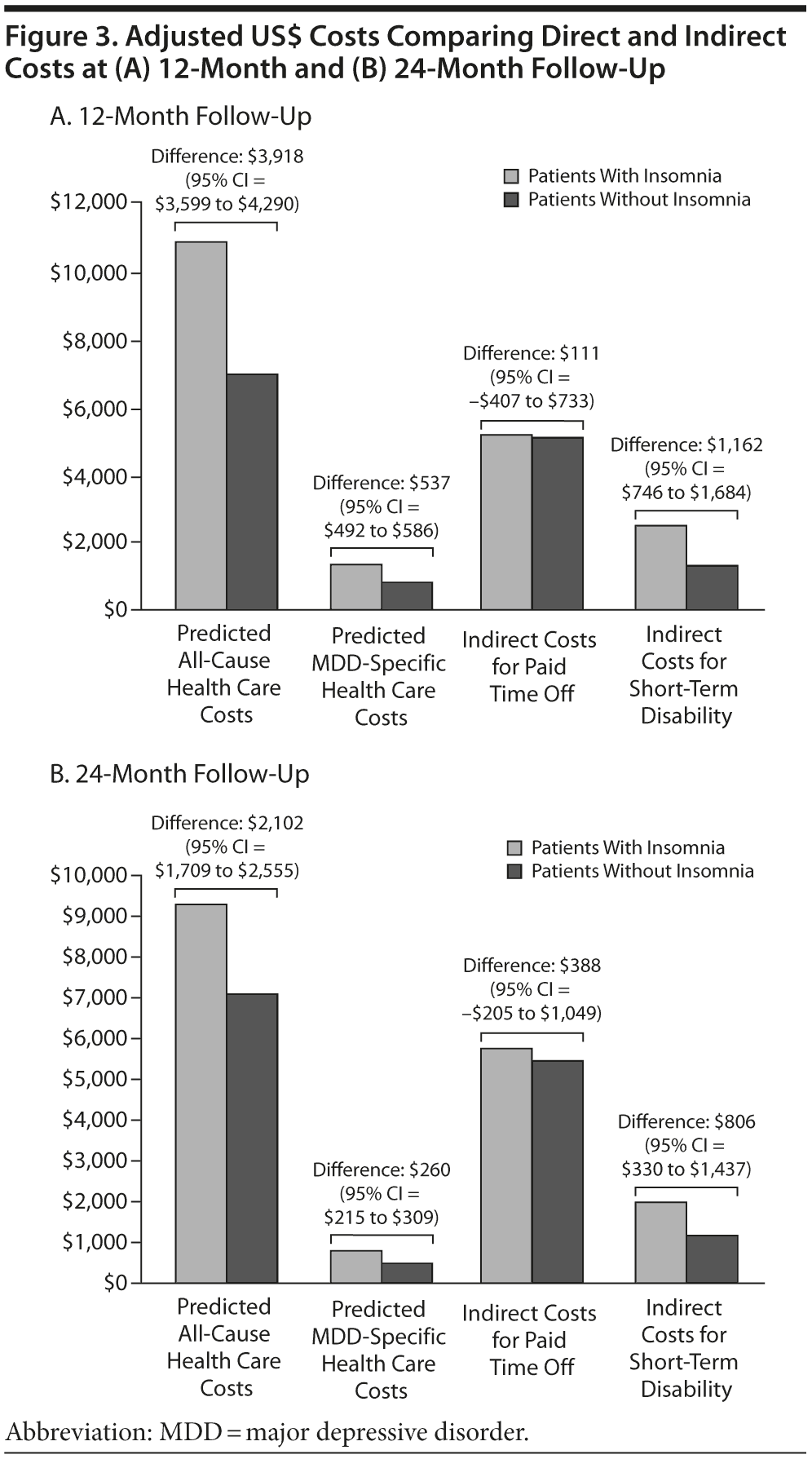
As shown in Table 2, all-cause and MDD-related resource use in the 12-month follow-up period was higher in the insomnia medication cohort for hospitalization (P < .0001), ER visits (P < .0001), and MDD-specific outpatient visits (P = .002). Unadjusted total health care costs in the 12-month follow-up period were also higher in the insomnia medication group (P < .0001). Similar findings were noted in the 24-month follow-up period (Table 2). After adjustment for baseline characteristics, patients taking insomnia medication were found to have more than 1.5 times the risk of MDD-related hospitalizations (odds ratio [OR] = 1.50, 95% CI = 1.43-1.57, P < .0001) and 2.5 times the risk of MDD-related ER visits (OR = 2.51, 95% CI = 2.24-2.81, P < .0001) compared to the control group (Figure 2). Similarly, patients taking insomnia medication had a higher risk for all-cause hospitalizations (OR = 1.84, 95% CI = 1.73-1.96, P < .0001) and were more than 4 times as likely to have all-cause ER visits (OR = 4.25, 95% CI = 3.65-4.95, P < .0001) during the 12-month follow-up period (Figure 2). As shown in Figure 3A, adjusted all-cause health care costs were $3,918 (95% CI = $3,599-$4,290) higher in the insomnia medication cohort compared with controls. MDD-related health care costs were $537 (95% CI = $492-$586) higher in the insomnia medication cohort compared with controls.

