Psychotic Disorders in Children and Adolescents: A Primer on Contemporary Evaluation and Management

LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital (MGH) sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Dr Stevens is physician-in-charge at Westpark (Dearborn), Henry Ford Health Systems, Dearborn, Michigan, and clinical assistant professor at Wayne State University, Detroit, Michigan. Dr Prince is director of child psychiatry at North Shore Medical Center in Salem, Massachusetts, and an instructor in psychiatry at Harvard Medical School, Boston, Massachusetts. Dr Prager is director of the Child Psychiatry Emergency Service at Massachusetts General Hospital, Boston, and assistant professor of psychiatry (child psychiatry) at Harvard Medical School, Boston, Massachusetts. Dr Stern is chief of the Avery D. Weisman Psychiatry Consultation Service at Massachusetts General Hospital, Boston, and the Ned H. Cassem professor of psychiatry in the field of psychosomatic medicine/consultation at Harvard Medical School, Boston, Massachusetts.
Dr Princehas received honoraria from the American Physician Institute and has provided expert legal reviews in malpractice cases. Dr Sternis an employee of the Academy of Psychosomatic Medicine, has served on the speaker’s board of Reed Elsevier, is a stock shareholder in WiFiMD (Tablet PC), and has received royalties from Mosby/Elsevier and McGraw Hill. Drs Stevens and Prager report no conflicts of interest related to the subject of this article.
Prim Care Companion CNS Disord 2014;16(2):doi:10.4088/PCC.13f01514
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: March 8, 2013; accepted November 26, 2013.
Published online: March 13, 2014.
Funding/support: None reported.
Corresponding author: Jonathan R. Stevens, MD, MPH, Henry Ford Health Systems, 5111 Auto Club Rd, Ste 112, Dearborn, MI 48126 ([email protected]).
Over the past decade, psychiatric illnesses and, in particular, psychotic disorders in children and adolescents have been recognized more frequently. This increase in recognition, combined with changing trends in the provision of mental health care, has led primary care physicians and pediatricians to increasingly serve as the principal treaters of psychiatrically ill youth and their families. Primary care providers and behavioral health services (eg, in colocated care, embedded care practices, patient-centered homes, and consultation) form the backbone of contemporary psychosis evaluation and management. It is unclear whether psychotic disorders in younger individuals are actually becoming more prevalent, or whether educational efforts directed at enhanced screening and treatment have improved case detection. Efficacious and safe interventions should be employed to maximize beneficial effects and to minimize medication-related complications, such as weight gain, diabetes, and heart disease.
The question for today’s health care providers is not whether psychotic symptoms will be encountered in daily practice, but how to confidently and effectively diagnose and treat the conditions in which they manifest. In order to frame this discussion, several areas of inquiry will be explored: “Have you ever been unsure about whether a young person in your practice was psychotic or simply imaginative? Have you wondered how to evaluate such a patient (eg, with laboratory testing or with neuropsychological testing) and what resources to recommend? Have you been concerned about using antipsychotics in young people? Does the potential for adverse side effects of antipsychotics in the short-term or long-term concern you?” For practitioners faced with these concerns, the following discussion should prove useful.
WHAT IS PSYCHOSIS AND HOW COMMON IS IT IN YOUNG PEOPLE?
Narrowly defined, psychosis denotes the presence of either delusions (false implausible beliefs) or hallucinations (false perceptions involving any sensory modality). Broader definitions of psychosis include manifestations of thought disorders, behavioral disorganization, or catatonia.1 Family members, particularly parents, are often concerned that children who manifest delusions or hallucinations might have schizophrenia. Fortunately, most forms of psychosis in children and adolescents are not a result of schizophrenia.
Population-based surveys show that the prevalence of psychotic symptoms may be far greater than had been previously considered, with a meta-analysis suggesting a prevalence rate of 5%-8% in the general population (which is nearly 10 times higher than the prevalence of diagnosed psychotic disorders).2 Prevalence rates of such symptoms may be even higher among young people. In 2000, Poulton and colleagues3 reported that 14% of 11-year-old children endorsed psychotic symptoms upon interview and showed that these symptoms were associated with a 5-fold to 16-fold increased rate of psychotic illness in early adulthood (depending on the strength of the initial symptoms). Since then, large, population-based studies surveying psychotic symptoms among adolescents have found rates of 9%-14% in interview-based studies4,5 and rates greater than 25% in some studies using self-report questionnaires.6-8 Positive answers on self-report questionnaires have been validated on clinical interview.8 This emerging body of research suggests that a sizable minority of young people experience psychotic symptoms.

- Hallucinatory experiences and delusions are more common in our patients than we may realize and occur frequently in the pediatric population. While not a benign occurrence, psychotic symptoms do not necessarily portend the future development of schizophrenia.
- Psychotic symptoms in children and adolescents can occur in the context of a bevy of psychiatric disorders other than schizophrenia (eg, depression, anxiety, attention-deficit/hyperactivity disorder, posttraumatic states, and autism spectrum disorders) or can be secondary to a wide variety of medical conditions.
- The onset of psychosis is usually preceded by a period of nonpsychotic symptoms known as prodromal symptoms. In recent years, there has been substantial research in early intervention efforts (eg, with psychotherapy or antipsychotic medicines) focused on the early stages of schizophrenia and on young people with prodromal symptoms. At present, outcome data are insufficient to draw definitive conclusions.
- The pharmacologic treatment of psychotic symptoms in a pediatric population is similar in many ways to the treatment of infection with antibiotics—the clinician needs to choose the proper medication at a sufficient dose and then await therapeutic results while monitoring for potential side effects.
The clinical significance of our increasing recognition of psychotic experiences in youth remains uncertain. Some clinicians may consider hearing voices, when the patient knows that they are not real, to be a benign phenomenon. Recent work, however, suggests that clinicians should not discount the importance of these symptoms.9 A longitudinal, questionnaire-based study of suicide prevention in high-school students found an unexpectedly robust association between experiencing auditory hallucinations and subsequent suicide attempts at follow-up (34% of students who previously endorsed psychopathology and hallucinations endorsed suicide attempts at follow-up).9
DOES THE PRESENCE OF PSYCHOTIC SYMPTOMS NECESSARILY PORTEND A DIAGNOSIS OF SCHIZOPHRENIA?
Schizophrenia is a chronic psychotic disorder with an estimated world-wide prevalence of 0.46%.10 It is a pervasive, often devastating, neuropsychiatric disorder associated with severe deficits in cognition, behavior, and social functioning.11 Its onset is generally between the ages of 14-35 years, with 50% of the cases diagnosed before the age of 25 years. The onset of schizophrenia between the ages of 13 and 18 years is referred to as early-onset schizophrenia or adolescent-onset schizophrenia. Schizophrenia diagnosed prior to age 13 years is variably referred to as very early-onset schizophrenia, prepubertal, or childhood-onset schizophrenia. Childhood-onset schizophrenia, now conceptualized as a childhood version of the same disorder exhibited by adolescents and adults, carries a poor prognosis. Fortunately, compared to adult-onset schizophrenia, childhood-onset schizophrenia is rare, with an incidence lower than 1 in 10,000 children12 The incidence of adolescent-onset schizophrenia is not as well established; however, up to 20% of adults who carry the diagnosis of schizophrenia became ill before the age of 18 years.13 Schizophrenia is feared by patients, their families, and clinicians, in part, because individuals who have schizophrenia are at high risk for suicide. Studies have shown that 90% of youth who commit suicide have a mental disorder, and up to 30% of those with schizophrenia will make a suicide attempt during their lifetime,1 making monitoring young people with schizophrenia for suicide risk extremely important.
