Association of Hypnotics With Stroke Risk: A Population-Based Case-Control Study

ABSTRACT
Background: The aim of this study was to determine what association, if any, hypnotics have on the risk of stroke events.
Method: In a nationwide population-based case-control study, cases were patients with incident stroke diagnosed between January 1, 2006, and December 31, 2006. Patients with hemorrhagic or ischemic stroke diagnosis codes (ICD-9-CM codes 430-438) and who had been hospitalized for further treatment were included in the study. Patients with any type of stroke diagnosed before 2006 were excluded. The authors selected 2,779 stroke patients and 27,790 controls matched for age, gender, physician visit date, and comorbidities. The impact of hypnotics on stroke was examined by multiple logistic regression models and sensitivity analyses.
Results: Individuals prescribed any hypnotic had elevated risk of stroke compared to those prescribed no hypnotics. For groups prescribed 1-27, 28-148, and ≥ 149 pills, odds ratios for stroke were 1.71 (95% CI, 1.49-1.96), 1.84 (95% CI, 1.62-2.11), and 1.45 (95% CI, 1.26-1.68), respectively. Adjusted odds ratios were elevated in separate analyses for zolpidem and estazolam. The observed results were robust with stratification by comorbidities, such as hypertension and diabetes, and using ischemic stroke as the case group.
Conclusions: This study shows that, in a case-control study matched for age, gender, and comorbidities using multiple logistic regression and sensitivity tests, zolpidem and estazolam were slightly associated with an increased risk of stroke. Further large-scale and in-depth studies should be performed. Use of hypnotics should always be determined by specialists, and adverse effects should be continuously monitored.
Prim Care Companion CNS Disord 2014;16(2):doi:10.4088/PCC.13m01583
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: September 24, 2013; accepted November 27, 2013.
Published online: March 27, 2014.
Corresponding author: Frank Huang-Chih Chou, MD, PhD, Department of Community Psychiatry, Kai-Syuan Psychiatric Hospital, No. 130, Kai-Syuan 2nd Rd, Ling-Ya District, Kaohsiung 802, Taiwan, ROC ([email protected]).
The clinical use of sedatives and hypnotics has gradually increased in recent years, and a 53% growth rate of its prescription over a 5-year period was reported in 2006.1 Medications that are commonly used to treat insomnia include benzodiazepines, nonbenzodiazepines, γ-aminobutyric acid agonists, melatonin receptor agonists, sedating antidepressants, and antihistamines.2 The common adverse effects associated with the benzodiazepines and nonbenzodiazepines are residual daytime sedation, drowsiness, dizziness, cognitive impairment, motor incoordination, and dependence.3-5 One recent study, which included the frequently prescribed hypnotics zolpidem and temazepam, reported that hypnotics could be associated with the development of cancer and death, even at prescription levels of less than 18 doses per year over a 2.5-year duration.6 Other studies have also reported significant association of hypnotic usage with increased mortality.7-10 Only a few studies reported association between cardiovascular event/mortality and hypnotic usage, but the results were inconsistent.9,11-13 The impact of hypnotics on stroke risk has not been clearly elucidated.
There were insufficient data on the benzodiazepines commonly used for the treatment of insomnia such as estazolam, flurazepam, and triazolam or benzodiazepine-like hypnotics such as zolpidem that now dominate the market in many countries. Given the suggestive but limited data available on hypnotics and the possible risk of stroke, we decided to conduct a case-control study using the National Health Insurance Research Database in Taiwan, a unique nationwide, population-based claims data system with information on the medical history of all citizens.
METHOD
Ethics Statement
This study was initiated after approval by the Institutional Review Board of Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan. Since all identifying personal information was stripped from the secondary files before analysis, the review board waived the requirement for written informed consent from the patients involved.
Database
Taiwan implemented its National Health Insurance program, which provides compulsory universal health insurance, in 1995. The program enrolls up to 99% of citizens and has contracts with 97% of all medical providers. The database contains comprehensive information on insured subjects, including dates of clinical visits, diagnostic codes, and details of prescriptions and expenditures. This study used the Longitudinal Health Insurance Dataset for 2004-2006 released by the Taiwan National Health Research Institutes. The patients in this dataset did not statistically significantly differ from the larger cohort in age, gender, or health care costs, as reported by the Taiwan National Health Research Institutes.

- Zolpidem and estazolam were slightly associated with an increased risk of stroke, as determined by multiple logistic regression and sensitivity tests.
- Use of hypnotics should always be determined by specialists, and adverse effects should be continuously monitored.
Study Population
For this study, cases were patients with incident stroke diagnosed between January 1, 2006, and December 31, 2006. Stroke patients with hemorrhagic or ischemic stroke diagnosis codes (ICD-9-CM 430-438) and who had been hospitalized for further treatment were included in the study. Patients with any type of stroke diagnosed before 2006 were excluded.
