Sublingual Buprenorphine and Dental Problems: A Case Series
To the Editor: Sublingual buprenorphine, a semisynthetic opioid with partial agonist activity at the mu receptor, has become an important treatment option for opioid dependence.1 While dental problems are frequently reported in drug users,2-5 we previously described a patient who experienced a significant decline in dental health following initiation of treatment with buprenorphine.6 To better characterize this phenomena, we sought to describe patients on buprenorphine maintenance treatment reporting worsening dental health after treatment initiation.
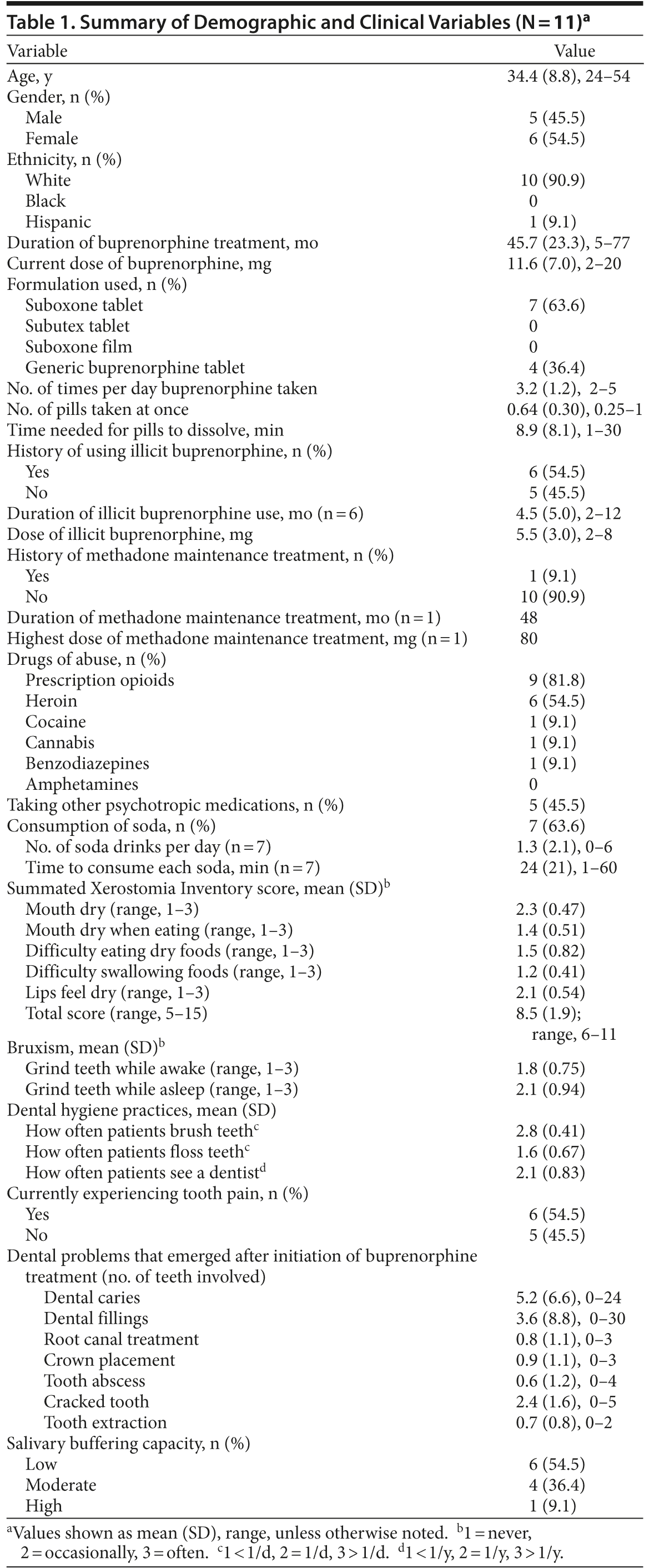
Case series. The Partners Human Research Committee approved this study, which was conducted in Boston, Massachusetts, between May and November 2012. This case series included only those patients with opioid dependence in treatment at Brigham and Women’s Hospital and who were reporting worsening dental health after initiation of buprenorphine. Eleven patients provided informed consent, and their clinical and dental health were reviewed. The results are summarized in Table 1.