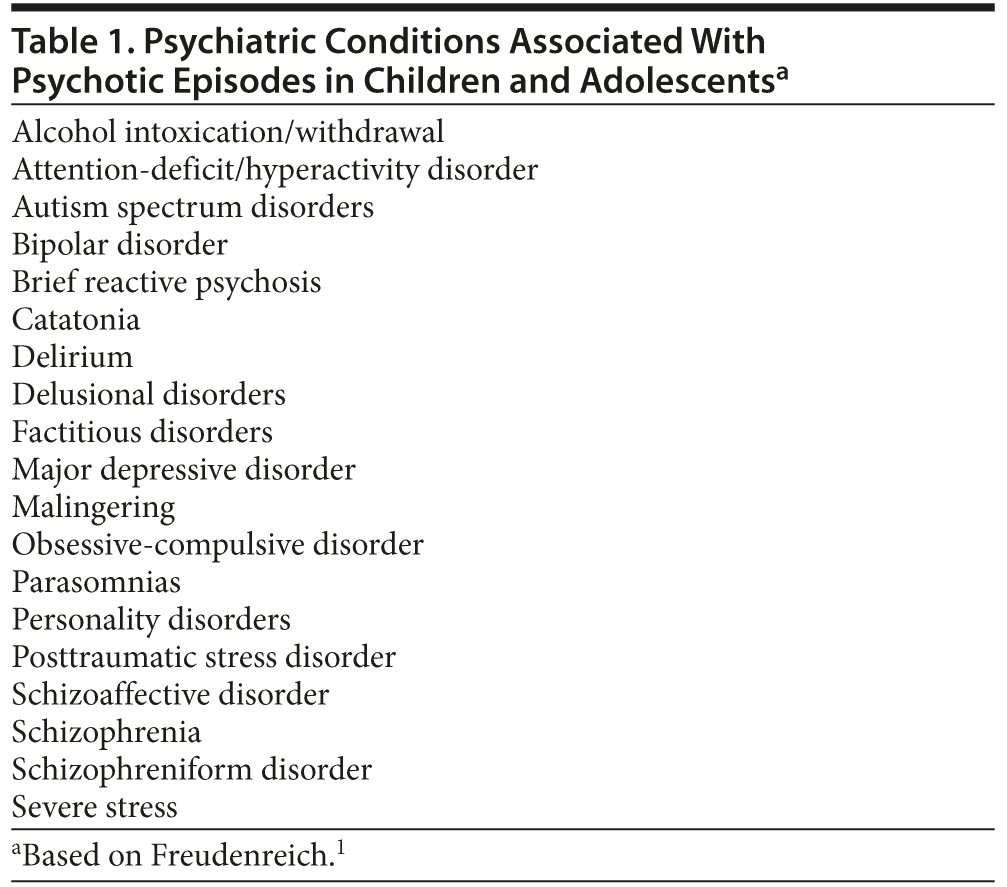
However, rather than being a harbinger of schizophrenia, psychotic symptoms in childhood and adolescence more likely accompany other psychiatric conditions (eg, major depression, bipolar disorder, or dissociative states). Table 1 lists psychiatric disorders that can be accompanied by psychosis. Differentiating early-onset schizophrenia or childhood-onset schizophrenia from other psychiatric disorders has important treatment implications. For example, a pediatric patient with bipolar disorder often experiences hallucinations and delusions but also has the clinical characteristics of mania, depression, or both.14 In children with psychological trauma-related hallucinations (typically associated with nightmares and trance-like states), psychotic symptoms may quickly abate with psychotherapeutic and/or social interventions.15 Compared to traumatized children, children with schizophrenia are more apt to display a formal thought disorder, negative symptomatology (eg, apathy, social isolation), and impulsive aggression.15 Like children and adolescents with schizophrenia, those with autism spectrum disorders may also have odd beliefs (eg, believing that they are able to communicate with valued inanimate toys).16 Children with milder forms of autism (eg, Asperger’s syndrome) may be misdiagnosed with psychosis due to their idiosyncratic beliefs, social awkwardness, and concrete thought process.16 Taking a comprehensive clinical history helps to disentangle diagnostic uncertainties.
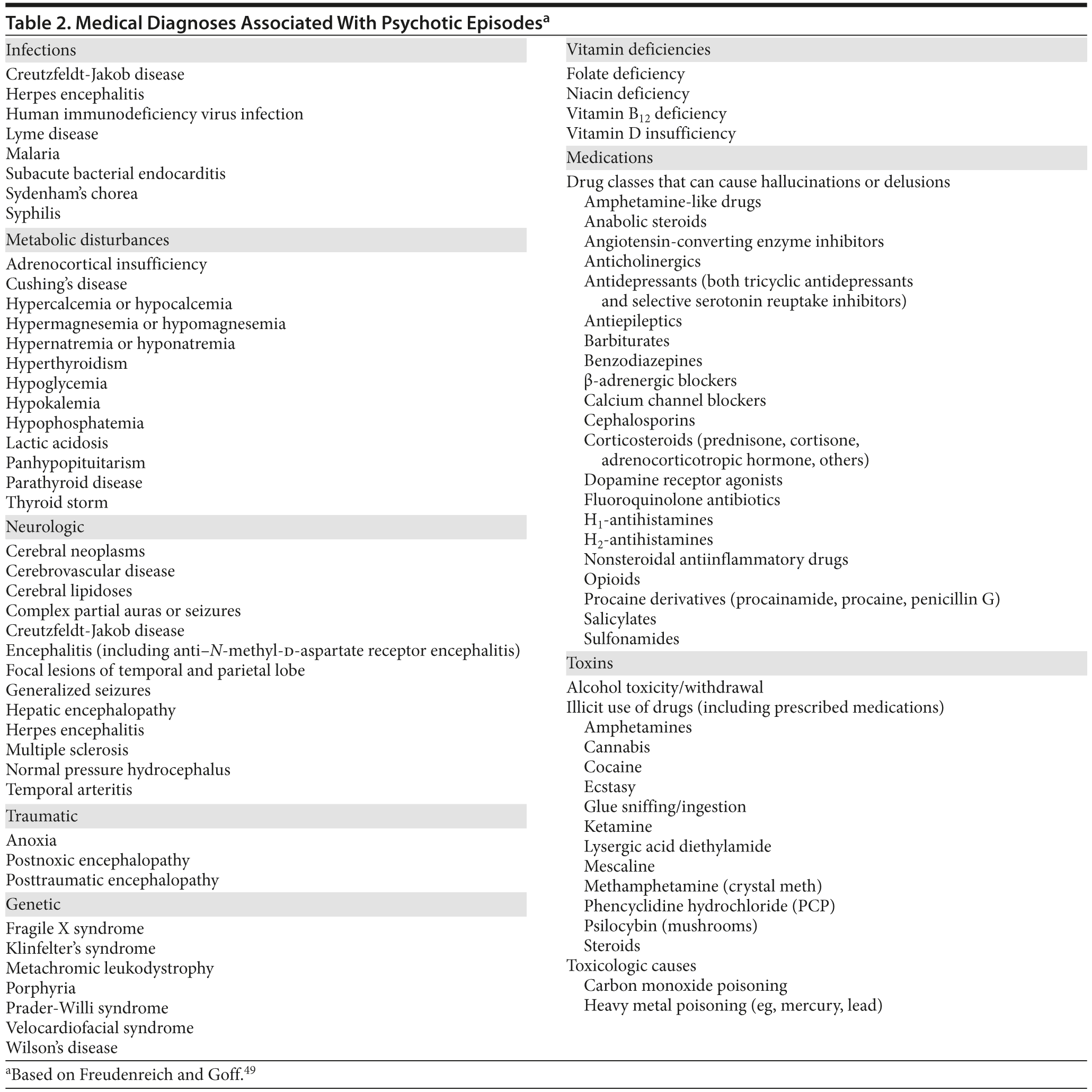
Psychotic symptoms have been associated with, or are secondary to, a wide variety of medical disorders as well. Psychosis may develop in response to central nervous system lesions, as a consequence of medical illness, or after traumatic injury or drug use. Recently, reports17 have linked a particularly severe form of new-onset psychosis—often associated with insomnia, catatonia, and autonomic instability—to antibodies against the glycine-binding NR1 subunits of the anti-N-methyl-d-aspartate (NMDA) receptor. Overall, studies from the adult literature show that about 3% of new-onset presentations of psychosis can be attributed to a medical condition.13,18 Therefore, before making a diagnosis of a primary psychotic disorder, secondary causes should be ruled out by a thorough—but not indiscriminate—medical workup.
WHAT ARE PRESENTING CHARACTERISTICS OF PSYCHOSIS IN CHILDREN AND ADOLESCENTS?
The most common manifestations in young people with psychosis are hallucinations, impaired functioning, flattened affect, and social withdrawal.11,19 Caregivers are more likely than the child patients themselves to report these problems to the clinician, as children often minimize, misinterpret, or avoid mentioning their symptoms. Hence, a comprehensive psychiatric assessment should include interviews with the child and his or her family members, review of records, information gathered from other involved adults (including a detailed description of the presentation and course of the psychotic symptoms), attention to developmental delays, a family psychiatric history, a history of abuse and/or neglect, and a mental status examination.11,20
Often, prior to the onset of psychosis, individuals who go on to develop schizophrenia show nonspecific, but irregular or unusual, social and cognitive development.21 This period is referred to as the prodrome—or the period linking premorbid functioning to full psychosis—and is often characterized by social withdrawal and anergic, eccentric, or suspicious behavior.22 Such prodromal symptoms may be misdiagnosed as part of a depressive disorder.19 Many researchers are interested in the possibility of making a diagnosis of schizophrenia in children during this prodromal period prior to the development of psychosis. Research groups22 have developed criteria and structured interviews to aid in diagnosis of prodromal psychotic symptoms (eg, the Comprehensive Assessment of Symptoms of At-Risk Mental States and Structured Interview for Prodromal Symptoms).22 Currently, these complex instruments have limited usefulness in clinical settings.