Each stroke patient was matched with 10 controls from the Longitudinal Health Insurance Database between January 1, 2006, and December 31, 2006. The controls were matched to cases on the basis of propensity score, which, in turn, was derived from gender, age, comorbidities (hypertension, coronary artery disease, diabetes, hyperlipidemia, atrial fibrillation, and chronic kidney disease, anxiety and depression and associated antidepressants including sertraline, citalopram, fluoxetine, paroxetine, venlafaxine, duloxetine, amitriptyline, clomipramine, imipramine, and nortriptyline), and year of physician visit.14,15 An SAS macro (SAS Institute, Inc, Cary, North Carolina) was applied to implement greedy matching on the basis of propensity score. Individuals younger than 18 years and patients who had previously suffered a stroke were excluded. In the end, there were 2,779 stroke patients and 27,790 matched controls in our series.
Definition of Exposure and Covariate Adjustment
The dosage, date of prescription, duration, and total number of pills dispensed from the outpatient pharmacy prescription database were recorded. We focused on hypnotics approved by the US Food and Drug Administration (http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationfor
patientsandproviders/ucm101557.htm). Among these, zolpidem, estazolam, triazolam, and flurazepam are the most commonly prescribed drugs in Taiwan. The defined daily dose for zolpidem, estazolam, triazolam, and flurazepam was 10 mg, 3 mg, 0.25 mg, and 30 mg, respectively. To examine the dose-effect relationship, we categorized hypnotic use into 4 groups in our series (no hypnotics and equally distributed tertiles of hyponotics users).
The patients’ age, gender, comorbidities (history of hypertension, coronary artery disease, diabetes, hyperlipidemia, atrial fibrillation, and chronic kidney disease), monthly income level as a proxy of socioeconomic status, level of urbanization, and geographic region of residence were also recorded. The individuals were classified into 3 groups: (1) low socioeconomic status (lower than US $571 per month; new Taiwan dollar [NT] $20,000), (2) moderate socioeconomic status (between US $571 and $1,141 per month; NT $20,000-40,000), and (3) high socioeconomic status (US $1,142 per month; NT $40,001 or more).16 The geographic regions and urbanization of residence were classified as previously described.17,18
Statistical Analysis
SPSS version 15 (SPSS Inc, Chicago, Illinois) was used for data analysis. Pearson’s χ2 test was used for categorical variables, demographic characteristics (age group and gender), and comorbidities. The multiple logistic regression model was