Beneficial Effects of the Sigma-1 Agonist Fluvoxamine for Tardive Dyskinesia in Patients With Postpsychotic Depressive Disorder of Schizophrenia: Report of 5 Cases

ABSTRACT
Objective: Tardive dyskinesia (TD) is characterized by involuntary, repetitive, purposeless movements that can affect different parts of the body. Tardive dyskinesia is a well-known side effect of conventional antipsychotics and commonly occurs after several years of treatment. The effective treatment of TD is unclear. Recently, the sigma-1 receptor agonist fluvoxamine was reported to be beneficial for hyperkinetic movement disorders.
Method: We report 5 cases with postpsychotic depressive disorder of schizophrenia and TD. All patients were given fluvoxamine 100 mg/d, and after the second week the dosage of fluvoxamine was increased to 200 mg/d. At the fourth week, patients were assessed in terms of TD and postpsychotic depressive disorder of schizophrenia.
Results: Fluvoxamine was found to be beneficial for TD and postpsychotic depressive disorder of schizophrenia in all patients by the fourth week.
Conclusions: Recently, the sigma-1 receptor agonist fluvoxamine has been considered beneficial for various neuropsychiatric disorders. However, data about the effects of fluvoxamine on hyperkinetic movement disorders are limited. In this report, we attempted to demonstrate the beneficial effects of fluvoxamine on TD, and we suggest that the mechanism of action might be due to sigma-1 agonism. Further detailed, double-blind studies should clarify the potential use of fluvoxamine in the treatment of hyperkinetic movement disorders.
Prim Care Companion CNS Disord 2012;14(6):doi:10.4088/PCC.12br01401
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: April 24, 2012; accepted May 29, 2012.
Published online: November 8, 2012.
Corresponding author: Yakup Albayrak, MD, Kırklareli Devlet Hastanesi, Psikiyatri Klinigi, Kırklareli, Turkey ([email protected]).
Tardive dyskinesia (TD) is characterized by involuntary, repetitive, purposeless movements that can affect different parts of the body.1,2 It is a well-recognized complication of conventional antipsychotics and usually occurs after several years of treatment. Sometimes antidepressants or calcium channel blockers may be responsible.3 Classically, the tongue, face, and neck muscles are involved, but the extremity muscles and the muscles controlling body posture and breathing can also be affected.4 The effective treatment of TD is still unclear; a number of medications have been tried with varying degrees of success.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that is approved for psychiatric disorders such as major depressive episodes by the European Medicines Agency and obsessive-compulsive disorder by the US Food and Drug Administration. Beside inhibition of serotonin reuptake, fluvoxamine is also a potent agonist of endoplasmic reticulum (ER) protein sigma-1 receptors, which play a role in the pathophysiology of a number of psychiatric and neurodegenerative disorders.5 It has been reported that fluvoxamine may be beneficial for neuropsychiatric disorders via sigma-1 receptor agonism.5 Here, we report 5 cases in which fluvoxamine was beneficial for both postpsychotic depressive disorder and TD in patients with schizophrenia.
CASE REPORTS
Case 1