On interview, youth with schizophrenia may be incoherent, have loose associations or tangentiality, be overinclusive, or demonstrate thought-blocking, echolalia, or neologisms.19 While their affect is generally flat or blunted, it may also be silly, goofy, or labile.19 Tests of orientation or memory are generally intact (which may help the interviewer to distinguish primary psychosis from delirium). The thought process shows prominent difficulties in abstraction. Thought content is notable for hallucinations (typically auditory in nature, as with adults). Delusions (especially ideas of reference) are the most frequently encountered psychotic symptoms.21 Other components of thought disorder (eg, perseveration, a lost sense of identity, poverty of content, illogical thinking, circumstantiality, audible thoughts) and Schneiderian first-rank symptoms may also be present.23 Moreover, these children rarely have insight into the significance of their symptoms, and, consequently, their judgment and impulse control are usually compromised (particularly around self-destructive or aggressive impulses).20
Unlike adults, children with psychosis rarely demonstrate waxy flexibility or become catatonic.14 On the other hand, they can be emotionally reactive or agitated.19 The majority of children with childhood-onset schizophrenia often exhibit “soft” neurologic signs, including primitive reflexes, abnormal stereognosis, 2-point discrimination, and dysdiadochokinesia (impaired rapid alternating movements).24 Affected youth may manifest either a decreased or increased rate of eye blinking, as well as paroxysmal saccadic eye movements (inability to follow an object with smooth eye movements).25
HOW ARE CHILDREN AND ADOLESCENTS DIAGNOSED WITH SCHIZOPHRENIA?
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) made several changes to the category of schizophrenia, including raising the symptom threshold. A diagnosis of schizophrenia now requires 2 of the following conditions: delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, or negative symptoms such as affective flattening or paucity of thought or speech (in the manual’s previous editions, the threshold was 1).26 To meet diagnostic criteria, symptoms must be present for at least 6 months (including at least 1 month of active symptoms); must result in deterioration in social, school-related, and self-care functioning; and should not be accounted for by a diagnosis of schizoaffective disorder, a mood disorder, a substance use disorder, or general medical conditions.26
Additionally, an “attenuated psychosis syndrome” was included in the research section as a category for persons who do not meet criteria for a full-blown psychotic disorder but exhibit minor variations of relevant symptoms.26 This category seeks to identify individuals with an increased risk for developing a psychotic disorder, which may be significant for the implementation of effective early interventions.
Clinicians also must be attuned to developmental, cultural, and intellectual factors that may influence assessment and diagnosis. Awareness of such factors allows the clinician to interpret clinical data correctly and to differentiate among appropriate and inappropriate behaviors. For example, research has shown that minority youth have a higher chance of being misdiagnosed with a behavior disorder or schizophrenia.27 There may be several reasons for this misdiagnosis; one explanation is that some distress idioms are more confined to particular racial and ethnic groups. In some cultures and religious groups, certain types of delusions and hallucinations (eg, hearing or seeing religious figures or spirits) are consistent with religious beliefs and are therefore not considered abnormal.11 If taken out of context, a patient’s tightly held cultural or religious beliefs could be misinterpreted by a clinician as possible psychosis.11
Personality tests and projective tests should not be used to diagnose schizophrenia in youth; research indicates that tests, such as the Rorschach, do not increase the diagnostic accuracy in early-onset schizophrenia.20
WHAT CAUSES SCHIZOPHRENIA IN CHILDHOOD AND ADOLESCENCE?
While there is no single confirmed etiology of childhood-onset or early-onset schizophrenia, it is likely that genetic, behavioral, and environmental factors all contribute to the development of this disease.28
Genetic risk has been well-studied. First-degree relatives of patients with childhood-onset schizophrenia have a higher prevalence rate of schizophrenia and schizophrenia spectrum disorders, as well as impairment in smooth pursuit eye movements similar to those observed in patients with adult-onset schizophrenia.29 One study found that healthy siblings of patients with childhood-onset schizophrenia had decreased cerebral gray matter in the same pattern as was seen in the probands.30 In an investigation of youth identified as being at “high risk,”31 the young relatives of patients with schizophrenia demonstrated an increased rate of Axis I psychopathology (eg, attention-deficit/hyperactivity disorder [ADHD], conduct disorder), soft neurologic signs, and a high level of outwardly expressed emotion. On neuropsychological examination, these “high-risk” nonprobands also demonstrated impairment in attention, spatial working memory, and executive function, as well as a volume reduction in the amygdala, hippocampus, and superior temporal gyrus.31
WHICH NEURODEVELOPMENTAL AND NEUROBIOLOGICAL ABNORMALITIES ARE ASSOCIATED WITH YOUTH WHO DEVELOP SCHIZOPHRENIA?
It is widely agreed that schizophrenia is a neurodevelopmental disorder; no single neurodevelopment model, however, explains the pathophysiology of the illness. Several studies have implicated complications during pregnancy and delivery as risk factors for the development of schizophrenia.32 The combination of genetic risk and evidence of acquired damage has suggested a neurodevelopmental theory with early central nervous system abnormalities that contribute to an increased vulnerability to schizophrenia later in life. Hypoxia-associated obstetrical complications also appear to increase the odds of developing earlier-onset schizophrenia.33
Structural brain abnormalities are an established feature of schizophrenia, and recent evidence suggests a progressive nature of this pathology.34 Like adults with schizophrenia, youth with childhood-onset schizophrenia show bilateral enlargement of the lateral ventricles on neuroimaging. However, unlike adults, the abnormalities in brain morphology in patients with childhood-onset schizophrenia evolve during adolescence. Rapoport and associates35,36 reported that adolescents with schizophrenia have significantly reduced frontal and temporal gray matter volumes compared with those observed in healthy age-matched controls. Moreover, youth with childhood-onset schizophrenia appear to lose more cortical gray matter compared to children who suffer from transient psychosis.34 Subsequent studies from this group have shown that the healthy siblings of afflicted patients also have reductions in cerebral volume and gray matter.30 Similar to patients with adult-onset schizophrenia, over the course of development, youth with childhood-onset schizophrenia appear to lose cortical thickness in prefrontal and temporal regions, regardless of their treatment with medications.37 In a systematic review of 66 articles comparing brain volume in patients with a first psychotic episode with the volume seen in healthy controls, a meta-analysis demonstrated that whole-brain and hippocampal volumes are reduced and that ventricular volumes are increased in affected patients compared to healthy controls.38 Future improvements in neuroimaging technology hold the potential to reveal more about neurobiological disturbances and their correlates in schizophrenia. Yet, at present, results of neuroimaging studies have not made the leap from bench to bedside.39
WHICH NEUROTRANSMITTERS ARE THOUGHT TO BE INVOLVED IN SCHIZOPHRENIA?
The neurotransmitter most commonly implicated in the pathophysiology of schizophrenia is dopamine. Drugs that increase dopaminergic receptor activity (eg, cocaine, amphetamines) may induce an acute psychotic episode, whereas drugs that block postsynaptic D2 receptors help to alleviate psychotic symptoms. Furthermore, persons with schizophrenia also have fewer D1 receptors in the prefrontal cortex.40 Disturbances in a variety of other neurotransmitters such as glutamate, serotonin, and γ-aminobutyric acid (GABA) have been implicated in the pathophysiology of schizophrenia. Researchers are also interested in glutamate because phencyclidine (PCP or angel dust) is an NMDA/glutamine antagonist; NMDA receptor dysfunction can also cause psychotic symptoms.41 As mentioned earlier, anti-NMDA receptor antibodies, sometimes secondary to teratomas or autoimmune dysfunction, can cause psychosis in children, often in conjunction with autonomic instability and seizures.42 Serotonin may also be important; most second-generation antipsychotics share relatively potent antagonism of serotonin 5-HT2A receptors coupled with relatively weaker antagonism of dopamine D2 receptors. Preliminary studies suggest that GABA may also play a role in the development of chronic psychotic syndromes. Overall, disrupted neurotransmission and cognitive functions are key components in the pathophysiology of schizophrenia; however, no single neurotransmitter is clearly responsible for the onset and progression of schizophrenia.
ARE THERE ENVIRONMENTAL RISKS FOR THE DEVELOPMENT OF SCHIZOPHRENIA?
It is clear now that “the grass is not greener,” as data from 6 longitudinal studies in 5 countries have shown that regular cannabis use predicts an increased risk for schizophrenia and symptoms of psychosis.43 Cannabis use during early adolescence coupled with a specific genetic vulnerability and changes in brain development are correlated with risk for the development of schizophrenia44 and overall cognitive decline.45 However, the direction of the effect has been called into question. Some suggest that individuals with psychosis use cannabis to alleviate their psychotic symptoms or to improve their mood.46 Others, however, suggest that cannabis causes or exacerbates psychotic symptoms.47 A pooled analysis of 35 studies showed a dose-response effect, with greater risk of psychosis in people who used cannabis most frequently.47 Moreover, individuals with schizophrenia who are moderate to heavy cannabis users show a greater brain volume reduction over a 5-year follow-up compared with nonusers.48
Despite the fact that fewer adolescents believe that regular cannabis use is harmful to their health and increasingly permissive state laws governing the use of medical marijuana, the medical literature presents clear evidence for a neurotoxic effect of cannabis on the adolescent brain. These findings highlight the importance of efforts targeting adolescent cannabis use, including policy measures and psychoeducation in the doctor’s office.