used to examine whether hypnotic use was an independent risk factor of stroke after adjusting for age, gender, comorbidities (history of hypertension, coronary artery disease, diabetes, hyperlipidemia, atrial fibrillation, and chronic kidney disease), level of urbanization, region of residence, and socioeconomic status. A P value < .05 was considered statistically significant.
Sensitivity Analyses
We evaluate whether the association between hypnotics and stroke could be due to underlying comorbidities with stratified analysis. Next, we further address the association between hypnotics and ischemic stroke with the procedures described previously.
RESULTS
Demographic Data
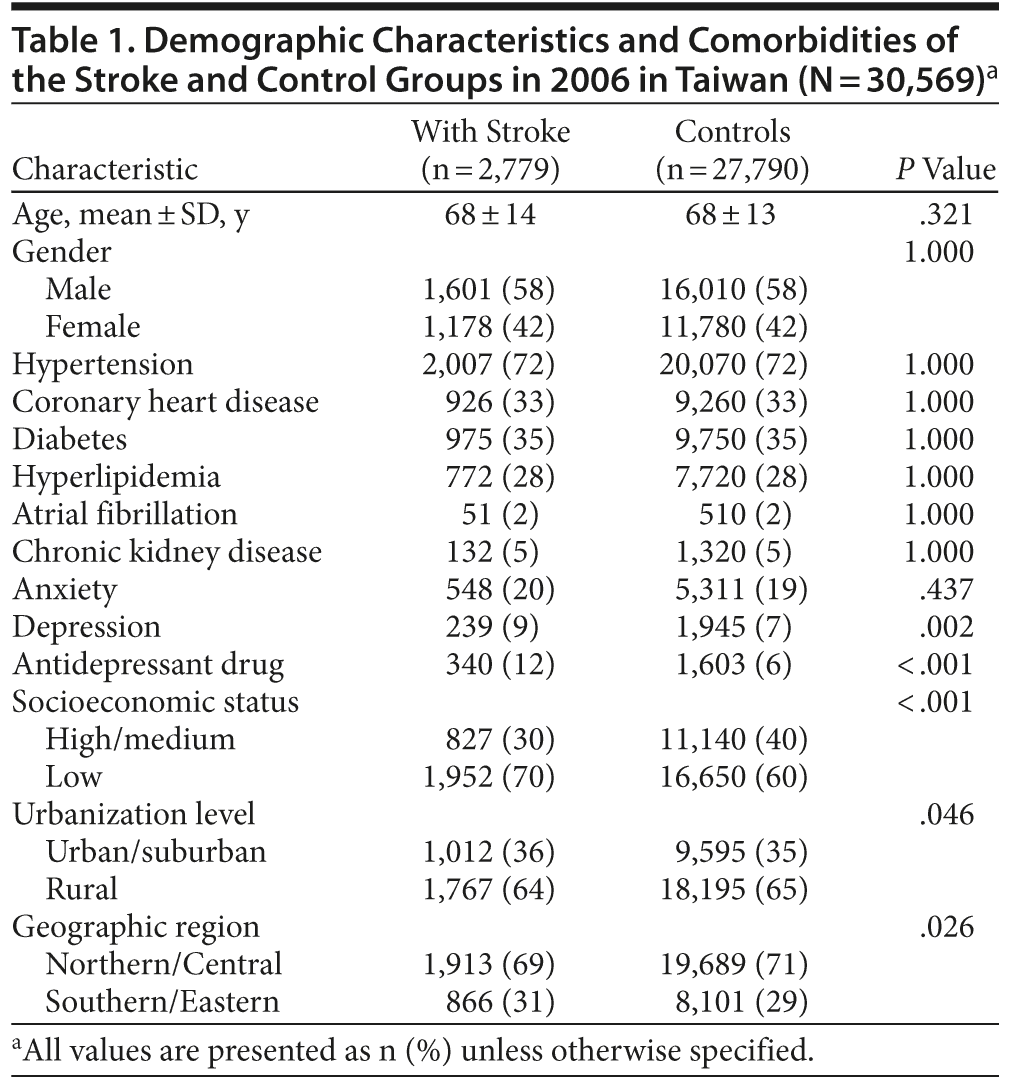
During 2006, patients with incident stroke (n = 2,779) and matched controls (27,790) for age, gender, comorbidities, and physician visit date were selected. The distribution of demographic characteristics and selected comorbidities between the 2 groups is shown in Table 1. There were significantly higher incidences of use of antidepressant drug and depression in the stroke group. The stroke group was more likely to be associated with low socioeconomic status.
Association Between Hypnotic Use and Stroke
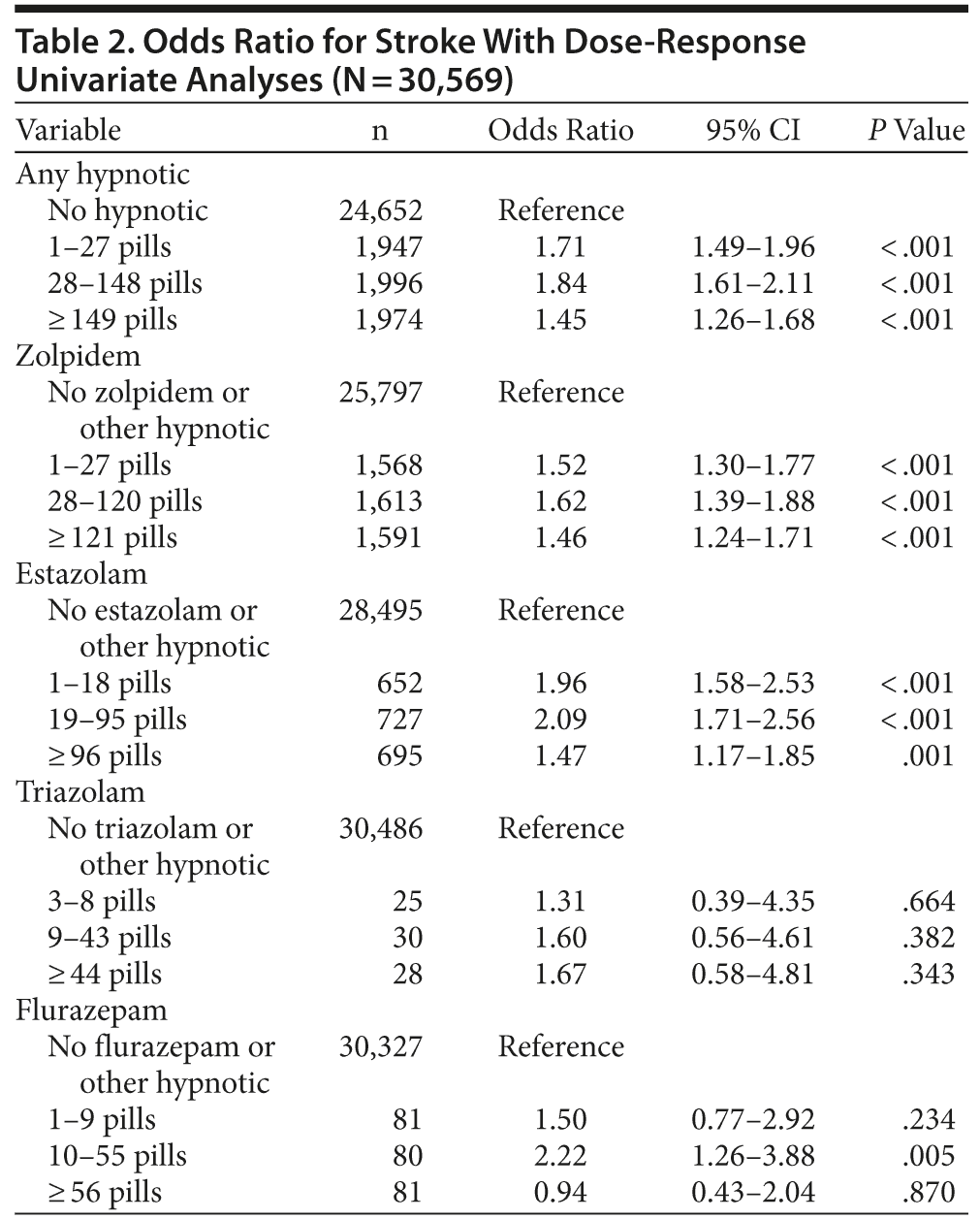