Ms A, a 53-year-old woman, had been diagnosed with schizophrenia for 30 years. She had been treated with various conventional antipsychotics such as haloperidol, zuclopenthixol, and chlorpromazine for almost 25 years. For the last 2 years, she has been treated with quetiapine 600 mg/d. The results of brain magnetic resonance imaging (MRI), electroencephalography (EEG), blood chemistry, a complete blood count, and thyroid function tests were all normal. On psychiatric and physical examination, depressed mood, anhedonia, insomnia, poor appetite, fatigue, difficulty concentrating, distressing oral dyskinesia, and choreic movements on bilateral fingers of the upper limbs were noted. Ms A was diagnosed with schizophrenia, residual type; postpsychotic depressive disorder of schizophrenia; and medication-induced movement disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).6
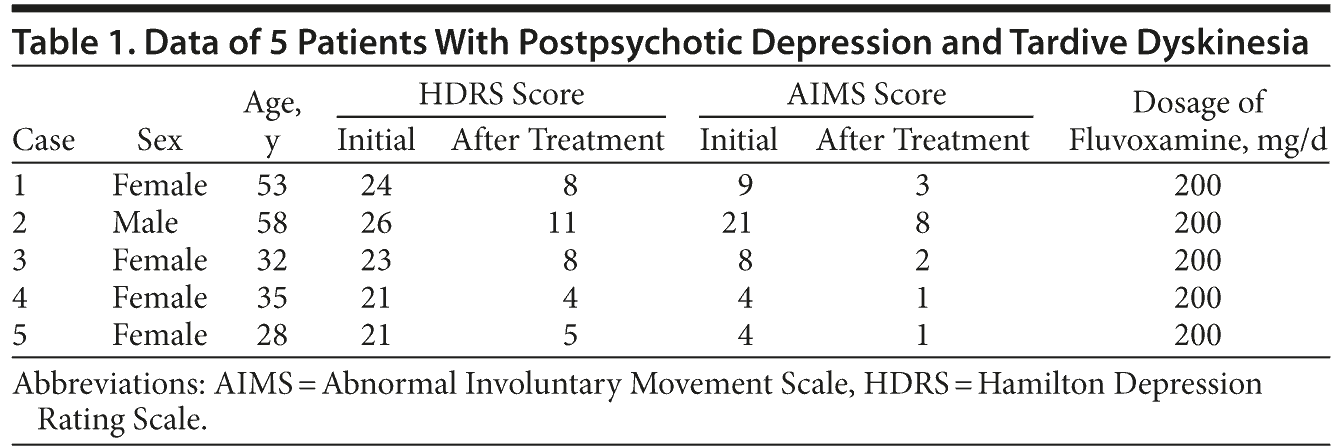
Ms A scored 24 on the Hamilton Depression Rating Scale (HDRS)7 and 9 on the Abnormal Involuntary Movement Scale (AIMS).8 Fluvoxamine 100 mg/d was added to her treatment, and the dosage of fluvoxamine was increased to 200 mg/d after 2 weeks. At the fourth week, the dyskinesia had improved gradually, and her symptoms of postpsychotic depressive disorder of schizophrenia were observed to be remitting based on scores of 8 on the HDRS7 and 3 on the AIMS8 (Table 1).

- There is still no effective treatment of tardive dyskinesia (TD).
- Fluvoxamine is a potent sigma-1 receptor agonist that is suggested to have various effects on neuropsychiatric disorders.
- Fluvoxamine might be an effective treatment for TD with fewer side effects than tetrabenazine.
Case 2
Mr B, a 58-year-old man, had been diagnosed with schizophrenia for 35 years. He had been treated with various conventional antipsychotics such as haloperidol decanoate, flupenthixol, and zuclopenthixol decanoate for almost 28 years. He had been treated with olanzapine 15 mg/d, biperiden 6 mg/d, and propranolol 60 mg/d for the last 5 years. The results of a brain MRI, EEG, blood chemistry, a complete blood count, and thyroid function tests were all normal. On psychiatric and physical examination, depressed mood, anhedonia, delusions of persecutions, fatigue, insomnia, and dyskinesias on lips, tongue and both upper limbs and dystonia on neck and shoulder were noted. Mr B was diagnosed with schizophrenia, paranoid type; postpsychotic depressive disorder of schizophrenia; and medication-induced movement disorder according to DSM-IV-TR.6
Mr B scored 26 on the HDRS7 and 21 on the AIMS.8 He was hospitalized, and fluvoxamine 100 mg/d was added to his treatment; however, biperiden 6 mg/d and propranolol 60 mg/d were stopped. The dosage of fluvoxamine was increased to 200 mg/d after 2 weeks. At the fourth week, the dyskinesia had improved gradually, and symptoms of postpsychotic depressive disorder of schizophrenia were observed to be remitting based on scores of 11 on the HDRS7 and 8 on the AIMS8 (Table 1); however, delusions of persecution were still persisting.
Case 3
Ms C, a 32-year-old woman, had been diagnosed with schizophrenia for 4 years. She had been treated with zuclopenthixol 30 mg/d for 3 years. She had been treated with risperidone 3 mg/d for the previous year. The results of a brain MRI, EEG, blood chemistry, a complete blood count, and thyroid function tests were all normal. On psychiatric and physical examination, depressed mood, anhedonia, insomnia, suicidal thoughts, difficulty concentrating, and dyskinesia on the tongue and choreic movements on bilateral fingers of upper limbs were noted. Ms C was diagnosed with schizophrenia, residual type; postpsychotic depressive disorder of schizophrenia; and medication-induced movement disorder according to DSM-IV-TR.6
Ms C scored 23 on the HDRS7 and 8 on the AIMS.8 Fluvoxamine 100 mg/d was added to her treatment, and the dosage of fluvoxamine was increased to 200 mg/d after 2 weeks. At the fourth week, her symptoms of postpsychotic depressive disorder of schizophrenia and TD were observed to be remitting based on scores of 8 on the HDRS7 and 2 on the AIMS8 (Table 1).