HOW CAN ONE DISTINGUISH PRIMARY PSYCHOSIS FROM SECONDARY PSYCHOSIS?
A thorough medical workup is essential for distinguishing between primary and secondary psychotic disorders. Table 2 lists medical disorders that have been associated with psychosis.49 Although there is no generally agreed-upon medical evaluation that every patient with psychosis should undergo,1,18 screening tests should be ordered on the basis of a patient’s personal and family history, as well as on the basis of one’s clinical suspicion of conditions. Blanket testing for rare disorders may increase the risk of false-positive or false-negative results. On the other hand, the search for medical etiologies of psychosis may be expanded in the face of atypical presentations or when cases are refractory to standard treatments.18
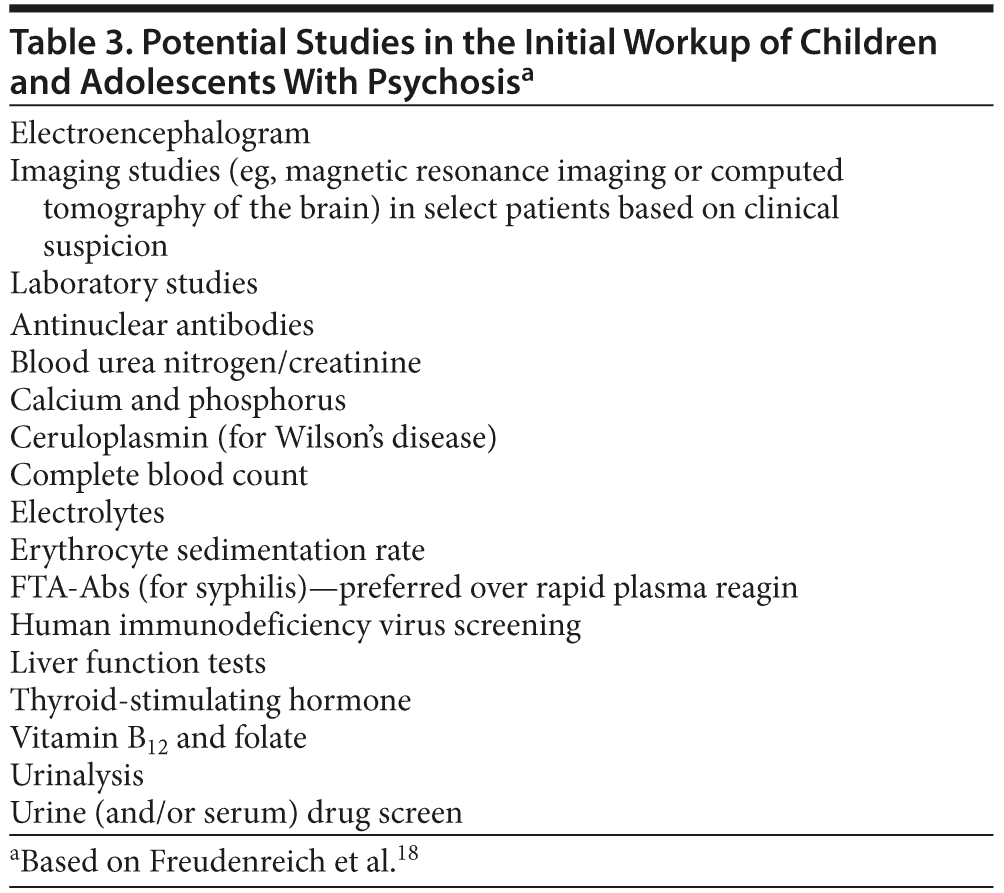
Key tests in the pediatric population (Table 3) may include drug screens (to rule out medication or substance-related psychosis), laboratory studies (to rule out infections, metabolic disturbances, certain genetic conditions, or vitamin deficiencies), an electroencephalogram (EEG), and structural neuroimaging (eg, magnetic resonance imaging [MRI] or computed axial tomography [CT] scanning). The clinical relevance of a given EEG tracing can be difficult to discern, as almost half of the patients with new-onset psychosis will have EEG abnormalities of unclear significance.50 Furthermore, EEGs read as “normal” may not capture an interictal psychosis. Likewise, incidental and non-clinically meaningful MRI findings are found in approximately 20% of the non-psychiatrically ill population.51 Recent guidelines suggest that structural neuroimaging should not be obtained routinely during an evaluation for a first episode of psychosis.52 Despite the low yield of neuroimaging, counter arguments supporting its judicious use emphasize that patients and families feel reassured by negative scans.18 Conversely, neuroimaging can rule out rare etiologies for psychosis (eg, brain tumors) and help patients and families understand and accept the psychotic symptoms as manifestations of a psychiatric disorder.18
HOW SHOULD PSYCHOSIS DUE TO AN UNDERLYING MEDICAL CONDITION BE TREATED?
The optimal treatment for secondary psychosis is treatment of the underlying medical or neurologic condition. Concomitant treatment of the secondary psychosis (usually with an antipsychotic agent) can protect both the patient and the treatment team while medical treatment is administered. Notably, most guidelines for dosing antipsychotics are based on the treatment of patients with schizophrenia and bipolar disorder. Patients treated for psychosis related to medical causes are often antipsychotic naive and sensitive to the sedating properties of lower-potency antipsychotics and to the extrapyramidal effects of higher-potency antipsychotics.
WHAT DOES THE FIRST EPISODE OF PSYCHOSIS INVOLVE?
Once functionally impairing psychotic symptoms develop—defined in terms of symptom severity and duration—a patient is no longer considered to be in the prodromal phase of illness, but instead diagnosed with an episode of psychosis. Clinically, the demarcation between prodromal (or “attenuated”) symptoms and frank psychosis is rarely clear-cut, particularly in a patient with brief or intermittent bouts of mild psychotic symptoms. Moreover, few patients receive emergency treatment for psychosis. The median duration of untreated psychosis refers to the lag between the development of symptoms and the initiation of treatment. This lag time can range from 4 to 50 weeks in first episodes53,54 and can extend longer when mood symptoms or illicit drug use cloud the diagnostic picture. The availability of mental health resources, socioeconomic factors, and cultural attitudes toward mental illness can also affect the time to treatment. Most studies show that a longer duration of untreated psychosis is associated with worse clinical outcomes.55 This observation has spurred increased research interest into interventions that target the period prior to, or immediately after, the development of a psychotic disorder. The hope is that these targeted interventions might forestall the onset of psychosis or improve long-term outcomes.
WHAT PSYCHOSOCIAL TREATMENTS ARE AVAILABLE FOR YOUTH WITH PSYCHOSIS OR SCHIZOPHRENIA?
Psychosocial interventions often involve altering the environment to minimize undue stress (which increases vulnerability to psychotic episodes) and matching the level of stimulation to the patient’s level of alertness and overall functioning. Identifying those factors that precipitate the patient’s clinical deterioration assists in determining the appropriate expectations in the home and classroom.56 Parents, teachers, and health care providers can help to identify the patient’s progression toward psychosis (eg, monitoring reality testing). In addition, specific strategies for addressing both positive (eg, hallucinations, delusions) and negative (eg, apathy, social withdrawal) symptoms may be useful. When a patient with psychosis is agitated, distressed, or unsure of what is and is not “real,” caregivers or parents should be encouraged to simplify the environment, decrease expectations of the child, and reduce environmental stimulation. For negative symptoms, regular social interactions with others and structured familiar activities (eg, eating lunch or listening to music) may diminish isolation.56
Cognitive-behavioral techniques that evaluate evidence for beliefs or thinking through explanations surrounding a patient’s perceptions can help to alter dysfunctional behaviors. A consistent framework with similar words/techniques used at home, at school, and with friends may allow the patient to employ a standardized approach to events across settings and to diminish misperceptions of daily events and interactions.57
Research data supporting psychosocial treatments in pediatric psychotic disorders are scant. There is a controlled study comparing cognitive-behavioral therapy (CBT) with no antipsychotic to a monitoring program in prodromal patients.58 CBT was associated with less progression to psychosis; however, the results of this study were limited by methodological flaws. The PACE (Personal Assessment and Crisis Evaluation) trial randomly assigned prodromal patients (aged 14-28 years) to risperidone plus CBT versus a needs-based intervention of supportive therapy and psychotropics other than antipsychotics.59 At 6 months, 3 of 31 patients in the risperidone plus CBT group converted to psychosis, whereas 10 of 28 patients in the supportive therapy and nonneuroleptic pharmacotherapy group converted to psychosis.59 Since the average risperidone dose used in this study was relatively low (1.3 mg/d), there is a suggestion that CBT was a vital ingredient to the protective effects seen in this study.59
Several studies related to adults with psychosis support the usefulness of psychoeducation to decrease medication noncompliance and relapse rates. A Cochrane review of 44 randomized trials that compared any form of psychoeducation to standard care in > 5,000 patients with schizophrenia (or a related serious mental illness) showed that psychoeducational approaches increased patients’ awareness of their illness and its treatment; these interventions were relatively brief and inexpensive.60 A similar review of 19 studies that employed cognitive-behavioral approaches showed promising—but ultimately inconclusive—results for schizophrenia.61 More recently, a study of young adults (mean age of 23 years) newly diagnosed with psychosis or major depression compared outcomes after a cognitive remediation program or treatment as usual. The results suggested that 10 weekly 2-hour sessions (consisting of psychoeducation about cognitive deficits, compensatory strategies, group activities, and computer-assisted cognitive training) could improve learning, memory, and overall psychosocial functioning.62 Taken together, these studies highlight the need for clinicians to be attentive to signs of or patient/family complaints about cognitive deficits that persist after the remission of psychosis. Psychosocial alterations, neuropsychological evaluation, and cognitive strategies may prove to be valuable modalities to improve functional outcomes.