Work Productivity Loss
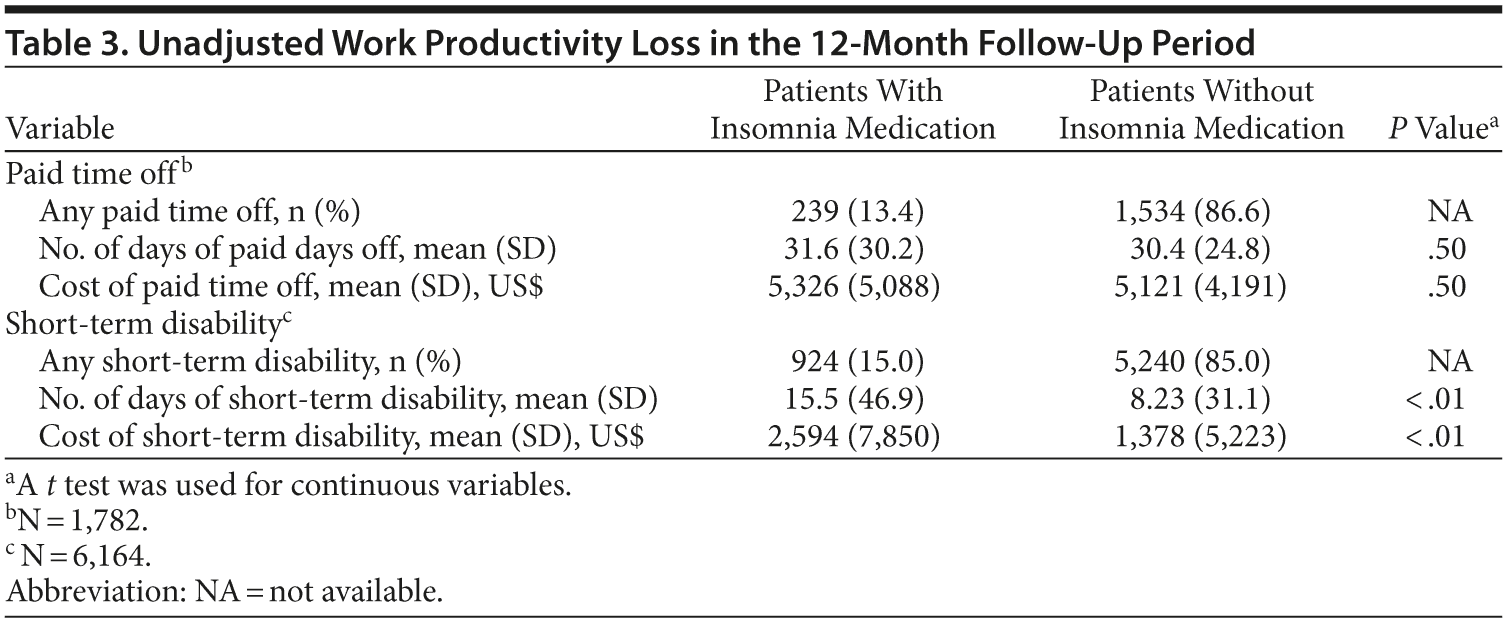
The sample size for patients with paid time off data in the first follow-up year was 1,782 (2.0% of the original sample, Figure 1). There were no statistically significant differences in the actual number of paid time off days (31.6 days vs 30.4 days, respectively, P = .50) and the costs of paid time off between patients with and without insomnia medication ($5,326 vs $5,121, respectively; P = .50). The adjusted costs of paid time off were not statistically different between the 2 groups (cost difference: $111, 95% CI = -$407 to $733, Figure 3A) in the first follow-up year.
