This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Prevalence of Substance Misuse in New Patients in an Outpatient Psychiatry Clinic Using a Prescription Monitoring Program

ABSTRACT
Objective: To investigate the value of a prescription monitoring program in identifying prescription drug misuse among patients presenting to a resident physician outpatient psychiatry clinic at an academic medical center.
Method: Participants were 314 new patients aged 18 years or older presenting to the clinic from October 2011 to June 2012. Resident physicians completed a data collection form for each participant using information from the patient interview and from the prescription monitoring program report. Prescription drug misuse was defined as having any 1 of the following 5 criteria in the prescription monitoring program report: (1) filled prescriptions for 2 or more controlled substances, (2) obtained prescriptions from 2 or more providers, (3) obtained early refills, (4) used 3 or more pharmacies, and (5) the prescription monitoring program report conflicted with the patient’s report.
Results: At least 1 indicator of prescription drug misuse was found in 41.7% of patients. Over 69% of the patients that the residents believed were misusing prescription drugs actually met 1 of the criteria for prescription drug misuse. The prescription monitoring program report changed the management only 2.2% of the time. Patients with prior benzodiazepine use (χ21 = 17.68, P < .001), prior opioid use (χ21 = 19.98, P < .001), a personality disorder (χ21 = 7.22, P < .001), and chronic pain (χ21 = 14.31, P < .001) had a higher percentage of prescription drug misuse compared to patients without these factors.
Conclusions: Using the prescription monitoring program to screen patients with prior benzodiazepine and opioid use, with a personality disorder, and/or with chronic pain may be useful in confirming the suspicion of prescription drug misuse identified at the initial evaluation.
Prim Care Companion CNS Disord 2014;16(1):doi:10.4088/PCC.13m01566
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: August 3, 2013; accepted October 1, 2013.
Published online: January 2, 2014.
Corresponding author: Elle M. Sowa, MD, University of Virginia, Department of Psychiatry and Neurobehavioral Sciences, 2955 Ivy Rd, Northridge Suite 210, Charlottesville, VA 22903 ([email protected]).
Prescription drug misuse is a growing problem. The rate of chronic nonmedical use of prescription pain relievers rose by nearly 75% between 2002-2003 and 2009-2010.1 People between the ages of 26 and 49 years and males experienced the greatest increase in prescription medicine abuse.1 The increase in prescription drug abuse has coincided with a 115% increase in the number of emergency department visits involving prescription drug misuse or abuse between 2004 and 2010.2 The increase in prescription drug abuse has also coincided with an increase in drug overdose mortality rates. The majority of overdose deaths in West Virginia in 2006 were associated with nonmedical use and diversion of prescription drugs, primarily opioid analgesics.3 Prescription monitoring programs have been implemented in response to these trends, although these programs are limited in that they only record prescription drugs obtained from prescribers. In the United States as of May 2012, every state or district except 3 had enacted legislation enabling a prescription monitoring program, and 43 states had an operational program.4,5 Currently, a significant gap exists between awareness of prescription monitoring program resources and informed utilization. In 1 state with a prescription monitoring program, 84% of physicians surveyed were aware of its existence, but less than 59% had ever used it.6
Regardless of the percentage of providers who use the prescription monitoring program, its implementation can affect the diversion of prescription drugs, trends in opioid abuse, and prescribing behavior. The indicators of doctor shopping decreased after the implementation of a prescription monitoring program for buprenorphine in a region in France.7 There was a smaller increase in poison center intentional opioid exposures and a smaller increase in opioid treatment admissions in states with a prescription monitoring program versus states without such a program.8 When emergency department physicians reviewed prescription monitoring program data before discharging patients, they altered opioid prescribing for 74 of 179 (41%) patients.9 However, prescription monitoring programs were not significantly associated with lower rates of drug overdose or opioid overdose mortality or lower rates of consumption of opioid drugs, indicating the need for more research on best practices.10,11 Several studies have noted an association between nonmedical use of opioids and mental health and pain comorbidities,12-15 including an association between personality disorder and prescription drug misuse.16,17 Psychiatric outpatient visits are also associated with a risk for prescription opioid abuse.18
This study examined the epidemiology of prescription drug misuse and the utility of the prescription monitoring program in identifying prescription drug misuse in the setting of a resident physicians’ outpatient psychiatry clinic at an academic medical center. Understanding this epidemiology will help guide the creation of effective screening tools and best-use policies for prescription monitoring program information.