WHAT DOES THE PHARMACOTHERAPY OF SCHIZOPHRENIA IN YOUTH INVOLVE?
Pharmacologic treatment for schizophrenia in youth remains an area of active research, with several large multisite trials completed within the past few years. Six, 6-week, randomized, placebo-controlled studies of second-generation antipsychotics for early onset schizophrenia have been completed thus far. They showed that aripiprazole, olanzapine, paliperidone, quetiapine, and risperidone—but not ziprasidone—were superior to placebo.13 In these controlled studies, aripiprazole,63 olanzapine,64 paliperidone,65 quetiapine,66 and risperidone67,68 were superior to placebo with regard to improvement in the Positive and Negative Syndrome Scale (PANSS) total score.
There have been 7 head-to-head trials comparing antipsychotics in adolescents using small sample sizes and short duration (4-8 weeks). In general—with the exception of clozapine—there were no differences in efficacy between most antipsychotics.13 One of these studies, The Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS), compared the efficacy and safety of 2 second-generation antipsychotics (olanzapine and risperidone) with a first-generation antipsychotic (molindone) in a double-blind multisite trial of patients with childhood-onset schizophrenia and schizoaffective disorder.69 Of 116 patients who received at least 1 antipsychotic dose, neither risperidone or olanzapine demonstrated superior efficacy over molindone.70 Adverse effects were frequent but differed among medications (eg, olanzapine and risperidone were associated with significantly greater weight gain; molindone led to more self-reports of akathisia). Of note, in 2010, the sole manufacturer of molindone discontinued production of this medicine.
In terms of atypical antipsychotic dosing, typically, these medications are initiated at a low dose and gradually titrated upward to achieve efficacy. Risperidone, for example, is generally started at 0.25 mg twice a day and can be increased every 1 to 2 days in association with close observation. In general, olanzapine and quetiapine are more sedating; their dosing is usually started at 2.5 to 5 mg/d and 25 to 50 mg/d, respectively. As most antipsychotic medications have a relatively long half-life, they typically do not need be administered more than twice daily. For patients maintained on antipsychotics, clinicians should monitor weight, vital signs, and relevant laboratory results (eg, triglycerides, cholesterol, fasting glucose, and prolactin levels).
Newer atypical antipsychotics (eg, asenapine, iloperidone, lurasidone) may eventually have a role in the treatment of patients with childhood-onset or early onset schizophrenia and in patients deemed at-risk of developing schizophrenia due to genetic and/or environmental factors. However, at present, there is little evidence to support the safety, tolerability, or efficacy of these agents in the pediatric population.
If trials with 2 atypical antipsychotics are ineffective or poorly tolerated, a trial with a typical agent (eg, chlorpromazine, perphenazine, haloperidol) should be considered. As suggested in the TEOSS study, second-generation (atypical) antipsychotics—excluding clozapine—are not necessarily more effective than first-generation (typical) agents for children and adolescents with early onset schizophrenia or schizoaffective disorder.70 Current practice parameters list both typical and atypical antipsychotics as “first-line” treatments for early-onset and childhood-onset schizophrenia.11 Still, many clinicians favor the use of second-generation antipsychotics on the basis of familiarity and the lower risk of neurologic side effects. There also have been approximately 3 times as many trials conducted using second-generation antipsychotics in the pediatric population than trials using first-generation medications.13 Nearly all of the trials of first-generation antipsychotics were performed in the 1990s or earlier; some were placebo-controlled, and several trials use medications rarely prescribed in current clinical practice (eg, trifluoperazine or thioridazine). The usual oral dosage of typical antipsychotics ranges between 3 and 6 mg/kg/d for the low-potency phenothiazine (chlorpromazine) and between 0.1 and 0.5 (up to 1.0) mg/kg/d for the high-potency antipsychotic (haloperidol).13 Most of the published trials of first-generation antipsychotics reported a decrease in psychotic symptoms.11
In sum, current pharmacologic research for early-onset and childhood-onset schizophrenia focuses on the identification of antipsychotic agents that will provide optimal efficacy without also causing undue adverse events. Many typical and atypical antipsychotics have been shown (based on open trials, randomized clinical trials, and head-to-head comparisons) to be efficacious in the treatment of early-onset and childhood-onset schizophrenia71; however, they are well known to cause side effects such as weight gain, extrapyramidal symptoms, and metabolic abnormalities.
WHAT IS THE ROLE OF LONG-ACTING (INJECTABLE) ANTIPSYCHOTICS IN CHILDHOOD-ONSET SCHIZOPHRENIA?
Nonadherence to oral antipsychotic medications is one of the most significant clinical challenges in the treatment of schizophrenia. Nonadherence rates may be as high as 50% in the first year of treatment and 75% during the first 2 years of treatment.72 Despite evidence that continuous antipsychotic treatment is more effective than interrupted treatment,73 long-acting therapy use in the United States remains low. Barriers to increased long-acting therapy use include physicians’ reluctance to administer injectable medications, confusing reimbursement procedures, and the unfounded belief that patients would reject an offer of this treatment modality.74
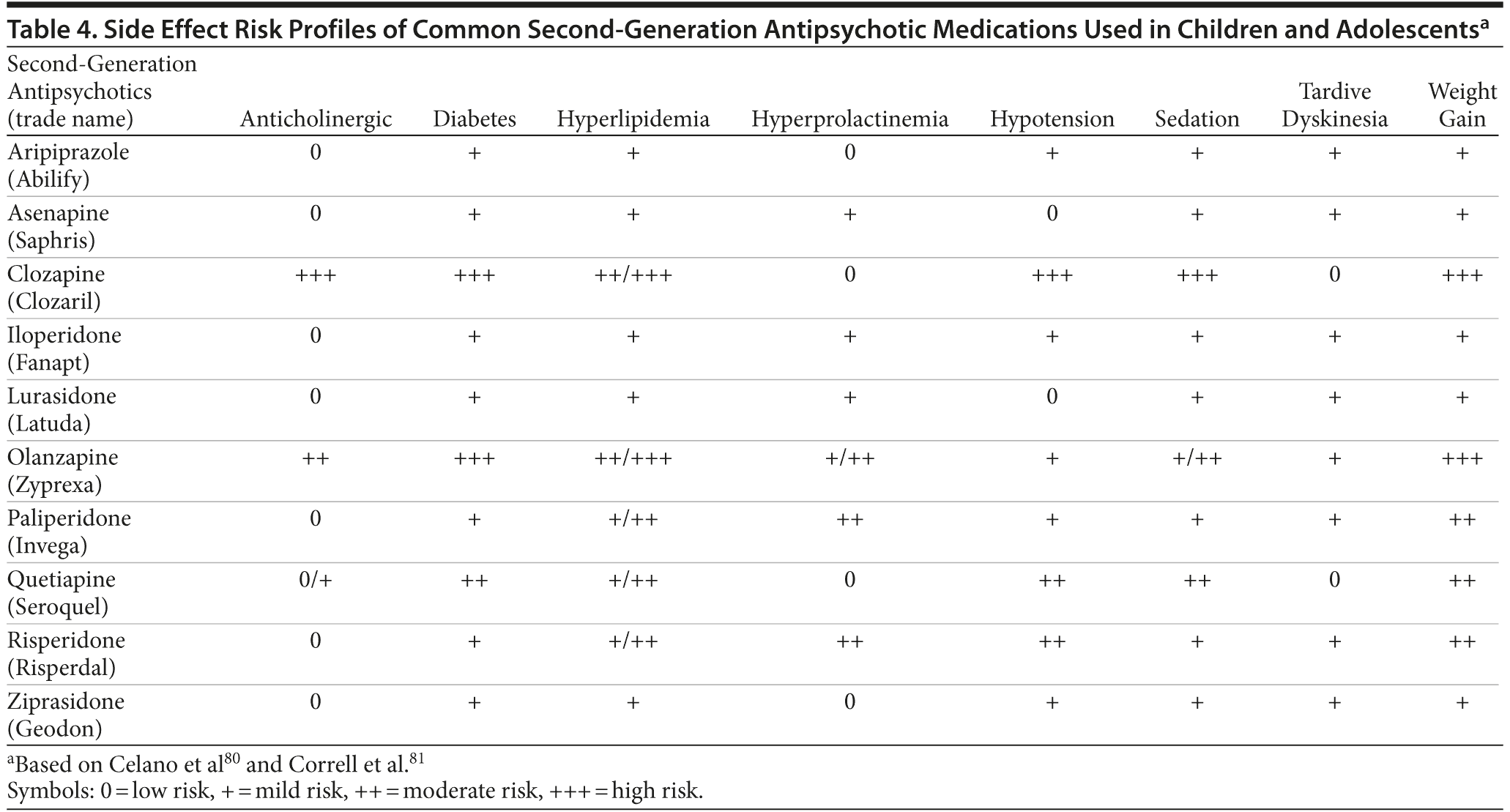
In the near future, as many as 4 new atypical antipsychotic long-acting therapy formulations may become available.74 In clinical situations wherein adherence with oral preparations has proven difficult, it is reasonable—especially in older adolescents—to consider using a long-acting medication. Risperidone long-acting therapy has been shown to be efficacious and well tolerated in the treatment of schizophrenia, including those with newly diagnosed schizophrenia.75 Short-term76 and long-term studies77 found that risperidone long-acting therapy was associated with low rates of discontinuation related to adverse events and few reports of pain from injections.78 Evidence for newer long-acting injectables (eg, olanzapine, paliperidone) remains limited.79 The available evidence suggests that long-acting injectable antipsychotics can be used safely and effectively in early stages of the illness and that they may be associated with better outcomes than with oral medications. However, this is largely supported by evidence from naturalistic cohort studies and by a small number of controlled trials of risperidone long-acting injection.79 Given the paucity of data of long-acting therapy in the adolescent population, the use of these preparations in adolescents remains empiric. Table 4 provides the side effect risk profiles of common second-generation antipsychotic medications used in children and adolescents.80,81
WHAT ARE THE ADVERSE EFFECTS OF ANTIPSYCHOTICS?