Mean patient age was 34.4 years (SD = 8.8); patients were predominantly white (91%) and had been taking buprenorphine for a mean of 45.7 months (SD = 23.3; range, 5-77) at a mean dose of 11.6 mg/d (SD = 7.0; range, 2-20 mg). Patients were mostly (63.6%) prescribed buprenorphine/naloxone combination tablets and took the medication a mean of 3.2 times a day (SD = 1.2; range, 2-5 times), taking a mean of 8.9 minutes (SD = 8.1; range, 1-30 min) to dissolve each tablet completely. The mean Summated Xerostomia Inventory7 score was 8.5 (SD = 1.9; range, 6-11).
Since initiating buprenorphine treatment, the subjects reported a mean of 5.2 dental caries (SD = 6.6; range, 0-24 caries), 3.6 dental fillings (SD = 8.8; range, 0-30 fillings), 2.4 cracked teeth (SD = 1.6; range, 0-5 teeth), 0.9 crown placements (SD = 1.1; range, 0-3 placements), 0.8 root canal treatments (SD = 1.1; range, 0-3 treatments), and 0.7 tooth extractions (SD = 0.8; range, 0-2 extractions). At the time of the assessment, the majority of subjects (54.5%) reported having toothache pain.
Salivary buffering capacity,8 measured using Dentobuff test strips (Orion Diagnostica, Espoo, Finland), was noted to be low, moderate, and high in 54.5%, 36.4%, and 9.1% of the patients, respectively.
Consistent with prior reports, patients in this case series reported a wide variety of dental problems requiring intervention.2-5 The majority of patients also reported experiencing toothache pain at the time of assessment. Indeed, in a study of 508 opioid-dependent patients receiving methadone or buprenorphine treatment in Australia, 41.1% desired dental treatment, making it the most common health issue for which they desired treatment.9 Nevertheless, the reasons for the patients’ perceived decline in oral health remain unclear. The majority of patients reported cigarette smoking, bruxism, regular soda consumption, and moderate dental hygiene practices as well as the use of other psychotropic medications—factors known to negatively impact oral health.10-13 On the other hand, patients reported xerostomia scores comparable to those of healthy community samples.7
The majority (> 90%) of the patients had a low or moderate salivary buffering capacity. Less than 50% of the general population (aged 18-65 years) are thought to have a low or moderate buffering capacity.14 Because the saliva plays an important role in the prevention of dental caries, the low buffering capacity may be an additional contributor to dental problems in these patients.15
Dental caries result from the activity of acids on tooth structure. The most common factor leading to the production of acids in the mouth is the activity of Streptococcus mutans on fermentable sugars.16 Dental caries and erosion of teeth would therefore also occur when teeth are exposed to an environment that has low pH—such as in the context of bulimia nervosa, chronic sipping of sodas with low pH, and chronic use of medications that have a low pH.10,11,17 Buprenorphine used for addiction treatment is given sublingually, and the properties of this treatment may have a direct and adverse effect on dentition. First, buprenorphine/naloxone is acidic, with a pH of 3.4 when dissolved in water (T. Baxter, Reckitt Benckiser Pharmaceuticals Inc, written communication, May 2012). Second, due to the poor oral bioavailability of buprenorphine, patients are specifically instructed to keep the tablet and the accumulating saliva in their oral cavity to maximize absorption through the mucosal surfaces. Patients in this case series reported taking the medication approximately 3 times a day, each tablet taking about 9 minutes to completely dissolve. The prolonged contact between tooth surfaces with buprenorphine/naloxone, therefore, may be a contributing factor in the alteration of tooth surface microbial profile and/or the pH to promote dental caries, similar to what has been previously reported in patients who use methamphetamine.18,19
Nevertheless, this case series has significant limitations. We used a small convenience sample taken from our own practice, making these findings very tentative and preliminary. Subjects in our case series also endorsed many potentially confounding factors that could contribute to the development of caries. As such, without a control group we are unable to determine if these dental problems predate the use of buprenorphine or are related to other lifestyle or biologic factors. Finally, patient self-report of dental problems may be inaccurate, making it difficult to ascertain if indeed dental problems worsened following treatment with buprenorphine.