Univariate analysis determined that hypnotics were associated with increased stroke risk (Table 2). For groups prescribed 1-27, 28-148, and ≥ 149 pills, odds ratios for stroke were 1.71 (95% CI, 1.49-1.96), 1.84 (95% CI, 1.62-2.11), and 1.45 (95% CI, 1.26-1.68), respectively. Odds ratios for stroke were elevated for zolpidem and estazolam.
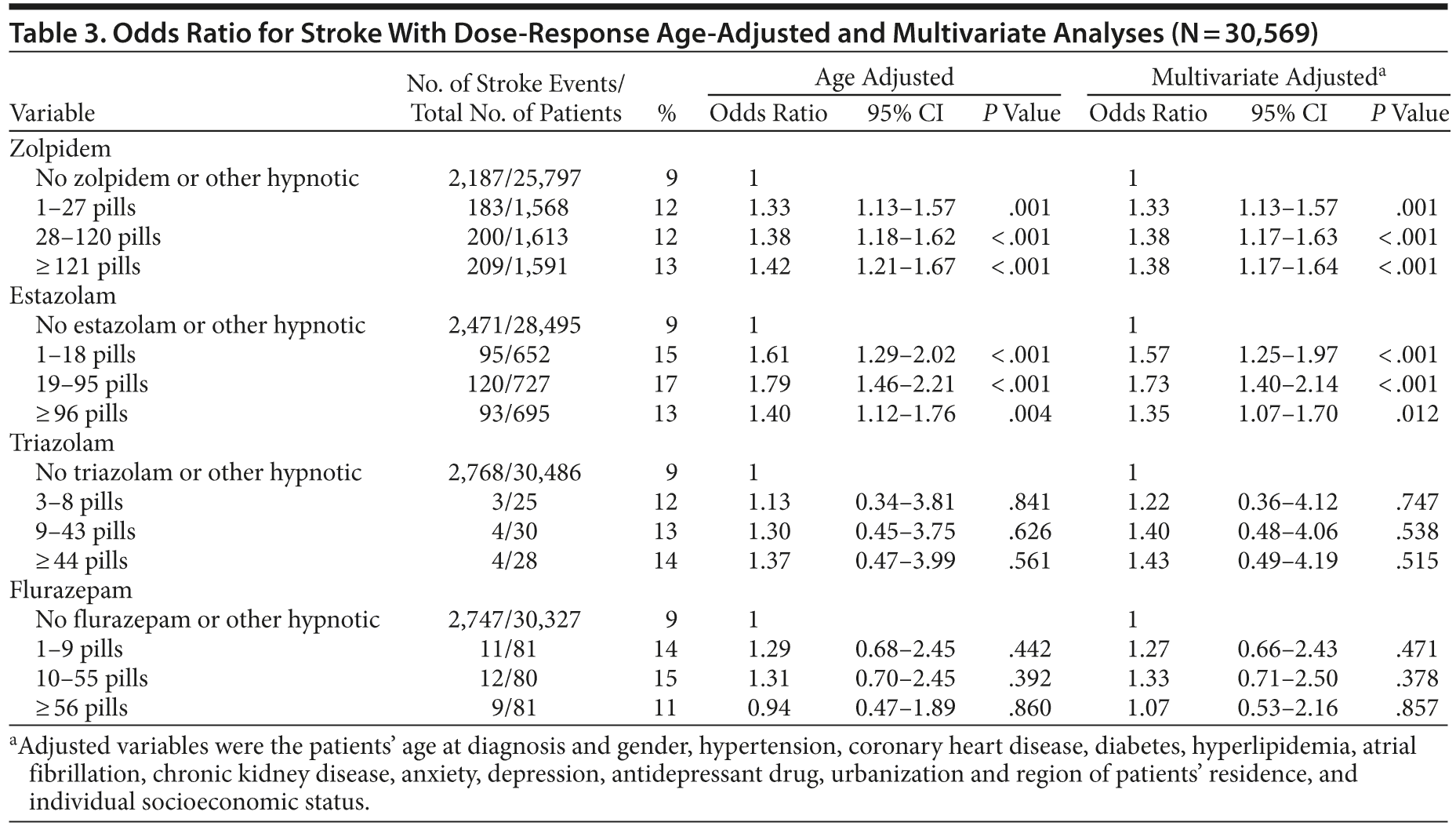
Patients prescribed hypnotics, such as zolpidem or estazolam, were associated with increased risk of stroke after adjustment of other factors. For the use of zolpidem, adjusted odds ratios for stroke were 1.33 (95% CI, 1.13-1.57), 1.38 (95% CI, 1.17-1.63), and 1.38 (95% CI, 1.17-1.64) for the lowest, medium, and highest tertile users compared with nonusers. For estazolam, adjusted odds ratios were 1.57 (95% CI, 1.25-1.97), 1.73 (95% CI, 1.40-2.14), and 1.35 (95% CI, 1.07-1.70) for the lowest, medium, and highest tertile users compared with nonusers. Use of triazolam and flurazepam was not statistically associated with elevated stroke risk (Table 3).
Sensitivity Analysis
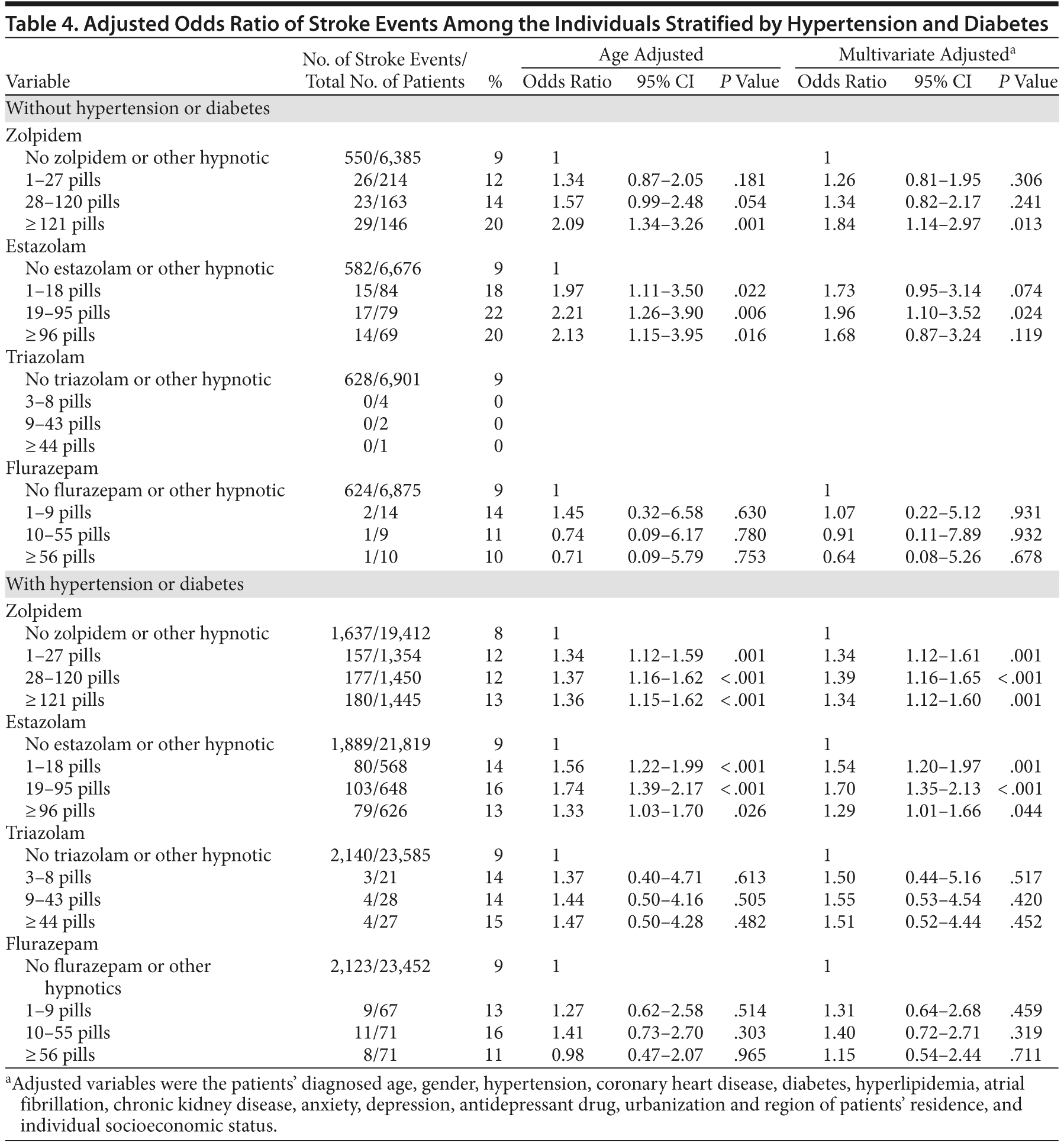
We evaluated whether the association between hypnotics and stroke could be altered due to underlying comorbidities such as hypertension and diabetes. We found that hypnotics, such as zolpidem and estazolam, were associated with increased stroke risk in individuals with or without hypertension or diabetes (Table 4).