In reviewing the available randomized, controlled studies and head-to-head comparisons of antipsychotics, Schimmelmann and colleagues82 noted, “Antipsychotics are efficacious in the treatment of early-onset schizophrenia. The lack of evidence for efficacy differences between various antipsychotics (except for clozapine for treatment-refractory early-onset schizophrenia) suggests that a primum non nocere principle should be applied when choosing and antipsychotic.”(p228) Therefore, the side effect profile of the antipsychotics often dictates the choice of pharmacologic agent for young people with psychotic symptoms. Antipsychotic therapy has been linked to a bevy of metabolic changes (eg, dyslipidemia, weight gain, plasma prolactin increases), sedation, extrapyramidal symptoms, and dyskinesias (see Table 4). With the exception of tardive dyskinesia,83 young people with schizophrenia seem more likely to experience these side effects from atypical neuroleptics compared with their adult counterparts. Children and adolescents may gain weight, even when treated with antipsychotics that are considered “weight-neutral” in adults.84 Similarly, there are reports of prolactin elevations in youth treated with prolactin-sparing agents such as quetiapine.85
Other common adverse effects of antipsychotics include drowsiness and anticholinergic effects (eg, dry mouth, nasal congestion, blurred vision), particularly with the low-potency phenothiazines. Caregivers can usually manage the short-term adverse effects of antipsychotics with adjustments of dose and timing of administration. One can avoid undue sedation by using less sedating agents and by prescribing most of the daily dose at bedtime. One should not confuse drowsiness with impaired cognition; drowsiness can usually be corrected by adjusting the dose and the timing of administration. Furthermore, there is no evidence that antipsychotics adversely affect cognition when used in low doses. One can minimize anticholinergic effects by choosing a medium-potency or high-potency antipsychotic.
Extrapyramidal Side Effects
Extrapyramidal side effects such as acute dystonia, akathisia (motor restlessness), and parkinsonism (bradykinesia, tremor, and lack of facial expressions) are more commonly seen with use of high-potency agents (eg, butyrophenones, thioxanthenes) and have been reported in up to 75% of children receiving these agents. The extent to which antiparkinsonian agents (eg, anticholinergic drugs such as benztropine, trihexyphenidyl, antihistamines, and the antiviral agent amantadine) should be used prophylactically when antipsychotics are introduced is controversial, although coadministration has been shown to reduce the incidence of extrapyramidal side effects by a factor of 7.86
Other Movement Disorders
Abrupt cessation of antipsychotic drugs can cause withdrawal dyskinesias (typically benign, but occasionally more pronounced) and a syndrome of deteriorating behavior. As with adults, the long-term administration of antipsychotic drugs can produce tardive dyskinesia. Although children appear generally less likely to develop tardive dyskinesia than adults,83 the fact that tardive dyskinesia is potentially irreversible prompts caregivers to evaluate the benefits of such medications, including documenting a clear indication and identifying target symptoms; to periodically discontinue the drug to assess continued need; and to monitor closely to ensure early detection. Current practice parameters recommend monitoring neurologic side effects with standardized scales (eg, Abnormal Involuntary Movement Scale or Simpson Angus Scale) at baseline and periodically during any treatment regimen requiring antipsychotic agents.11
Akathisia
Akathisia is a movement disorder characterized by anxiety and an inability to sit still; it can be a side effect of either antipsychotics or antidepressants. Akathisia may be particularly problematic in young patients because of underrecognition or misdiagnosis as ADHD or agitation. Caregivers should consider akathisia in the differential when a child or adolescent starts an antipsychotic and becomes acutely agitated with an associated inability to sit still (and/or with aggressive outbursts).
Treatment of akathisia involves reduction of the offending agent to the lowest effective dose and then using either a benzodiazepine (eg, lorazepam, 0.5 to 1 mg 3 times per day) or a β-blocker. Although all β-blockers are likely to be effective, propranolol, initiated at 10 mg 2 or 3 times per day with an increase every few days until the desired effect is achieved, is a good choice. Alternately, betaxolol hydrochloride, a long-acting β1-selective adrenergic antagonist with few medication interactions, may be useful.
Neuroleptic Malignant Syndrome
Little is known about the potentially lethal neuroleptic malignant syndrome in children and adolescents; however, preliminary evidence indicates that its presentation is similar to that in adults. This syndrome may be difficult to distinguish from primary central nervous system pathology, concurrent infection, anti-NMDA receptor encephalitis, or other, more benign, side effects of antipsychotic treatment, including extrapyramidal side effects or anticholinergic toxicity. Neuroleptic syndrome is a life-threatening medical emergency; treatment is largely supportive but can include dopamine reuptake inhibitors like amantadine, similar to strategies used in adults.
WHAT STRATEGIES ARE HELPFUL FOR NONRESPONDERS?
A trial of clozapine should be considered for patients who do not respond to trials with first-line atypical or typical antipsychotics or for those who experience significant dyskinesia from these medications. In the United States and Europe, there has been considerable experience with use of clozapine in adolescents. Clozapine’s superiority has been shown in 2 randomized clinical trials compared to a typical (ie, haloperidol)87 and atypical (ie, olanzapine)12 antipsychotic for childhood-onset schizophrenia. During the course of these trials, clozapine was associated with a range of adverse events (including tachycardia, hypotension, lipid abnormalities, agranulocytosis, seizure, and nocturnal enuresis).
A unique head-to-head randomized controlled trial of olanzapine and clozapine in the treatment of patients with childhood-onset schizophrenia showed that clozapine led to a statistically significant reduction in all outcome measures (particularly negative symptoms of schizophrenia) even when compared to another atypical agent.12
Established dosing parameters for clozapine have not yet been determined in the pediatric population. In 1 open study of clozapine for youth with schizophrenia, doses from 125 to 825 mg/d (mean = 375 mg/d) for up to 6 weeks were necessary for efficacy.88 Although clozapine is remarkably effective in chronic treatment-resistant schizophrenia and affective psychosis, there is a dose-related risk of seizures and an increased risk of leukopenia and agranulocytosis in adolescents that is similar to the risk in adults. As a result, using clozapine requires close monitoring. There is a small, but emerging, literature that supports the use of adjunctive lithium in the prevention and management of neutropenia in children treated with clozapine.89
WHAT STRATEGIES ARE AVAILABLE FOR EARLY INTERVENTION AND PREVENTION OF CHILDHOOD-ONSET SCHIZOPHRENIA?