Case 4
Ms D was a 35-year-old woman who had been diagnosed with schizophrenia for 10 years. She had been treated with haloperidol 20 mg/d and biperiden 6 mg/d for 7 years. She had been treated with olanzapine 20 mg/d and biperiden 6 mg/d for the last 2 years. The results of a brain MRI, EEG, blood chemistry, a complete blood count, and thyroid function tests were all normal. On psychiatric and physical examination, depressed mood, anhedonia, insomnia, decreased appetite, difficulty concentrating, and choreic movements on bilateral fingers of upper limbs were noted. Ms D was diagnosed with schizophrenia, residual type; postpsychotic depressive disorder of schizophrenia; and medication-induced movement disorder according to the DSM-IV-TR.6
Ms D scored 21 on the HDRS7 and 4 on the AIMS.8 Fluvoxamine 100 mg/d was added to her treatment, and the dosage of fluvoxamine was increased to 200 mg/d after 2 weeks. At the fourth week, the dyskinesia had improved gradually, and her symptoms of postpsychotic depressive disorder of schizophrenia were observed to be remitting based on scores of 4 on the HDRS7 and 1 on the AIMS8 (Table 1).
Case 5
Ms E, a 28-year-old woman, had been diagnosed with schizophrenia for 4 years. She had been treated with pimozide 4 mg/d and biperiden 4 mg/d for 2 years. She had been treated with risperidone 5 mg/d and biperiden 2 mg/d for the last 2 years. The results of a brain MRI, EEG, blood chemistry, a complete blood count, and thyroid function tests were all normal. On psychiatric and physical examination, anhedonia, insomnia, decreased appetite, delusions of reference, and distressing oral dyskinesia were noted. Ms E was diagnosed with schizophrenia, paranoid type; postpsychotic depressive disorder of schizophrenia; and medication-induced movement disorder according to the DSM-IV-TR.6
Ms E scored 21 on the HDRS7 and 4 on the AIMS.8 Fluvoxamine 100 mg/d was added to her treatment, and the dosage of fluvoxamine was increased to 200 mg/d after 2 weeks. At the fourth week, the dyskinesia had improved gradually, and her symptoms of postpsychotic depressive disorder of schizophrenia were observed to be remitting based on scores of 5 on the HDRS7 and 1 on the AIMS8 (Table 1); however, delusions of persecution were still persisting.
DISCUSSION
Depression is a commonly occurring symptom in schizophrenia. There are a number of important differential diagnoses of depressive symptoms in schizophrenia. Differential diagnoses include schizoaffective disorder, organic conditions, and the “negative” symptoms of schizophrenia. The negative features of schizophrenia have many clinical similarities to the syndrome of depression. Lack of energy, anhedonia, and social withdrawal might cause particular problems when attempting to differentiate between the 2 syndromes.9 Observed sadness is a strong indicator of depression in schizophrenia. Prominent subjectively low mood, suggesting depression, and prominent blunting of affect, suggesting negative symptoms, are the 2 features that are most helpful in differentiating the 2 syndromes.10 Because not all patients in our study had prominent blunting of affect and prominent depressed mood and anhedonia existed in all patients, we considered the diagnosis of postpsychotic depressive disorder of schizophrenia according to the DSM-IV-TR.6 Clinical trials of SSRIs have been in favor of an effect on depressive symptoms in schizophrenia.9 Consistent with the literature, all of our patients benefited from fluvoxamine in terms of postpsychotic depressive disorder of schizophrenia.