METHOD
Participants
Eligible participants were 559 consecutive new patients aged ≥ 18 years presenting from October 2011 to June 2012 to a resident physician outpatient psychiatry clinic at an academic medical center. Patients who had been living in the state of Virginia for less than 3 months were excluded due to the potential for no available information in the prescription monitoring program. The prescription monitoring program lists the patient’s name, the pharmacy where the prescription was filled, the provider who wrote the prescription, and the date that the prescription was filled for all class II substances. Notation in the prescription monitoring program may take up to 2 weeks. All providers in the state may see the results for their patients. Patient permission is not required.

- Every new patient presenting to an outpatient psychiatry clinic need not be screened using the prescription monitoring program.
- Prior benzodiazepine use, prior opioid use, having a personality disorder, and having chronic pain were associated with a higher percentage of prescription drug misuse.
- It would be logical to use the prescription monitoring program to screen these selected patients and to confirm the suspicion of prescription drug misuse identified at the initial evaluation.
Participants with incomplete data collection forms (described below) were excluded. Deidentified information from 314 completed data collection forms (56% of eligible participants) was entered into a statistical software database for analysis. There were no statistically significant differences (P ≤ .01) in age, sex, or presenting complaint between the 314 participants with complete data and the 245 participants with incomplete data.
Procedures
Following the institutional review board-approved protocol, which did not require informed consent, the PGY-III resident physician completed a data collection form with information including demographics, substance use history, history of chronic pain, social history (disability, legal charges), psychiatric diagnoses (Axes I and II), whether the patient asked for a prescription drug by name, and whether the resident thought the patient was misusing prescription drugs. After the patient interview, the resident examined the prescription monitoring program report encompassing the year prior to the encounter and completed the remainder of the data collection form using the information contained in the report including the number of providers, controlled substances, and pharmacies reported; the presence of early refills (within 25 days of having been dispensed a controlled substance); whether there was more than 1 substance prescribed in a single drug class; whether the prescription monitoring program report matched the patient’s report; and whether the prescription monitoring program report changed the management of the patient.
Prescription drug misuse. Participants were judged as having prescription drug misuse if any 1 of the predetermined criteria were present in the prescription monitoring program report: (1) filled prescriptions for 2 or more controlled substances, (2) obtained prescriptions from 2 or more providers, (3) obtained early refills, (4) used 3 or more pharmacies, and (5) the prescription monitoring program report conflicted with the patient’s report.
History of substance use. Substance use history consisted of tobacco use, history of illicit substance abuse, history of alcohol abuse, prior detoxification/rehabilitation, prior benzodiazepine use, prior opioid use, and prior stimulant use.
Data Analysis
Risk criteria included demographic and substance use characteristics associated with prescription opioid abuse19: aged 18-34 years, male sex, and prior diagnostic history of substance abuse, depression, and posttraumatic stress disorder (PTSD). Given the relatively small number of participants, univariate χ2 analyses (including odds ratios [ORs], as an estimate of relative risk, with 95% CIs) and t tests, as appropriate, were used to investigate the relationship of each risk factor to prescription drug misuse (P ≤ .01 to minimize type 1 error). Further exploratory analyses investigated the association of other factors of interest to prescription drug misuse and the association of each risk factor to each of the 5 individual criteria for prescription drug misuse. Due to small cell sizes, several of these exploratory analyses have large confidence intervals, and others do not merit inclusion.
RESULTS
Patient Characteristics
Participants were 188 women and 126 men aged 18-70 years (mean age = 38.6 years). Nearly 18% of patients were receiving disability payments; 69 patients (22.0%) had children living in their home; and 48 (15.3%) had a history of criminal charges. Sixty-seven patients (21.3%) asked for a prescription drug by name.