Future research should focus on recruiting larger sample sizes, confirming dental problems using dental examinations and radiographs, and using a control group and a prospective design to eliminate the confounding issue of preexisting dental problems. The potential association between dental problems and the salivary buffering capacity in patients maintained on buprenorphine treatment should also be examined further.
References
1. Fiellin DA, O’ Connor PG. Clinical practice: office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347(11):817-823. PubMed doi:10.1056/NEJMcp013579
2. D’ Amore MM, Cheng DM, Kressin NR, et al. Oral health of substance-dependent individuals: impact of specific substances. J Subst Abuse Treat. 2011;41(2):179-185. PubMed doi:10.1016/j.jsat.2011.02.005
3. Titsas A, Ferguson MM. Impact of opioid use on dentistry. Aust Dent J. 2002;47(2):94-98. PubMed doi:10.1111/j.1834-7819.2002.tb00311.x
4. Rees TD. Oral effects of drug abuse. Crit Rev Oral Biol Med. 1992;3(3):163-184. PubMed
5. Nathwani NS, Gallagher JE. Methadone: dental risks and preventive action. Dent Update. 2008;35(8):542-544, 547-548. PubMed
6. Suzuki J, Park EM. Buprenorphine/naloxone and dental caries: a case report. Am J Addict. 2012;21(5):494-495. PubMed doi:10.1111/j.1521-0391.2012.00254.x
7. Thomson WM, van der Putten G-J, de Baat C, et al. Shortening the Xerostomia Inventory. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(3):322-327. PubMed doi:10.1016/j.tripleo.2011.03.024
8. Söderling E, Le Bell Y, Alanen P, et al. Salivary buffer systems and the Dentobuff test. Proc Finn Dent Soc. 1985;81(5-6):284-287. PubMed
9. Winstock AR, Lea T, Sheridan J. Patients’ help-seeking behaviours for health problems associated with methadone and buprenorphine treatment. Drug Alcohol Rev. 2008;27(4):393-397. PubMed doi:10.1080/09595230802093745
10. Ciancio S. Medications: impact on dental diagnosis and treatment planning. Compend Contin Educ Dent. 2001;22(2, spec no):24-28. PubMed
11. Tahmassebi JF, Duggal MS, Malik-Kotru G, et al. Soft drinks and dental health: a review of the current literature. J Dent. 2006;34(1):2-11. PubMed doi:10.1016/j.jdent.2004.11.006
12. Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003;326(4):179-182. PubMed doi:10.1097/00000441-200310000-00005
13. Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults: results after 6 years. J Clin Periodontol. 1981;8(3):239-248. PubMed doi:10.1111/j.1600-051X.1981.tb02035.x
14. Dentobuff [package insert]. Espoo, Finland: Orion Diagnostica; 2008.
15. Leone CW, Oppenheim FG. Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. J Dent Educ. 2001;65(10):1054-1062. PubMed
16. Russell RR. Changing concepts in caries microbiology. Am J Dent. 2009;22(5):304-310. PubMed
17. Brown S, Bonifazi DZ. An overview of anorexia and bulimia nervosa, and the impact of eating disorders on the oral cavity. Compendium. 1993;14(12):1594, 1596-1602, 1604-1608, quiz 1608. PubMed
18. Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15(1):27-37. PubMed doi:10.1111/j.1601-0825.2008.01459.x
19. Klasser GD, Epstein J. Methamphetamine and its impact on dental care. J Can Dent Assoc. 2005;71(10):759-762. PubMed
Author affiliations: Brigham and Women’s Hospital (all authors); Harvard Medical School (all authors); and Harvard School of Dental Medicine (Dr Woo), Boston, Massachusetts.
Author contributions: The authors alone are responsible for the content and writing of this report.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Published online: October 3, 2013.
Prim Care Companion CNS Disord 2013;15(5):doi:10.4088/PCC.13l01533
© Copyright 2013 Physicians Postgraduate Press, Inc.