The pathophysiology of TD is complex and remains unclear.11 The most common theory on the pathophysiology of TD supposes a supersensitivity of dopamine receptors from prolonged receptor blockade or up-regulation of these receptors.12 Other theories include striatal disinhibition of the thalamocortical pathway from an imbalance of the D1 and D2 receptors caused by the chronic use of dopamine blocking agents. Additionally, neurodegeneration secondary to lipid peroxidation or excitotoxic mechanisms may contribute to TD.12 Because of the relatively poor understanding of the pathophysiology, no effective treatment for TD is currently available. The best approach to management remains prevention, including restricting the use of antipsychotic medications to established indications and the use of alternative treatments when possible. When neuroleptic medications are needed, they should be prescribed in the smallest therapeutic doses for the shortest possible time.13 There is still no effective treatment for TD, although tetrabenazine ameliorates TD most effectively. Vitamin B6, vitamin E, donepezil, levetiracetam, and botulinum toxin are also other treatment options. In extreme cases, surgical interventions and deep brain stimulation have shown promising results, but further studies are needed.14
Fluvoxamine is shown to be a potent sigma-1 receptor agonist.5 A positron emission tomography study using a selective sigma-1 receptor agonist, [11C]SA4503, showed that fluvoxamine bound to sigma-1 receptors in the living human brain at therapeutic doses, suggesting that sigma-1 receptors play a role in the mechanism of action of fluvoxamine.15 There is also conflicting evidence that sigma-1 receptor agonists increase and reduce dopamine levels in the striatum.16 The sigma-1 receptors are concentrated in specific areas of the limbic system and brainstem motor structures of the human brain.17 The density of sigma-1 receptors is moderate to high in the caudate, putamen, septum, nucleus accumbens, and amygdala in rats18 and humans.16 Currently, there are some case reports showing that fluvoxamine was effective in the hyperkinetic movement disorders. Furuse and Hashimoto reported that fluvoxamine was effective in the blonanserin-induced19 and aripiprazole-induced20 akathisia in patients with schizophrenia. Furthermore, fluvoxamine was reported to be effective for treating both hemiballism and depression in a patient with depressive disorder, and it was suggested that fluvoxamine might act as reducer of dopamine in striatal area.21 Recently, we reported that fluvoxamine was effective in the treatment of duloxetine-induced TD.22 Precise mechanisms underlying the beneficial effects of fluvoxamine for TD are currently unclear, but the chaperone activity of sigma-1 receptors on ER might play a role in its mechanism.5,23,24 Sigma-1 receptor agonists, including fluvoxamine, might modulate dopaminergic neurotransmission in the limbic area via chaperone activity because sigma-1 receptors are known to modulate a number of neurotransmitter systems.25
Accumulating evidence suggests the role of ER stress in the pathophysiology of neurodegenerative disorders such as TD.26,27 The major function of molecular chaperones at the ER is to lead proper folding of newly synthesized proteins. These actions of molecular chaperones for ER stress are important for cells to prevent accumulation of toxic protein aggregates, thus facilitating cellular survival under cellular stress.28 The mechanism regulating chaperone activity of the sigma-1 receptors involves the physical protein-protein interaction between sigma-1 receptors and another ER chaperone, immunoglobulin heavy chain-binding protein BiP/GRP78.23 When the sigma-1 receptors form a conjugate with BiP/GRP78, the chaperone activity is minimized. In contrast, the sigma-1 receptors dissociated from BiP/GRP78 exert the maximum chaperone activity. Importantly, several synthetic compounds (eg, fluvoxamine) possessing the agonist property of the sigma-1 receptors promote the dissociation of the sigma-1 receptors from BiP; thus sigma-1 receptor agonists gain the chaperone activity of the sigma-1 receptors.24,25 Considering the neurodegenerative and excitotoxic theories of TD, the sigma-1 receptor agonist fluvoxamine might produce therapeutic effects via chaperone activity on ER.
CONCLUSION
This report describes the beneficial effects of sigma-1 receptor agonist fluvoxamine for TD in patients with schizophrenia. Therefore, the beneficial effects of fluvoxamine on hyperkinetic movement disorders would be useful as an alternative treatment without serious and distressing side effects. Nonetheless, further detailed, double-blind studies should clarify the potential use of fluvoxamine in the treatment of hyperkinetic movement disorders.
Drug names: aripiprazole (Abilify), biperiden (Akineton), donepezil (Aricept and others), duloxetine (Cymbalta), fluvoxamine (Luvox and others), haloperidol (Haldol and others), levetiracetam (Keppra and others), olanzapine (Zyprexa), pimozide (Orap), propranolol (Inderal, InnoPran, and others), quetiapine (Seroquel), risperidone (Risperdal and others), tetrabenazine (Xenazine).
Author affiliations: Department of Psychiatry, Kırklareli State Hospital, Kırklareli, Turkey (Dr Albayrak) and Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan (Dr Hashimoto).
Potential conflicts of interest: None reported.
Funding/support: None reported.