The sample size for patients with STD data in the first follow-up year was 6,164 (7.1% of the original sample, Figure 1). Both the actual number of STD days (15.5 days vs 8.2 days, respectively; P < .01) and STD costs ($2,594 vs $1,378, respectively; P < .01) were higher in the insomnia medication cohort compared to the cohort without insomnia medication (Table 3). After adjustment for baseline characteristics, patients taking insomnia medication had $1,162 more indirect costs for STD compared to the control group (95% CI = $746-$1,684; Figure 3A) in the first follow-up year.
Sensitivity Analysis
Sensitivity analysis using a 24-month follow-up period revealed similar findings to those using a 12-month follow-up. Patients with insomnia medication were 46% more likely to have an all-cause ER visit (OR = 1.46, 95% CI=1.39-1.55, P < .0001) and had more than twice the risk of an MDD-related ER visit (OR = 2.39, 95% CI=2.08-2.76, P < .0001) (data not shown). Patients in the insomnia medication group had an 82% higher likelihood of an all-cause hospitalization visit (OR = 1.82, 95% CI=1.69-1.97, P < .0001). Similarly, patients with insomnia medication had greater than 4 times the likelihood of having an MDD-related hospitalization (OR = 4.14, 95% CI=3.43-5.0, P < .0001) in the 24-month follow-up after controlling for baseline characteristics (data not shown).
In a 24-month follow-up, 1,543 patients were available for evaluation of paid time off (data not shown). There were no statistically significant differences in the actual number of paid time off days (33.2 days vs 31.2 days, respectively; P = .22) and the costs of paid time off ($5,771 vs $5,411, respectively; P = .22) between patients with and without insomnia medication. The adjusted cost difference of paid time off between the 2 groups in the 24-month follow-up was $388 (95% CI=-$205 to $1,049; Figure 3B).
The sample size for patients with STD data in the second follow-up year was 4,483 (data not shown). Compared to the cohort without insomnia medication, both the actual number of STD days (11.5 days vs 6.6 days, respectively; P < .01) and STD costs ($2,002 vs $1,135, respectively; P < .01) were higher in the insomnia medication cohort. After adjustment for baseline characteristics, patients taking insomnia medication had $806 more indirect costs for STD compared to the controls (95% CI=$330-$1,437; Figure 3B) in the second follow-up year.
DISCUSSION
This study found that the use of insomnia medications in newly treated patients with MDD is associated with increased health care resource utilization and higher total and MDD-related direct and indirect medical costs over a 2-year postindex time frame. The high cost differential was especially pronounced for MDD-related hospitalizations and ER visits. This finding could suggest that the use of insomnia medications results in greater depression or more likely indicates that patients with insomnia have more severe depression. This hypothesis is supported by a study of 40,791 men and women that found an association between more insomnia symptoms and greater incident treatment of depression.35 In addition, in the first follow-up year of this study, patients with comorbid MDD and insomnia had significantly higher work productivity loss costs, including STD costs. Likewise, over both years in this study, patients taking insomnia medications were more likely than patients with MDD alone to require STD leave and had significantly higher associated costs.
Insomnia has been found to be related to higher medical costs and impaired work performance, both independently12,18 and in conjunction with depression.16 Studies conducted to compare work-related disability rates among patients with insomnia and depression found that, compared with depression, insomnia was a potent driver of disability contributing to retirement, particularly for younger patients.12,16 One potential explanation for the relationship between insomnia and increased cost in MDD is that insomnia is an independent driver of cost in MDD. However, most costs associated with insomnia alone are indirect costs rather than costs related to health care utilization.18,26,27 A more likely hypothesis for the high direct and indirect costs associated with insomnia in patients with MDD is that insomnia is a marker for treatment-resistant depression. Sleep disturbances are extremely common in patients with MDD and are a frequent residual symptom in treatment-resistant depression and/or patients with partial response to antidepressant treatment.7,14,36 Furthermore, in a study of 74,977 people, sleeping problems were seen as a risk factor for suicide and this association was thought to be due to the occurrence of both sleeping problems and mixed anxiety or depression.37 Future research using clinical data may supplement our findings by incorporating elements such as severity of depression and insomnia, symptom patterns, and lifestyle factors that may contribute to the outcomes we observed in this study.
Previous research has shown that in patients with MDD, suboptimal response may be associated with worse increased health resource utilization and costs. Knoth et al6 studied patients with depression and reported that partial response and nonresponse to treatment were predictive of ER department utilization and hospitalization. In a claims database study, treatment resistance among depressed patients was associated with 40% higher medical care costs.7 Greenberg et al21 stated similar results and found that patients with treatment-resistant depression were more likely than those with stable MDD to incur excess medical and work loss-related costs, as well as high rates of depression-associated comorbidities. Other studies have shown that treatment-resistant depression is expensive.7,18,38 Corey-Lisle et al38 demonstrated that patients who were most likely treatment resistant used almost twice as many medical services as did patients who were not likely to be treatment resistant and incurred significantly greater indirect costs (P < .0001).
