Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Hyperkinetic movement disorder is a rare and transient complication of nonketotic hyperglycemia. The typical description consists of acute-onset unilateral or bilateral involuntary movements seen in the wake of uncontrolled hyperglycemia; accompanied with computed tomography (CT) or magnetic resonance imaging (MRI) changes in basal ganglia. The movement disorder consists of a combination of chorea, athetosis, and ballismus.
Alcohol Dependence, Hypoglycemia, and Transient Movement Disorders
To the Editor: Hyperkinetic movement disorder is a rare and transient complication of nonketotic hyperglycemia. The typical description consists of acute-onset unilateral or bilateral involuntary movements seen in the wake of uncontrolled hyperglycemia; accompanied with computed tomography (CT) or magnetic resonance imaging (MRI) changes in basal ganglia. The movement disorder consists of a combination of chorea, athetosis, and ballismus. The exact pathophysiology of this syndrome is unknown.1
We report 2 cases of acute-onset movement disorder after an episode of neuroglycopenia in alcohol-dependent patients with no evidence of preceding hyperglycemia. Both of these cases presented to a tertiary addiction medicine center. These atypical cases may shed light on the pathophysiology of movement disorder associated with severe perturbations of blood glucose.
Case report 1. A 42-year-old man presented with a history of alcohol dependence since the age of 27 years. In the last 6 months, he was consuming 120-140 g of ethanol every day. He also had nicotine dependence since the age of 16 years. History and medical records did not suggest diabetes, hypertension, coronary artery disease, or hepatic decompensation.
He had been found unconscious in bed by his family members one morning and was rushed to the hospital. He had consumed an unknown quantity of alcohol the preceding night. The family reported decreased food intake for the past 2 to 3 months, and they were not sure if he had eaten anything at all in the last 24 hours.
On examination, we found tachycardia (110 beats/min), supine hypotension (100/60 mm Hg), spontaneous regular breathing (14 breaths/min), and bilateral dilated but responsive pupils. The patient did not respond to verbal or tactile stimuli but did to localized painful stimuli (sternal rub). Pulse oximetry revealed 97% oxygen saturation and capillary blood glucose (CBG) was 14 mg/dL (normal range, 80-160 mg/dL). We made a diagnosis of neuroglycopenia (ICD-10-CM); he was resuscitated with 75 mL of 20% dextrose given intravenously over 10 minutes. Before this intervention, he received 100 mg of thiamine intramuscularly. Concurrently, 500 mg of thiamine diluted in 100 mL of isotonic saline was infused intravenously. The patient regained normal sensorium within 15 minutes—CBG was noted to be 120 mg/dL and 100 mg/dL at the end of infusion and 30 minutes later, respectively. He reported that he had not had food due to dyspepsia for the last 72 hours and had consumed approximately 140 g of alcohol the previous night. He had no recollection of events after he went to sleep. He denied using any medications, ruling out the possibility of an accidental or intentional overdose of oral hypoglycemic agents. Physical examination revealed malnutrition (body mass index was 15.1 kg/m2). He had dry scaly skin, cheilitis, and atrophic glossitis ("bald tongue") indicating multiple vitamin deficiencies. The patient had no focal neurologic deficits. Laboratory investigations done at the time of presentation included serum sodium, chloride, potassium, calcium, phosphate, creatinine, urea, albumin, bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) concentration, all of which were within normal limits, while γ-glutamyl transferase (GGT) was 217 U/L, which is higher than normal (36 U/L). Hemogram results revealed no significant abnormalities. The HbA1c fraction was 5.1%, indicating the absence of undiagnosed diabetes mellitus (normal range, 4%-5.6%). Urine drug screening was negative for amphetamines, cocaine, benzodiazepines, opioids, and barbiturates. The patient was advised to stay for inpatient care, but he opted to come for evaluation the following day instead.
Approximately 12 hours after resolution of the hypoglycemia, the patient reported an abrupt onset of involuntary movements of both hands. He was brought back to the hospital, where, on examination, he was found to have continuous flowing movements of both upper limbs interspersed with high amplitude flailing movements. He had eaten a bowl of rice broth in the last 12 hours, and his CBG was 156 mg/dL. Over the next 2 hours, he developed dyskinetic movements of neck, tongue, and toes. The patient was visibly exhausted and agitated but oriented at this time. He received 10 mg of haloperidol intravenously with minimal improvement. Four hours later, he was noted to be increasingly agitated, disoriented, and incoherent. He also developed hypoglycemia (CBG = 50 mg/dL), which was promptly treated. Even after correction of hypoglycemia, he continued to have autonomic arousal and was gesturing as if in an animated conversation with a friend, lighting a cigarette, or picking some insects from the bed sheet. A provisional ICD-10-CM diagnosis of delirium tremens and hyperkinetic movement disorder was made, and he received 100 mg of intravenous diazepam over the next 6 hours to achieve mild sedation. In the next 48 hours, abnormal movements disappeared completely, and there were no cognitive or motor residual deficits. He also received Ringer lactate and dextrose infusions with hourly monitoring of CBG. A diagnostic workup for acute-onset chorea and hypoglycemia was undertaken.