History of Substance Use Associated With Prescription Drug Misuse
Of the participants, 109 (34.7%) had previously used some form of controlled prescription drug. The distinction between using prescription drugs obtained by prescription or through other means was not recorded. Sixty-nine participants (22.0%) had used benzodiazepines, 46 (14.6%) had used opioids, and 48 (15.3%) had used prescription stimulants. An appreciable minority had a history of substance abuse (45.5%), reporting at least 1 of the following: history of illicit substance abuse (31.2%), history of alcohol abuse (34.7%), and prior detoxification or rehabilitation (7.3%). Seventy-five (23.9%) participants were currently using or had a history of using tobacco. Approximately 7.3% of participants (23/314) had a DSM-IV Axis I substance-related disorder.20
At least 1 of the 5 indicators of prescription drug misuse was found in 41.7% of patients (15.0% met 1 criterion, 15.9% met 2 criteria, 7.0% met 3 criteria, 3.2% met 4 criteria, and 0.6% met all 5 criteria). Three substance use factors had statistically significant associations with prescription drug misuse: prior benzodiazepine use (χ21 = 17.68, P < .001), prior opioid use (χ21 = 19.98, P < .001), and prior benzodiazepine, opioid, and/or stimulant use (χ21 = 17.75, P < .001).
Diagnoses Associated With Prescription Drug Misuse
For DSM-IV Axis I diagnoses, 62.1% (195/314) of patients had a mooddisorder, 43.9% (138/314) had amajor depressive disorder, and 15.0% (47/314) had bipolar disorder. One-third (104/314) had an anxietydisorder, with 5.4% (17/314) of all patients having PTSD specifically. Attention-deficit/hyperactivity disorder was diagnosed in 9.6% (30/314) of participants. Over 85% (269/314) had no Axis II diagnosis or diagnosis was deferred; 0.3% (1/314) had a cluster A diagnosis, 8.9% (28/314) had a cluster B diagnosis, 1.0% (3/314) had a cluster C diagnosis, and 4.1% (13/314) had a diagnosis of personality disorder not otherwise specified.20 Additionally, 17.2% (54/314) of patients had chronic pain.
Three diagnoses had statistically significant associations with prescription drug misuse: anxiety disorder (χ21 = 7.67, P < .001), personality disorder (χ21 = 7.22, P < .001), and chronic pain (χ21 = 14.31, P < .001). Having a personality disorder or chronic pain was associated with a higher probability of prescription drug misuse, while having an anxiety disorder reduced such risk. Fewer patients with an anxiety disorder than without an anxiety disorder (30.8% vs 47.1%, respectively) met criteria for prescription drug misuse. More patients with a personality disorder than without a personality disorder (60.0% vs 38.7%, respectively) met criteria for prescription drug misuse. More patients with chronic pain than without chronic pain (46.3% vs 25.4%, respectively) met criteria for prescription drug misuse.
Resident Assessment
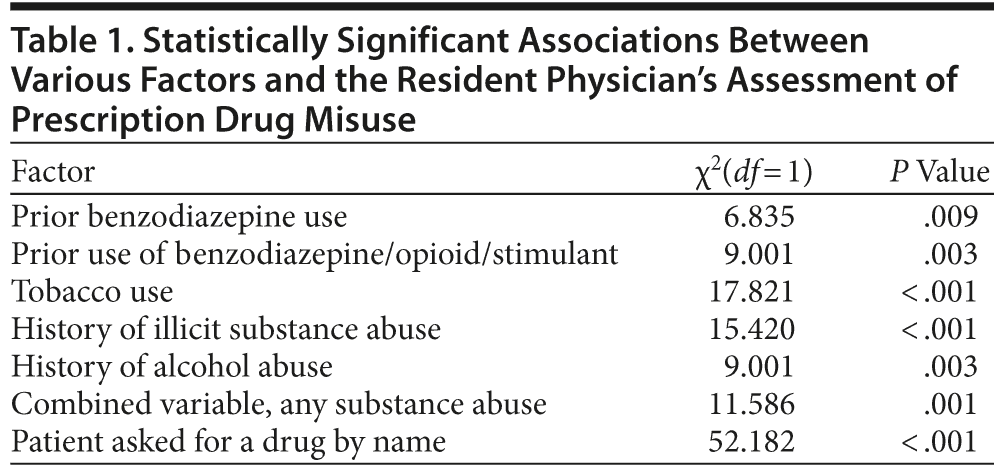
Prior to viewing the prescription monitoring program report, the resident predicted that 8.3% (26/314) of the patients were misusing prescription drugs. This assessment was associated with multiple factors (Table 1).