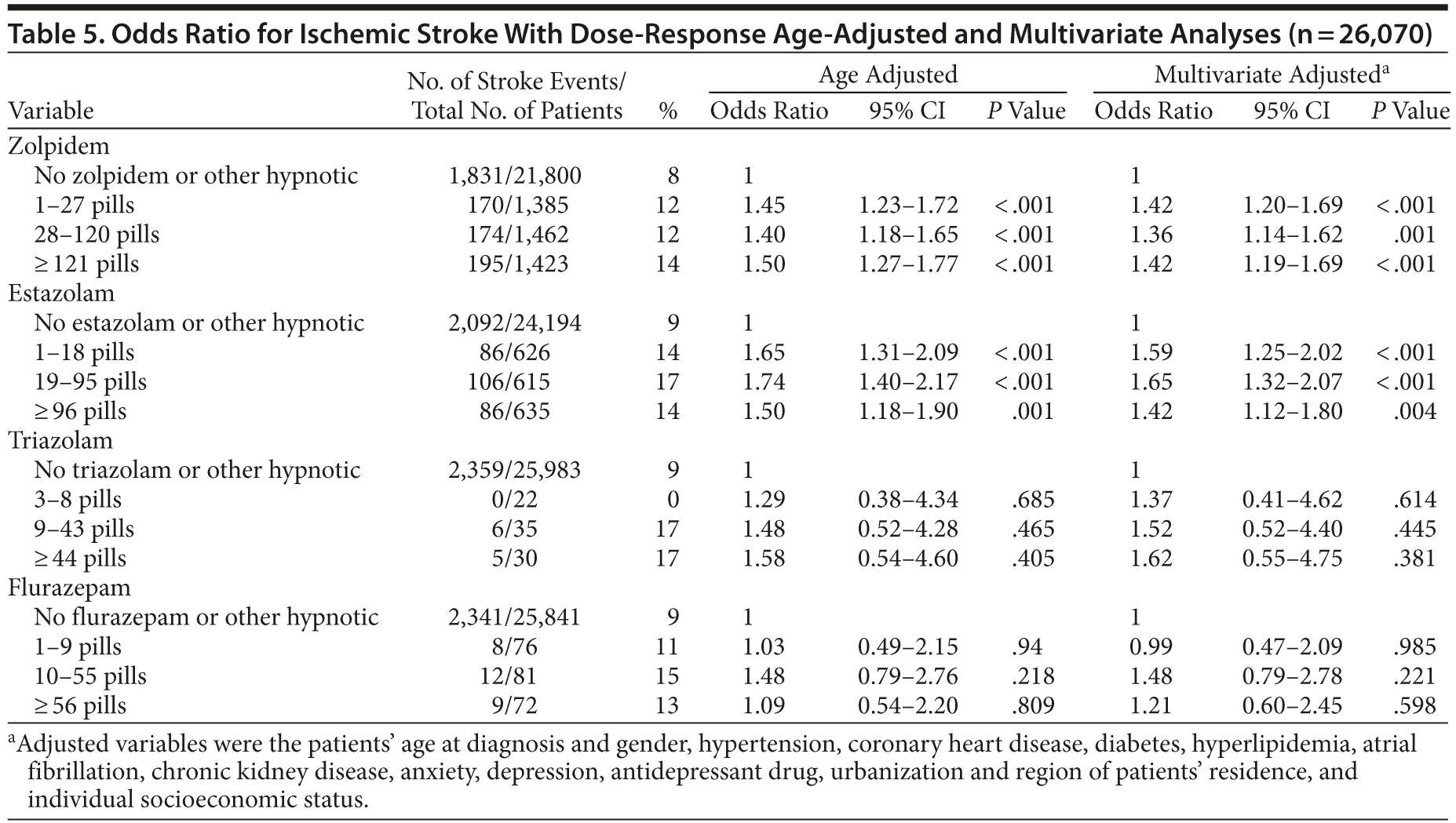
To further address the association between hypnotics and ischemic stroke, we conducted analyses with the above-mentioned procedures using patients with ischemic stroke (ICD-9-CM codes 433-438) as the case group and reselected the controls with 1:10 matching. After adjusting other factors, the ischemic stroke risk was evident for zolpidem and estazolam (Table 5).