REFERENCES
1. Jeste DV, Rockwell E, Harris MJ, et al. Conventional vs newer antipsychotics in elderly patients. Am J Geriatr Psychiatry. 1999;7(1):70-76. PubMed
2. Laporta M, Archambault D, Ross-Chouinard A, et al. Articulatory impairment associated with tardive dyskinesia. J Nerv Ment Dis. 1990;178(10):660-662. PubMed doi:10.1097/00005053-199010000-00008
3. Jankovic J. Tardive syndromes and other drug-induced movement disorders. Clin Neuropharmacol. 1995;18(3):197-214. PubMed doi:10.1097/00002826-199506000-00001
4. Schwartz M, Silver H, Tal I, et al. Tardive dyskinesia in northern Israel: preliminary study. Eur Neurol. 1993;33(3):264-266. PubMed doi:10.1159/000116951
5. Hashimoto K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. Cent Nerv Syst Agents Med Chem. 2009;9(3):197-204. PubMed
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington DC: American Psychiatric Association. 2000.
7. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
8. Guy W. Abnormal Involuntary Movement Scale (AIMS). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept of Health, Education and Welfare; 1976:534-537.
9. Mulholland C, Cooper S. The symptoms of depression in schizophrenia and its management. Adv Psychiatr. 2000;6(3):169-177. doi:10.1192/apt.6.3.169
10. Siris SG. Depressive symptoms in the course of schizophrenia. In: DeLisi LE, ed. Depression in Schizophrenia. Washington, DC: American Psychiatric Press; 1990:3-23.
11. Casey DE. Tardive dyskinesia: pathophysiology and animal models. J Clin Psychiatry. 2000;61(suppl 4):5-9. doi:10.1007/s002130000670 PubMed
12. Lavalaye J, Sarlet A, Booij J, et al. Dopamine transporter density in patients with tardive dyskinesia: a single photon emission computed tomography study. Psychopharmacology (Berl). 2001;155(1):107-109. doi:10.1007/s002130000670 PubMed
13. Brasic JR, Bronson B. Tardive dyskinesia. medscape.com/article/1151826-overview. Accessed June 18, 2012.
14. Soares KV, McGrath JJ. The treatment of tardive dyskinesia—a systematic review and meta-analysis. Schizophr Res. 1999;39(1):1-16, discussion 17-18. PubMed doi:10.1016/S0920-9964(99)00021-3
15. Kenney C, Hunter C, Davidson A, et al. Metoclopramide, an increasingly recognized cause of tardive dyskinesia. J Clin Pharmacol. 2008;48(3):379-384. PubMed doi:10.1177/0091270007312258
16. Ishikawa M, Ishiwata K, Ishii K, et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C]SA4503. Biol Psychiatry. 2007;62(8):878-883. PubMed doi:10.1016/j.biopsych.2007.04.001
17. Gudelsky GA. Effects of sigma receptor ligands on the extracellular concentration of dopamine in the striatum and prefrontal cortex of the rat. Eur J Pharmacol. 1995;286(3):223-228. PubMed doi:10.1016/0014-2999(95)00415-8
18. Alonso G, Phan V, Guillemain I, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97(1):155-170. PubMed doi:10.1016/S0306-4522(00)00014-2
19. Furuse T, Hashimoto K. Fluvoxamine for blonanserin-associated akathisia in patients with schizophrenia: report of five cases. Ann Gen Psychiatry. 2010;9(1):17. PubMed doi:10.1186/1744-859X-9-17
20. Furuse T, Hashimoto K. Fluvoxamine for aripiprazole-associated akathisia in patients with schizophrenia: a potential role of sigma-1 receptors. Ann Gen Psychiatry. 2010;9(1):11. PubMed doi:10.1186/1744-859X-9-11
21. Cayköyl×¼ A, Albayrak Y, UÄŸurlu GK, et al. Beneficial effects of fluvoxamine for hemiballism in a patient with depressive disorder: a case report. Acta Neurol Belg. 2011;111(1):62-65. PubMed
22. Albayrak Y, Ekinci O. Duloxetine associated tardive dyskinesia resolved by fluvoxamine: case report. J Clin Psychopharmacol. 2012. In press.
23. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74(1):739-789. PubMed doi:10.1146/annurev.biochem.73.011303.074134
24. Hayashi T, Tsai SY, Mori T, et al. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2011;15(5):557-577. PubMed
25. Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets. 2008;12(1):45-58. PubMed doi:10.1517/14728222.12.1.45
26. Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12(30):3857-3876. PubMed doi:10.2174/138161206778559614
27. Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385-392. PubMed doi:10.1038/sj.cdd.4401778
28. Doyle KM, Kennedy D, Gorman AM, et al. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15(10):2025-2039. PubMed doi:10.1111/j.1582-4934.2011.01374.x