When the resident predicted that the patient was misusing prescription drugs, the prescription monitoring program report matched the patient’s report 57.7% (15/26) of the time compared to 93.4% (269/288) of the time when the resident believed that the patient was not misusing prescription drugs. The resident’s assessment was associated with prescription drug misuse (χ21 = 8.824; P = .003; RR = 3.485; 95% CI, 1.466-8.282), with 69.2% of the patients that the resident believed were misusing prescription drugs meeting 1 of the criteria for prescription drug misuse; 39% of those whom the resident believed were not misusing prescription drugs met 1 of the criteria.
The Prescription Monitoring Program Report
Overall, the prescription monitoring program report matched the patient’s report 90.4% (284/314) of the time. A substantial minority of patients (45.5%) were not filling prescriptions for any controlled substances according to the prescription monitoring program report. Of the 171 patients who were obtaining controlled substances, the mean number of health care providers was 2.15 (SD = 1.50; range, 1-9), the mean number of pharmacies used was 1.86 (SD = 1.42; range, 1-14), and the mean number of controlled substances was 1.98 (SD = 1.30; range, 1-8). The prescription monitoring program revealed that 46.8% had 1 provider, 19.9% had 2 providers, and 33.3% had 3 or more prescribing providers. Over 90% used 1 to 3 pharmacies, and 88.3% used 1 to 3 controlled substances. The number of providers was positively correlated with the number of controlled substances (R = 0.835, P < .001) and the number of pharmacies (R = 0.750, P < .001). The number of controlled substances was positively correlated with the number of pharmacies (R = 0.738, P < .001). Nearly 20% of patients were prescribed more than 1 controlled substance in the same class; only a small percentage (4.5%) obtained an early refill.
The prescription monitoring program report changed the planned management only 2.2% (7/314) of the time. Even when the prescription monitoring program report did not match the patient’s report, the prescription monitoring program did not typically change planned patient management (93.3%, 28/30).
Exploratory Analyses
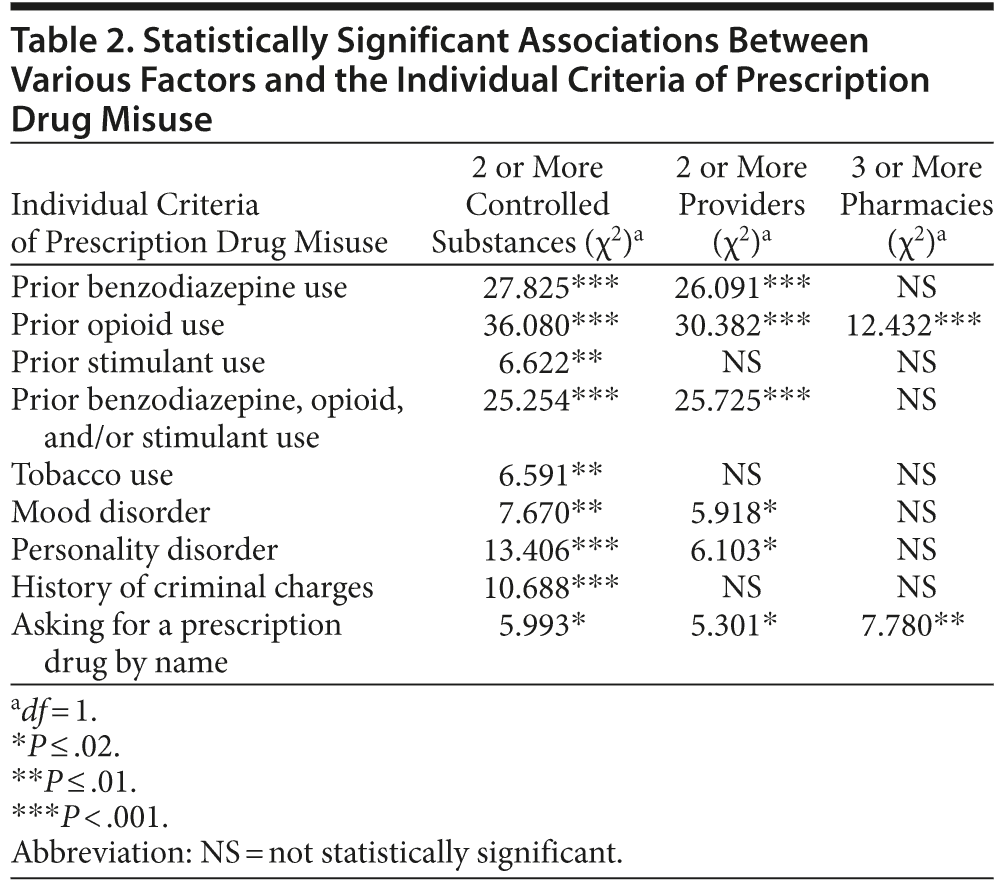
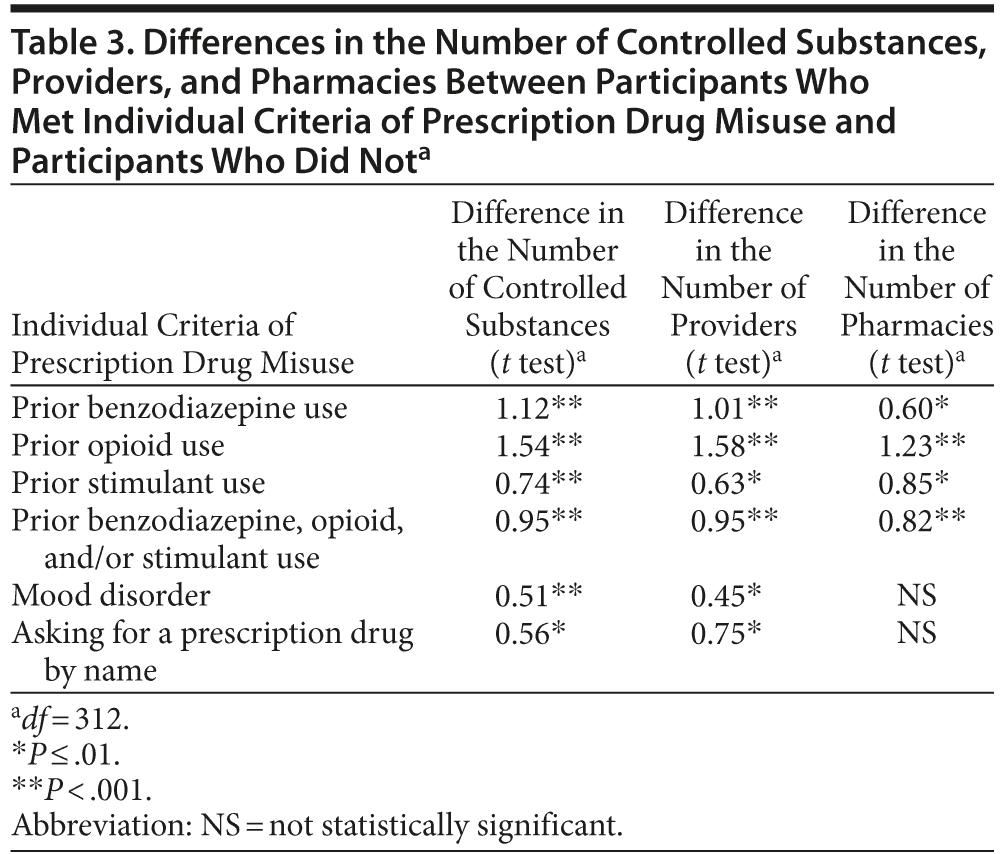
Multiple factors were associated with individual criteria of prescription drug misuse (Table 2). Table 3 shows the mean differences in the number of controlled substances, providers, and pharmacies between participants who met individual criteria and participants who did not.