DISCUSSION
Limited data exist regarding whether hypnotics increase the rate of cardiovascular events. Real-world data gathered through the day-to-day operations of medical establishments may provide the answer. Our study assessed the potential association between hypnotic use and the risk of stroke in a population-based case-control study. The likelihood of individuals treated with zolpidem or estazolam developing stroke events was statistically significant. Using sensitivity analysis, we evaluated whether the positive association between hypnotics and stroke could be due to underlying diseases such as hypertension and diabetes. We found that hypnotics remained an independent factor for stroke irrespective of a prior history of hypertension or diabetes.
The strengths of our study are that this was a large population-based case-control study (n = 30,569) with nearly complete follow-up information of any drug prescriptions among the whole study population (99%) and the National Health Insurance Bureau of Taiwan randomly reviews the charts of 1 per 100 ambulatory and 1 per 20 inpatient claimed cases and interviews patients to verify the accuracy of the diagnosis according to its Web site (http://nhird.nhri.org.tw/en/). Because the accuracy of records in the National Health Insurance Research Database for the diagnosis of ischemic stroke is as high as 95%, we further validated our study using ischemic stroke as the case control.19 After analyzing with the above-mentioned steps and adjusting other factors, the results were robust. Zolpidem and estazolam were associated with increased risk of ischemic stroke.
Hartz and Ross13 reported that the use of hypnotics is slightly associated with future poor health and mortality. The association of hypnotic use with stroke was insignificant after adjusting for the most highly confounded variables in the study. However, another result from the GAZEL cohort study12 found that taking sleep medication was associated with higher cardiovascular disease mortality. A main limitation of the above 2 studies is that the drugs included as hypnotics were not specifically identified. Studies have shown that the use of over-the-counter sleep aids is common, which usually contain diphenhydramine or a combination of diphenhydramine plus pain relievers.20,21 The question “Did you take any medication to help sleep?” did not necessarily refer to true hypnotics in those studies. In our study, we reported specific hypnotics and quantities of hypnotic drugs when available. Since the data were collected from a database, they were not affected by recall or reporting bias.
Randomized, controlled trials cannot be undertaken in all situations in which evidence is needed to provide treatment guidelines. Observational studies with adequate statistical analysis and a low level of bias are necessary to evaluate population effectiveness. Postmarketing surveillance is an important issue that could provide physicians, patients, and pharmaceutical companies with useful information about severe adverse effects. The National Health Insurance Research Database in Taiwan provides the opportunity for outcomes and health service research. In this case-control study, multiple logistic regression with subgroups analyses were used in an effort to eliminate selection bias for observable factors; it was subsequently revealed that zolpidem or estazolam was associated with increased stroke risk. However, the association does not necessarily imply a causal relationship. It is possible that higher-risk patients take more hypnotics, and it is this higher risk rather than the hypnotics that contributes to stroke. Although we corrected most medical and psychiatric comorbidities, the severity of the comorbidities was not available. As with all sleep disorders, a thorough sleep history is required to evaluate and make the diagnosis of insomnia.
There are several limitations to this study. First, diagnoses of stroke and any other comorbid conditions were completely dependent on ICD-9-CM codes. However, the National Health Insurance Bureau of Taiwan conducts randomized reviews of the charts and interviews patients to verify the accuracy of these diagnoses. Hospitals with outlier charges or practices are subject to auditing, and heavy penalties are levied in the event that malpractice or discrepancies are found. Furthermore, the accuracy of the National Health Insurance Research Database when recording the diagnosis of ischemic stroke is as high as 95%.19 Second, the database does not contain information on smoking, dietary habits, and body mass index, which may also be risk factors for vascular events. Third, detailed information on strokes cannot be precisely extracted from ICD-9-CM codes, which prevents subsequent subgroup analysis. Further studies linking administrative data and primary hospitalization information are warranted.
CONCLUSION
This study shows that, in a case-control study matched for age, gender, and comorbidities using multiple logistic regression and sensitivity tests, zolpidem and estazolam were slightly associated with an increased risk of stroke. Further large-scale and in-depth studies should be performed. Use of hypnotics should always be determined by specialists, and adverse effects should be continuously monitored.