Outcome studies ranging from several years to up to 42 years after diagnosis of childhood-onset schizophrenia indicate that the long-term function of patients with childhood onset is poor compared to those with adolescent-onset or adult-onset schizophrenia.86 In general, the earlier that childhood-onset schizophrenia develops, the worse the prognosis. Higher premorbid intelligence, having more positive than negative symptoms, and having family members cooperate in treatment confer a better prognosis.90
In a randomized trial involving 60 outpatients (median age 16 years) with prodromal symptoms randomized to olanzapine (5-15 mg/d) or placebo for 1 year and followed for 1 additional year,91 olanzapine was not associated with a reduction in the conversion to psychosis in patients with prodromal symptoms. The results of this study were complicated by a high dropout rate. Olanzapine was associated with a greater improved mean score for prodromal positive symptoms.91
Antipsychotics may not be the only (or best) tool available to aid patients at high risk for developing psychosis. A randomized trial of 81 treatment-seeking patients (aged 13-25 years) considered at ultrahigh risk of psychotic disorder were randomized to omega-3 polyunsaturated fatty acids (1.2 g/d) or placebo for 12 weeks and then followed for 1 year.92 Omega-3 polyunsaturated fatty acid supplementation appeared to delay the transition to psychotic disorder in these patients without any significant difference in adverse events compared to placebo.92 However, a recent Cochrane review54 of 18 randomized trials involving early interventions for psychosis found that no intervention has been efficacious in 2 randomized trials, which speaks to the emerging nature of this research.
The notion of early intervention and perhaps even prevention of schizophrenia remains at the forefront of early-onset psychosis research. Further research in this area is ongoing. However, at present, the literature does not support clear treatment guidelines for these vulnerable patients.
CONCLUSION
Clinicians should be vigilant and consider psychotic illnesses when evaluating children and adolescents with emotional and behavioral disturbances. Given the high developmental, financial, and functional toll associated with chronic psychotic illness, clinicians should employ a variety of diagnostic and treatment modalities to mitigate the risks associated with this illness. These modalities may include performing careful psychiatric and physical examinations, relevant laboratory testing, neuropsychological testing, and employing relevant cognitive therapies, as well as the use of antipsychotic and other pharmacologic interventions. Advances in the understanding of childhood-onset and early-onset schizophrenia, as well as the elucidation of prodromal psychotic symptomatology, will benefit both children and adults.
REFERENCES
1. Freudenreich O. Psychotic Disorders: A Practical Guide. Philadelphia, PA: Lippincott Williams and Wilkens; 2008.
2. van Os J, Linscott RJ, Myin-Germeys I, et al. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis-proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179-195. PubMed
3. Poulton R, Caspi A, Moffitt TE, et al. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053-1058. doi:10.1001/archpsyc.57.11.1053 PubMed
4. Bartels-Velthuis AA, Jenner JA, van de Willige G, et al. Prevalence and correlates of auditory vocal hallucinations in middle childhood. Br J Psychiatry. 2010;196(1):41-46. doi:10.1192/bjp.bp.109.065953 PubMed
5. Horwood J, Salvi G, Thomas K, et al. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. Br J Psychiatry. 2008;193(3):185-191. doi:10.1192/bjp.bp.108.051904 PubMed
6. Laurens KR, Hodgins S, Maughan B, et al. Community screening for psychotic-like experiences and other putative antecedents of schizophrenia in children aged 9-12 years. Schizophr Res. 2007;90(1-3):130-146. doi:10.1016/j.schres.2006.11.006 PubMed
7. Yung AR, Nelson B, Baker K, et al. Psychotic-like experiences in a community sample of adolescents: implications for the continuum model of psychosis and prediction of schizophrenia. Aust N Z J Psychiatry. 2009;43(2):118-128. doi:10.1080/00048670802607188 PubMed
8. Kelleher I, Harley M, Murtagh A, et al. Are screening instruments valid for psychotic-like experiences? a validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37(2):362-369. doi:10.1093/schbul/sbp057 PubMed
9. Kelleher I, Corcoran P, Keeley H, et al. Psychotic symptoms and population risk for suicide attempt: a prospective cohort study. JAMA Psychiatry. 2013;70(9):940-948. doi:10.1001/jamapsychiatry.2013.140 PubMed
10. Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi:10.1371/journal.pmed.0020141 PubMed
11. McClellan J, Werry J; American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl):4S-23S. doi:10.1097/00004583-200107001-00002 PubMed
12. Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: a double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63(7):721-730. doi:10.1001/archpsyc.63.7.721 PubMed
13. Maloney AE, Yakutis LJ, Frazier JA. Empirical evidence for psychopharmacologic treatment in early-onset psychosis and schizophrenia. Child Adolesc Psychiatr Clin N Am. 2012;21(4):885-909. doi:10.1016/j.chc.2012.07.011 PubMed
14. Pavuluri MN, Herbener ES, Sweeney JA. Psychotic symptoms in pediatric bipolar disorder. J Affect Disord. 2004;80(1):19-28. doi:10.1016/S0165-0327(03)00053-3 PubMed
15. Kaufman J, Birmaher B, Clayton S, et al. Case study: trauma-related hallucinations. J Am Acad Child Adolesc Psychiatry. 1997;36(11):1602-1605. PubMed
16. Unenge Hallerbäck M, Lugneg×¥rd T, Gillberg C. Is autism spectrum disorder common in schizophrenia? Psychiatry Res. 2012;198(1):12-17. doi:10.1016/j.psychres.2012.01.016 PubMed
17. van de Riet EH, Esseveld MM, Cuypers L, et al. Anti-NMDAR encephalitis: a new, severe and challenging enduring entity. Eur Child Adolesc Psychiatry. 2013;22(5):319-323. PubMed
18. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18. doi:10.1111/j.1751-7893.2008.00105.x PubMed
19. Schaeffer JL, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnostic and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538-545. doi:10.1097/00004583-200205000-00011 PubMed
20. McDonell M, McClellan J. Early-onset schizophrenia. In: Mash E, Barkley R, eds. Assessment of Childhood Disorders. 4th ed. New York, NY: Guilford Press; 2007:526-550.
21. Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(suppl 3):S162-S167. doi:10.1055/s-2003-45125 PubMed
22. Thomas LE, Woods SW. The schizophrenia prodrome: a developmentally informed review and update for psychopharmacologic treatment. Child Adolesc Psychiatr Clin N Am. 2006;15(1):109-133. doi:10.1016/j.chc.2005.08.011 PubMed
23. Bearden CE, Wu KN, Caplan R, et al. Thought disorder and communication deviance as predictors of outcome in youth at clinical high risk for psychosis. J Am Acad Child Adolesc Psychiatry. 2011;50(7):669-680. doi:10.1016/j.jaac.2011.03.021 PubMed
24. Karp BI, Garvey M, Jacobsen LK, et al. Abnormal neurologic maturation in adolescents with early-onset schizophrenia. Am J Psychiatry. 2001;158(1):118-122. doi:10.1176/appi.ajp.158.1.118 PubMed
25. Kumra S, Sporn A, Hommer DW, et al. Smooth pursuit eye-tracking impairment in childhood-onset psychotic disorders. Am J Psychiatry. 2001;158(8):1291-1298. doi:10.1176/appi.ajp.158.8.1291 PubMed
26. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Press; 2013.
27. DelBello MP, Lopez-Larson MP, Soutullo CA, et al. Effects of race on psychiatric diagnosis of hospitalized adolescents: a retrospective chart review. J Child Adolesc Psychopharmacol. 2001;11(1):95-103. doi:10.1089/104454601750143528 PubMed
28. Kodish I, McClellan J. In: Hersen M, Reitman D, eds. Handbook of Psychological Assessment, Case Conceptualization, and Treatment: Vol. 2, Children and Adolescents. Hoboken, NJ: John Wiley & Sons; 2008:405-443.
29. Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58(6):581-588. doi:10.1001/archpsyc.58.6.581 PubMed
30. Gogtay N, Sporn A, Clasen LS, et al. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am J Psychiatry. 2003;160(3):569-571. doi:10.1176/appi.ajp.160.3.569 PubMed
31. Keshavan MS, Diwadkar VA, Montrose DM, et al. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3(3):163-168. PubMed
32. Byrne M, Agerbo E, Bennedsen B, et al. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res. 2007;97(1-3):51-59. doi:10.1016/j.schres.2007.07.018 PubMed
33. Fish B, Kendler KS. Abnormal infant neurodevelopment predicts schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol. 2005;15(3):348-361. doi:10.1089/cap.2005.15.348 PubMed
34. Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2011;29(3):251-258. doi:10.1016/j.ijdevneu.2010.10.003 PubMed
35. Rapoport JL, Giedd JN, Blumenthal J, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56(7):649-654. doi:10.1001/archpsyc.56.7.649 PubMed
36. Rapoport JL, Addington AM, Frangou S, et al. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10(5):434-449. doi:10.1038/sj.mp.4001642 PubMed
37. Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003-1012. doi:10.1111/j.1469-7610.2006.01658.x PubMed
38. Steen RG, Mull C, McClure R, et al. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188(6):510-518. doi:10.1192/bjp.188.6.510 PubMed
39. Ahmed AO, Buckley PF, Hanna M. Neuroimaging schizophrenia: a picture is worth a thousand words, but is it saying anything important? Curr Psychiatry Rep. 2013;15(3):345. doi:10.1007/s11920-012-0345-0 PubMed
40. Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634-636. doi:10.1038/385634a0 PubMed
41. Clinton SM, Haroutunian V, Davis KL, et al. Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2003;160(6):1100-1109. doi:10.1176/appi.ajp.160.6.1100 PubMed
42. Millichap JJ, Goldstein JL, Laux LC, et al. Ictal asystole and anti-N-methyl-d-aspartate receptor antibody encephalitis. Pediatrics. 2011;127(3):e781-e786. doi:10.1542/peds.2010-2080 PubMed
43. Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99(4):425-430. doi:10.1111/j.1360-0443.2004.00683.x PubMed
44. Schimmelmann BG, Conus P, Cotton SM, et al. Cannabis use disorder and age at onset of psychosis—a study in first-episode patients. Schizophr Res. 2011;129(1):52-56. doi:10.1016/j.schres.2011.03.023 PubMed
45. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657-E2664. doi:10.1073/pnas.1206820109 PubMed
46. Kolliakou A, Joseph C, Ismail K, et al. Why do patients with psychosis use cannabis and are they ready to change their use? Int J Dev Neurosci. 2011;29(3):335-346. doi:10.1016/j.ijdevneu.2010.11.006 PubMed
47. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328. doi:10.1016/S0140-6736(07)61162-3 PubMed
48. Rais M, Cahn W, Van Haren N, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165(4):490-496. doi:10.1176/appi.ajp.2007.07071110 PubMed
49. Freudenreich O, Goff DC. Psychotic patients. In: Stern TA, Fricchione GH, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. Amsterdam, The Netherlands: Elsevier Science Publishers; 2010. doi:10.1016/B978-1-4377-1927-7.00012-1
50. Manchanda R, Norman R, Malla A, et al. EEG abnormalities and two year outcome in first episode psychosis. Acta Psychiatr Scand. 2005;111(3):208-213. doi:10.1111/j.1600-0447.2004.00490.x PubMed
51. Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282(1):36-39. doi:10.1001/jama.282.1.36 PubMed
52. Structural neuroimaging in first-episode psychosis. London (UK): National Institute for Health and Clinical Excellence (NICE); 2008 Feb. 24 p. (technology appraisal guidance; no. 136). http://www.guideline.gov/content.aspx?id=12284. Accessed December 4, 2013.
53. Marshall M, Lewis S, Lockwood A, et al. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62(9):975-983. doi:10.1001/archpsyc.62.9.975 PubMed
54. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. PubMed
55. Ruhrmann S, Schultze-Lutter F, Maier W, et al. Pharmacological intervention in the initial prodromal phase of psychosis. Eur Psychiatry. 2005;20(1):1-6. doi:10.1016/j.eurpsy.2004.11.001 PubMed
56. Stevens JR, Prince JB. Schooling students with psychotic disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(1):187-200, xi. doi:10.1016/j.chc.2011.09.008 PubMed
57. Turkington D, Kingdon D, Turner T; Insight into Schizophrenia Research Group. Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. Br J Psychiatry. 2002;180(6):523-527. doi:10.1192/bjp.180.6.523 PubMed
58. Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185(4):291-297. doi:10.1192/bjp.185.4.291 PubMed
59. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921-928. doi:10.1001/archpsyc.59.10.921 PubMed
60. Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database Syst Rev. 2011;(6):CD002831. PubMed
61. Jones C, Cormac I, Silveira da Mota Neto JI, et al. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev. 2004;(4):CD000524. PubMed
62. Lee RS, Redoblado-Hodge MA, Naismith SL, et al. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychol Med. 2013;43(6):1161-1173. PubMed
63. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432-1441. doi:10.1176/appi.ajp.2008.07061035 PubMed
64. Kryzhanovskaya L, Schulz SC, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(1):60-70. doi:10.1097/CHI.0b013e3181900404 PubMed
‘ 65. Singh J, Robb A, Vijapurkar U, et al. A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol Psychiatry. 2011;70(12):1179-1187. doi:10.1016/j.biopsych.2011.06.021 PubMed
66. Findling RL, McKenna K, Earley WR, et al. Efficacy and safety of quetiapine in adolescents with schizophrenia investigated in a 6-week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2012;22(5):327-342. doi:10.1089/cap.2011.0092 PubMed
67. Haas M, Eerdekens M, Kushner S, et al. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: double-blind study. Br J Psychiatry. 2009;194(2):158-164. doi:10.1192/bjp.bp.107.046177 PubMed
68. Haas M, Unis AS, Armenteros J, et al. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009;19(6):611-621. doi:10.1089/cap.2008.0144 PubMed
69. McClellan J, Sikich L, Findling RL, et al. Treatment of early-onset schizophrenia spectrum disorders (TEOSS): rationale, design, and methods. J Am Acad Child Adolesc Psychiatry. 2007;46(8):969-978. doi:10.1097/CHI.0b013e3180691779 PubMed
70. Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165(11):1420-1431. doi:10.1176/appi.ajp.2008.08050756 PubMed
71. Yan J. FDA advisors back additional antipsychotics for use in teens. Psychiatr News. 2009;44(13):1-29.
72. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63(12):1121-1128. doi:10.4088/JCP.v63n1206 PubMed
73. Kane JM. Schizophrenia. N Engl J Med. 1996;334(1):34-41. doi:10.1056/NEJM199601043340109 PubMed
74. Lasser RA, Schooler NR, Kujawa M, et al. A new psychosocial tool for gaining patient understanding and acceptance of long-acting injectable antipsychotic therapy. Psychiatry (Edgmont). 2009;6(4):22-27. PubMed
75. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl):5-14. doi:10.1177/0269881105056513 PubMed
76. Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125-1132. doi:10.1176/appi.ajp.160.6.1125 PubMed
77. Simpson GM, Mahmoud RA, Lasser RA, et al. A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2006;67(8):1194-1203. doi:10.4088/JCP.v67n0804 PubMed
78. Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol. 2005;20(4):213-221. doi:10.1097/00004850-200507000-00004 PubMed
79. Emsley R, Chiliza B, Asmal L, et al. Long-acting injectable antipsychotics in early psychosis: a literature review. Early Interv Psychiatry. 2013;7(3):247-254. doi:10.1111/eip.12027 PubMed
80. Celano CM, Beach SR, Huffman JC, et al. Side effects profiles of psychotropic medications from cardiovascular and other side effects of psychotropic medications. In: Stern TA, Herman JB, Gorrindo T, eds. The Massachusetts General Hospital Psychiatry Update and Board Preparation. 3rd ed. Boston, MA: MGH Psychiatry Academy Publishing; 2012.
81. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206. doi:10.1016/j.chc.2005.08.007 PubMed
82. Schimmelmann BG, Schmidt SJ, Carbon M, et al. Treatment of adolescents with early-onset schizophrenia spectrum disorders: in search of a rational, evidence-informed approach. Curr Opin Psychiatry. 2013 Mar;26(2):219-230. doi: 10.1097/YCO.0b013e32835dcc2a PubMed </jrn>
83. Correll CU, Kane JM Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656. doi:10.1089/cap.2006.0117 PubMed
84. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158. doi:10.1016/j.eurpsy.2010.09.011 PubMed
85. Stevens JR, Kymissis PI, Baker AJ. Elevated prolactin levels in male youths treated with risperidone and quetiapine. J Child Adolesc Psychopharmacol. 2005;15(6):893-900. doi:10.1089/cap.2005.15.893 PubMed
86. Stern TA, Anderson WH. Benztropine prophylaxis of dystonic reactions. Psychopharmacology (Berl). 1979;61(3):261-262. doi:10.1007/BF00432269 PubMed
87. Kumra S, Frazier JA, Jacobsen LK, et al. Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry. 1996;53(12):1090-1097. doi:10.1001/archpsyc.1996.01830120020005 PubMed
88. Frazier JA, Gordon CT, McKenna K, et al. An open trial of clozapine in 11 adolescents with childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1994;33(5):658-663. doi:10.1097/00004583-199406000-00006 PubMed
89. Mattai A, Fung L, Bakalar J, et al. Adjunctive use of lithium carbonate for the management of neutropenia in clozapine-treated children. Hum Psychopharmacol. 2009;24(7):584-589. doi:10.1002/hup.1056 PubMed
90. Eggers C, Bunk D, Krause D. Schizophrenia with onset before the age of eleven: clinical characteristics of onset and course. J Autism Dev Disord. 2000;30(1):29-38. doi:10.1023/A:1005408010797 PubMed
91. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790-799. doi:10.1176/appi.ajp.163.5.790 PubMed
92. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146-154. doi:10.1001/archgenpsychiatry.2009.192 PubMed