Gender
Gender was not associated with prescription drug misuse (χ21 = 2.351, P = .125), prior benzodiazepine use (χ21 = 3.458, P = .063), prior opioid use (χ21 = 0.031, P = .860), or whether the prescription monitoring program report matched the patient’s report (χ21 = 0.637, P = .425). There was no difference in the number of controlled substances (t311.958 = 2.266, P = .024), number of providers (t310.642 = 1.890, P = .60), or number of pharmacies (t311.840 = 1.471, P = .142) between men and women.
Age
Differences by age were analyzed using the cut-point of age 35 years. Patients less than 35 years were significantly less likely to have prior stimulant use (χ21 = 8.241; P = .004; OR = 0.391; 95% CI, 0.203-0.754) and used fewer providers (t293.931 = −3.276, P < .001), controlled substances (t291.473 = −3.898, P < .001), and pharmacies (t312 = −2.856, P = .005). Patients aged ≥ 35 years were significantly more likely to use 2 or more controlled substances (χ21 = 9.361; P = .002; OR = 2.187; 95% CI, 1.318-3.629) and more likely to have early refills (χ21 = 7.344; P = .007; OR = 6.329; 95% CI, 1.393-28.763).
DISCUSSION
The rate of substance abuse (45.5%) among new patients presenting to an outpatient psychiatry clinic emphasizes the need for careful prescribing and monitoring of controlled prescription drugs, especially among patients with prior use of opioids and benzodiazepines. Tobacco use, a history of illicit substance abuse, a history of alcohol abuse, prior detoxification/rehabilitation, an Axis I diagnosis of a substance-related disorder, and a history of criminal charges may be less important factors when screening for prescription drug misuse in this population.
Suspicion should be increased when patients have diagnoses of personality disorder and/or chronic pain because a higher proportion of these patients met this study’s criteria for prescription drug misuse. Interestingly, a smaller proportion of patients with an anxiety disorder (including PTSD) showed prescription drug misuse compared to patients without an anxiety disorder perhaps suggesting their use of controlled substances may be appropriate and monitored. Suspicion should also be heightened when a patient asks for a prescription drug by name. Gender was not a significant risk factor, but age group was associated with several individual criteria of prescription drug misuse.
The resident’s prediction of prescription drug misuse was statistically associated with prior use of benzodiazepine/opioid/stimulant, tobacco use, history of illicit substance abuse, history of alcohol abuse, any substance use history, and whether the patient asked for a substance by name. Nearly 70% of the patients that the resident believed were misusing prescription drugs actually met 1 of the criteria for prescription drug misuse. However, almost 40% of the patients that the resident believed were not misusing prescription drugs met 1 of the criteria for prescription drug misuse. Additionally, when the resident predicted that the patient was misusing prescription drugs, the prescription monitoring program report matched the patient’s report 57.7% (15/26) of the time compared to 93.4% (269/288) of the time when the resident predicted appropriate use.
The results of the prescription monitoring program reports in this study were consistent with a study of Massachusetts prescription monitoring program records for schedule II opioids, which found that most individuals (87.5%) used 1 to 2 prescribers, used 1 to 2 pharmacies, and had no early refills.19 The likelihood of using multiple providers was associated with receiving prescriptions for multiple different controlled drugs, which replicated findings from another study.21 Use of multiple providers was also associated with use of multiple pharmacies. If there is suspicion that the patient is misusing, the provider should obtain a prescription monitoring program report.
Limitations
The major limitation of this study was the nonrandom sampling of new patients because data collection forms were not completed for every consecutive new patient, primarily related to time constraints in the busy resident physician clinic; noncompletion rates were similar across residents. The study also did not use confirmatory urine drug screening. Generalizability of results to private practice outpatient psychiatry clinics is limited, although this academic center is located in a small city with no other large psychiatric outpatient service. The patient population seen at this clinic therefore is more likely to be representative of patients seen in general outpatient psychiatric clinics in urban areas. Studies vary in the definition of prescription drug misuse. Others have used a greater number of providers, controlled substances, and pharmacies.3,22 This study did not include information on drug doses or prescription drugs obtained from sources other than prescribers.23,24 Prescription monitoring programs in general do not record illicit procurement of these substances.