Drug names: citalopram (Celexa and others), clomipramine (Anafranil and others), diphenhydramine (Benadryl and others), duloxetine (Cymbalta), fluoxetine (Prozac and others), flurazepam (Dalmane and others), imipramine (Tofranil and others), nortriptyline (Pamelor, Aventyl, and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), temazepam (Restoril and others), triazolam (Halcion and others), venlafaxine (Effexor and others), zolpidem (Ambien, Edluar, and others).
Author affiliations: Department of Otolaryngology (Dr Lee), Department of Pediatrics (Dr Hung), and Division of Neurology, Department of Internal Medicine (Dr Huang), Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan; Community Medicine Research Center and Institute of Public Health, National Yang-Ming University, Taipei, Taiwan (Dr Lee); Department of Community Psychiatry, Kai-Syuan Psychiatric Hospital, Kaohsiung City, Taiwan (Drs Lee, Tsai, and Chou); School of Medicine, Tzu Chi University, Hualian, Taiwan (Drs Lee and Hung); and Graduate Institute of Health Care (Dr Chou) and Department of Nursing (Drs Chou and Tsai), Meiho University, Ping-Tong County, Taiwan.
Author contributions: All authors contributed equally to the manuscript.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Disclaimer: The interpretation and conclusions contained herein do not represent those of the aforementioned institutions.
Additional information: This study is based in part on data from the National Health Insurance Research Database (NHIRD; http://nhird.nhri.org.tw/en/) provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes, Taiwan (Serial No. 100363). Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply for the NHIRD. The use of NHIRD is limited to research purposes only.
REFERENCES
1. Mooallem J. The sleep-industrial complex. NY Times Magazine; November 18, 2007.
2. Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. 5th ed. St Louis, MO: Elsevier; 2010:483-491.
3. Nowell PD, Mazumdar S, Buysse DJ, et al. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278(24):2170-2177. PubMed doi:10.1001/jama.1997.03550240060035
4. Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225-233. PubMed
5. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335-1350. PubMed doi:10.1007/s11606-007-0251-z
6. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. PubMed doi:10.1136/bmjopen-2012-000850
7. Kripke DF, Klauber MR, Wingard DL, et al. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43(9):687-693. PubMed doi:10.1016/S0006-3223(97)00292-8
8. Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Saf. 2007;16(8):913-918. PubMed doi:10.1002/pds.1417
9. Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10(3):279-286. PubMed doi:10.1016/j.sleep.2008.12.004
10. Belleville G. Mortality hazard associated with anxiolytic and hypnotic drug use in the National Population Health Survey. Can J Psychiatry. 2010;55(9):558-567. PubMed
11. Merlo J, Hedblad B, Ogren M, et al. Increased risk of ischemic heart disease mortality in elderly men using anxiolytics-hypnotics and analgesics: results of the 10-year follow-up of the prospective population study “Men born in 1914,” Malmo, Sweden. Eur J Clin Pharmacol. 1996;49(4):261-265. PubMed doi:10.1007/BF00226325
12. Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: results from the GAZEL cohort study. Am J Epidemiol. 2011;173(3):300-309. PubMed doi:10.1093/aje/kwq371
13. Hartz A, Ross JJ. Cohort study of the association of hypnotic use with mortality in postmenopausal women. BMJ Open. 2012;2(5):e001413. PubMed doi:10.1136/bmjopen-2012-001413
14. Kennedy SH. A review of antidepressant therapy in primary care: current practices and future directions. Prim Care Companion CNS Disord. 2013;15(2):doi:10.4088/PCC.12r01420. doi:10.4088/PCC.12r01420 PubMed
15. Vis R, Hassink JJ, Vinkers CH. Tricyclic antidepressant plasma levels in depression: a practical guide [article in Dutch]. Tijdschr Psychiatr. 2013;55(9):695-705. PubMed
16. Chou FH, Tsai KY, Su CY, et al. The incidence and relative risk factors for developing cancer among patients with schizophrenia: a nine-year follow-up study. Schizophr Res. 2011;129(2-3):97-103. PubMed doi:10.1016/j.schres.2011.02.018
17. Lee CC, Su YC, Ho HC, et al. Risk of stroke in patients hospitalized for isolated vertigo: a four-year follow-up study. Stroke. 2011;42(1):48-52. PubMed doi:10.1161/STROKEAHA.110.597070
18. Chang CM, Huang KY, Hsu TW, et al. Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: a nationwide population-based study in Taiwan. PLoS ONE. 2012;7(7):e40590. PubMed doi:10.1371/journal.pone.0040590
19. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
20. Johnson EO, Roehrs T, Roth T, et al. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21(2):178-186. PubMed
21. Roehrs T, Hollebeek E, Drake C, et al. Substance use for insomnia in metropolitan Detroit. J Psychosom Res. 2002;53(1):571-576. PubMed doi:10.1016/S0022-3999(02)00448-8