This study has several limitations. A substantial baseline imbalance existed between the 2 patient groups (those treated with insomnia medication vs untreated) in terms of rates of concurrent comorbidity (CCI), ER visits, and total health care costs. These differences could potentially have accounted for postbaseline differences (as opposed to the presence or absence of insomnia medication). Multivariate regression was applied to adjust for baseline differences and preindex costs, but residual confounding due to unobserved factors may have contributed to the observed differences. The claims-based nature of this analysis prevented an assessment of other clinical factors such as specific depressive symptoms, lifestyle factors such as alcohol consumption, and the nature and severity of insomnia. Future studies using electronic health records would be informative and would provide additional insights into the role of insomnia in predicting treatment outcomes. In addition, our sample selection process did not use diagnostic codes to identify insomnia and may have underestimated the prevalence of insomnia in this patient population. Previous studies have found that the prevalence of insomnia in individuals with comorbid depression can range from 35% to 60%.12 In this study, the prevalence of medication-treated insomnia in patients with MDD was 11.8% because our interest was in the effects of sleep disturbances of sufficient magnitude to warrant prescription sleep medications. The relative small proportion of patients with paid time off data and STD data may limit the generalization of the study results.
CONCLUSIONS
Sleep disturbance is an integral part of MDD, and insomnia is a common complaint among depressed patients.8-11,19 Although the relationship between MDD and insomnia is well-documented,8-11,15 limited prior research exists on the effects of comorbid depression and insomnia on health-related expenditures and productivity loss. This study confirms the incremental effects of comorbid insomnia on the total burden of illness in depression and suggests that significant sleep disturbances among newly treated patients with MDD may be indicative of a more complex and costly disease state that may be less responsive to standard therapies. Further research on the nature of this relationship is warranted in order to guide effective treatment selection earlier in the course of depression.
Drug names: eszopiclone (Lunesta), flurazepam (Dalmane and others), pentobarbital (Nembutal), quazepam (Doral), secobarbital (Seconal), temazepam (Restoril and others), triazolam (Halcion and others), zaleplon (Sonata and others), zolpidem (Ambien, Edluar, and others).
Author affiliations: Department of Multiple Sclerosis, Neuroscience, Psychiatry and Health Economics and Outcomes Research (Drs Abouzaid and Kim); Department of CER and Communications (Ms Gabriel); and Outcomes Research Methods and Analytics (Drs Tian and Kahler), Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
Potential conflicts of interest: All authors are employees of Novartis Pharmaceuticals Corporation.
Funding/support: This research was funded internally by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
Previous presentation: Presented at the Academy of Managed Care Pharmacy (AMCP) Educational Conference; October 19-21, 2001; Atlanta, Georgia.
Acknowledgments: Editorial support for the preparation of this manuscript was provided by Michelle A. Adams, BSJ, MA, and Meredith Rogers, MS (Write All, Inc, Princeton, New Jersey), who received funding for services from Novartis.
Additional information: The MarketScan® databases are available from Thomson Reuters (Healthcare) Inc at http://marketscan.thomsonreuters.com/marketscanportal/.
Supplementary material: See accompanying pages for Appendix 1.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
2. Hasin DS, Goodwin RD, Stinson FS, et al. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097-1106. PubMed doi:10.1001/archpsyc.62.10.1097
3. Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465-1475. PubMed doi:10.4088/JCP.v64n1211
4. Stewart WF, Ricci JA, Chee E, et al. Cost of lost productive work time among US workers with depression. JAMA. 2003;289(23):3135-3144. PubMed doi:10.1001/jama.289.23.3135
5. Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. Washington, DC: American Psychiatric Association. 2010.
6. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196. PubMed
7. Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370-377. PubMed
8. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254-1269. PubMed doi:10.4088/JCP.v66n1008
9. Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(suppl 16):27-32. PubMed
10. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225. PubMed doi:10.4088/JCP.v60n0403
11. Nelson JC, Portera L, Leon AC. Residual symptoms in depressed patients after treatment with fluoxetine or reboxetine. J Clin Psychiatry. 2005;66(11):1409-1414. PubMed doi:10.4088/JCP.v66n1110
12. Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379-389. PubMed doi:10.1016/j.smrv.2010.01.003
13. AÄŸarg×¼n MY, Kara H, Solmaz M. Sleep disturbances and suicidal behavior in patients with major depression. J Clin Psychiatry. 1997;58(6):249-251. PubMed doi:10.4088/JCP.v58n0602
14. Spitzer RL, Kroenke K, Linzer M, et al. Health-related quality of life in primary care patients with mental disorders: results from the PRIME-MD 1000 study. JAMA. 1995;274(19):1511-1517. PubMed doi:10.1001/jama.1995.03530190025030
15. Léger D, Guilleminault C, Bader G, et al. Medical and socio-professional impact of insomnia. Sleep. 2002;25(6):625-629. PubMed
16. Overland S, Glozier N, Sivertsen B, et al. A comparison of insomnia and depression as predictors of disability pension: the HUNT study. Sleep. 2008;31(6):875-880. PubMed
17. Gartlehner G, Gaynes BN, Hansen RA, et al. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med. 2008;149(10):734-750. PubMed
18. National Institutes of Health. National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28(9):1049-1057. PubMed
19. Pollack M, Seal B, Joish VN, et al. Insomnia-related comorbidities and economic costs among a commercially insured population in the United States. Curr Med Res Opin. 2009;25(8):1901-1911. PubMed doi:10.1185/03007990903035505
20. Sarsour K, Kalsekar A, Swindle R, et al. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443-450. PubMed
21. Greenberg P, Corey-Lisle PK, Birnbaum H, et al. Economic implications of treatment-resistant depression among employees. Pharmacoeconomics. 2004;22(6):363-373. PubMed doi:10.2165/00019053-200422060-00003
22. Whooley MA, Kiefe CI, Chesney MA, et al; CARDIA Study. Depressive symptoms, unemployment, and loss of income: the CARDIA Study. Arch Intern Med. 2002;162(22):2614-2620. PubMed doi:10.1001/archinte.162.22.2614
23. Birnbaum HG, Ben-Hamadi R, Greenberg PE, et al. Determinants of direct cost differences among US employees with major depressive disorders using antidepressants. Pharmacoeconomics. 2009;27(6):507-517. PubMed doi:10.2165/00019053-200927060-00006
24. Birnbaum HG, Ben-Hamadi R, Kelley D, et al. Assessing the relationship between compliance with antidepressant therapy and employer costs among employees in the United States. J Occupat Environ Med. 2010;52(2):115-124. PubMed
25. US Food and Drug Administration. US Department of Health and Human Services. Drugs: Sleep Disorder (Sedative-Hypnotic) Drug Information. http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm101557.htm. Updated March 2, 2010. Accessed June 20, 2012.
26. Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263-273. PubMed
27. Asche CV, Joish VN, Camacho F, et al. The direct costs of untreated comorbid insomnia in a managed care population with major depressive disorder. Curr Med Res Opin. 2010;26(8):1843-1853. PubMed doi:10.1185/03007995.2010.488037
28. O’ Sullivan AK, Sullivan J, Higuchi K, et al. Health care utilization and costs for cystic fibrosis patients with pulmonary infections. Manag Care. 2011;20(2):37-44. PubMed
29. Asche CV, Singer ME, Jhaveri M, et al. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16(9):703-712. PubMed
30. US Bureau of Labor Statistics. Establishment Data: Historical Hours and Earnings. 2012. ftp://ftp.bls.gov/pub/suppl/empsit.ceseeb2.txt. Accessed July 25, 2012.
31. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed doi:10.1016/0895-4356(92)90133-8
32. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed doi:10.1016/0021-9681(87)90171-8
33. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2000. doi:10.1002/0471722146
34. Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23(3):525-542. PubMed doi:10.1016/j.jhealeco.2003.10.005
35. Salo P, Sivertsen B, Oksanen T, et al. Insomnia symptoms as a predictor of incident treatment for depression: prospective cohort study of 40,791 men and women. Sleep Med. 2012;13(3):278-284. PubMed doi:10.1016/j.sleep.2011.06.022
36. van Mill JG, Hoogendijk WJ, Vogelzangs N, et al. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry. 2010;71(3):239-246. PubMed doi:10.4088/JCP.09m05218gry
37. Bj׸rngaard JH, Bjerkeset O, Romundstad P, et al. Sleeping problems and suicide in 75,000 Norwegian adults: a 20 year follow-up of the HUNT I study. Sleep. 2011;34(9):1155-1159. PubMed
38. Corey-Lisle PK, Birnbaum HG, Greenberg PE, et al. Identification of a claims data “signature” and economic consequences for treatment-resistant depression. J Clin Psychiatry. 2002;63(8):717-726. PubMed doi:10.4088/JCP.v63n0810