Future Practice and Research
Future research should examine best practices for use of prescription monitoring programs to identify prescription drug misuse and examine interventions for the patients with prescription drug misuse (41.7% of all patients in this study). Diversion of prescription opioids may be reduced by prescriber education focusing on recognition of patient misuse and/or diversion.25 The US Food and Drug Administration is now requiring pharmaceutical companies that manufacture extended-release/long-acting opioid analgesics to provide education for prescribers via continuing education activities supported by pharmaceutical company educational grants.26 The best way to utilize prescription monitoring program data must be part of this educational process.
CONCLUSION
Every new patient presenting to an outpatient psychiatry clinic need not be screened using the prescription monitoring program. The prescription monitoring program report matched the patient’s report 90.4% of the time, and the prescription monitoring program report changed the management only 2.2% of the time. However, it would be logical to use the prescription monitoring program report to screen selected patients, such as those who reported previous use of some form of controlled prescription substance (benzodiazepines, opioids, and/or stimulants) and those with diagnoses of personality disorder and/or chronic pain.
Drug names: buprenorphine (Subutex, Suboxone, and others).
Author affiliations: Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville (Drs Sowa, Santa Cruz, Hidalgo, Lee, and Clayton); Department of Addiction Psychiatry, Oregon Health and Science University, Portland (Dr Fellers); Psychosomatic Medicine, Virginia Commonwealth University Health System, West Hospital, Richmond (Dr Raisinghani); Child Psychiatry, University of Connecticut, West Hartford (Dr Martinez); and Public Health Sciences, The National Social Norms Institute at the University of Virginia, Charlottesville (Dr Keller).
Potential conflicts of interest: Dr Keller is full-time salaried faculty of the University of Virginia and has been since 1991. The National Social Norms Institute (NSNI) was established at the University of Virginia with a gift from the Anheuser Busch companies and its charitable foundation in 2006. Since that time, NSNI has received additional funding from a variety of sources, including the Centers for Disease Control. There are no contractual constraints on publishing imposed by any funder. Dr Clayton, during the last 12 months (as of April 2013), has received grants from BioSante, Forest Research Institute, Palatin Technologies, Pfizer, Takeda, and Trimel; advisory board and consultant fees from Apricus Biosciences, Euthymics, Forest Research Institute, Lundbeck, Palatin Technologies, Pfizer, S1 Biopharmaceuticals, Sprout, Takeda Global Research and Development, and Trimel; royalties/copyright from Ballantine Books/Random House, Changes in Sexual Functioning Questionnaire, and Guilford Publications; and shares/restricted stock units from Euthymics and S1 Biopharmaceuticals. Drs Sowa, Fellers, Raisinghani, Santa Cruz, Hidalgo, Lee, and Martinez report no conflicts of interest related to the subject of this article.
Funding/support: None reported.
Previous presentations: Resident Research Day; Department of Psychiatry and Neurobehavioral Sciences; University of Virginia Health System; May 9, 2013; Charlottesville, Virginia; and the 166th Annual Meeting of the American Psychiatric Association; May 18-22, 2013; San Francisco, California.
Acknowledgments: The authors thank Michelle E. Zavage, MD, Shenandoah Community Health, Martinsburg, West Virginia; Justin B. Smith, MD, Department of Psychiatry, Massachusetts General Hospital, Belmont; and Renu J. Shah, MD, Forensic Psychiatry Fellow, Institute of Law, Psychiatry and Public Policy, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia Health System, Charlottesville. Drs Zavage, Smith, and Shah report no conflicts of interest related to the subject of this article.
REFERENCES
1. Jones CM. Frequency of prescription pain reliever nonmedical use: 2002-2003 and 2009-2010. Arch Intern Med. 2012;172(16):1265-1267. PubMed doi:10.1001/archinternmed.2012.2533
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. http://www.samhsa.gov/data/2k12/DAWN096/SR096EDHighlights2010.pdf Updated July 2, 2012. Accessed October 7, 2013.
3. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed doi:10.1001/jama.2008.802
4. National Alliance for Model State Drug Laws (NAMSDL). Status of state prescription monitoring programs. http://www.namsdl.org/library/2F9ADA8B-65BE-F4BB-A2961A3B323852DB/ Updated May 18, 2012. Accessed November 4, 2012.
5. National Alliance for Model State Drug Laws (NAMSDL) executive summary. Prescription drug monitoring programs: a brief overview. http://www.namsdl.org/library/2C1D3D84-1372-636C-DD7AA3FC63B30DB9/. Updated March 2011. Accessed November 14, 2012.
6. Feldman L, Williams KS, Coates J, et al. Awareness and utilization of a prescription monitoring program among physicians. J Pain Palliat Care Pharmacother. 2011;25(4):313-317. PubMed doi:10.3109/15360288.2011.606292
7. Pradel V, Frauger E, Thirion X, et al. Impact of a prescription monitoring program on doctor-shopping for high dosage buprenorphine. Pharmacoepidemiol Drug Saf. 2009;18(1):36-43. PubMed doi:10.1002/pds.1681
8. Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13(3):434-442. PubMed doi:10.1111/j.1526-4637.2012.01327.x
9. Baehren DF, Marco CA, Droz DE, et al. A statewide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56(1):19-23, e1-e3. PubMed doi:10.1016/j.annemergmed.2009.12.011
10. Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747-754. PubMed doi:10.1111/j.1526-4637.2011.01062.x
11. Brushwood DB. Maximizing the value of electronic prescription monitoring programs. J Law Med Ethics. 2003;31(1):41-54. doi:10.1111/j.1748-720X.2003.tb00058.x
12. Amari E, Rehm J, Goldner E, et al. Nonmedical prescription opioid use and mental health and pain comorbidities: a narrative review. Can J Psychiatry. 2011;56(8):495-502. PubMed
13. Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse, and dependence on prescription opioids among US adults: psychiatric, medical, and substance use correlates. Drug Alcohol Depend. 2008;94(1-3):38-47. PubMed doi:10.1016/j.drugalcdep.2007.09.018
14. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782. PubMed doi:10.1111/j.1360-0443.2010.03052.x
15. Sullivan MD, Edlund MJ, Zhang L, et al. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166(19):2087-2093. PubMed doi:10.1001/archinte.166.19.2087
16. Hall MT, Howard MO, McCabe SE. Prescription drug misuse among antisocial youths. J Stud Alcohol Drugs. 2010;71(6):917-924. PubMed
17. Sansone RA, Wiederman MW. The abuse of prescription medications: borderline personality patients in psychiatric versus non-psychiatric settings. Int J Psychiatry Med. 2009;39(2):147-154. PubMed doi:10.2190/PM.39.2.c
18. White AG, Birnbaum HG, Schiller M, et al. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15(12):897-906. PubMed
19. Katz N, Panas L, Kim M, et al. Usefulness of prescription monitoring programs for surveillance: analysis of schedule II opioid prescription data in Massachusetts, 1996-2006. Pharmacoepidemiol Drug Saf. 2010;19(2):115-123. PubMed doi:10.1002/pds.1878
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
21. Wilsey BL, Fishman SM, Gilson AM, et al. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112(1-2):99-106. PubMed doi:10.1016/j.drugalcdep.2010.05.007
22. Parente ST, Kim SS, Finch MD, et al. Identifying controlled substance patterns of utilization requiring evaluation using administrative claims data. Am J Manag Care. 2004;10(11 pt 1):783-790. PubMed
23. Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-44, HHS PublicationNo. (SMA) 12-4713. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
24. Inciardi JA, Surratt HL, Kurtz SP, et al. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8(2):171-183. PubMed doi:10.1111/j.1526-4637.2006.00255.x
25. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med. 2009;10(3):537-548. PubMed doi:10.1111/j.1526-4637.2009.00603.x
26. Food and Drug Administration. 2012. Introduction for the FDA blueprint for prescriber education for extended-release and long-acting opioid analgesics. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM277916.pdf. Accessed November 14, 2012.