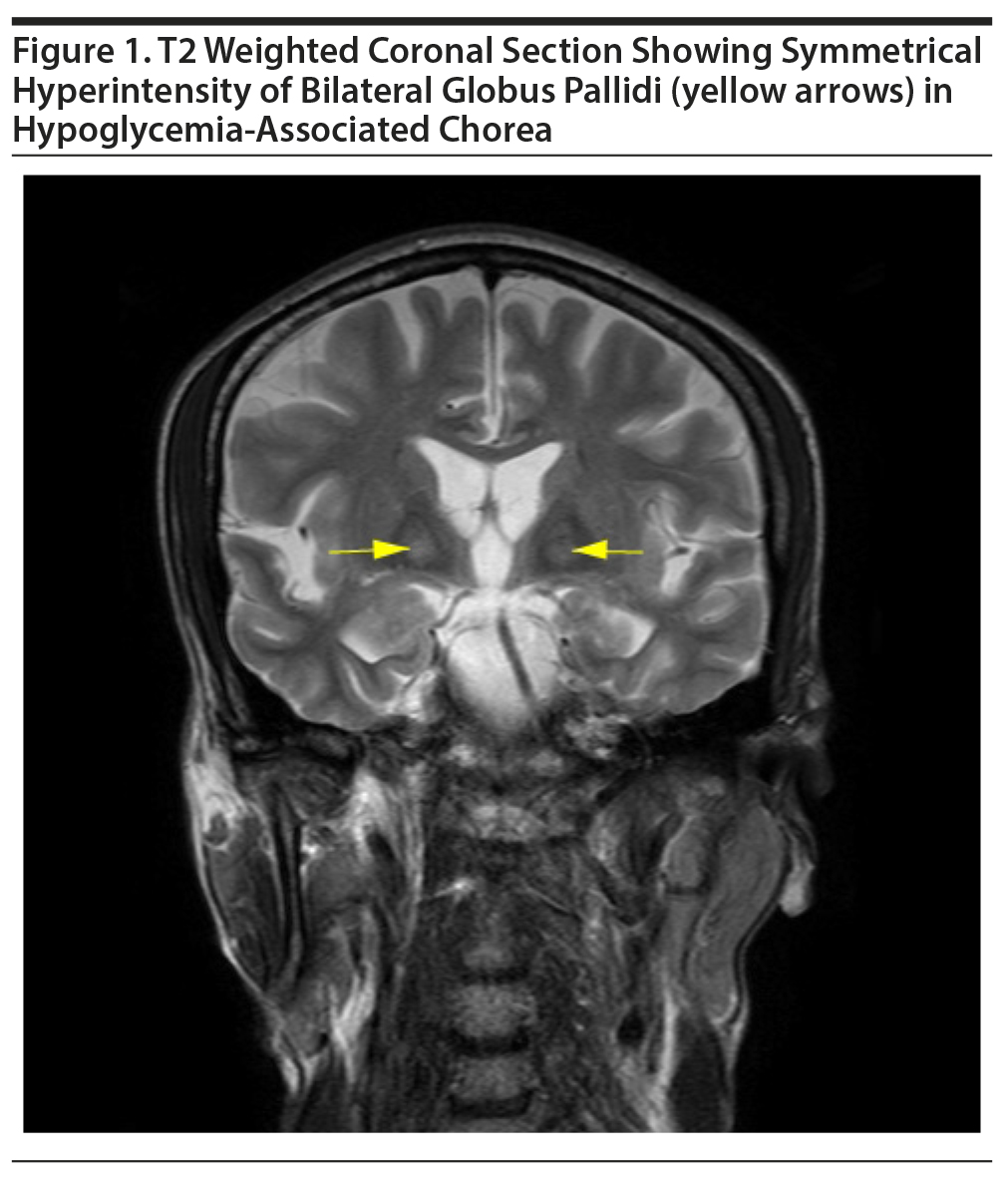
Three-generation family history revealed no history of movement disorder, and the patient had not been exposed to neuroleptics or antiemetics. There was no preceding history of joint pains, skin lesions, or renal diseases. Magnetic resonance imaging (MRI) of the brain revealed bilateral symmetrical T2 and FLAIR (fluid-attenuated inversion recovery) hyperintensities in globus pallidi without diffusion restriction or postcontrast enhancement (Figure 1). Slit lamp examination revealed no Kayser-Fleischer ring, and the saline dilution test result for acanthocytes was negative. Erythrocyte sedimentation rate (ESR) was within normal limits and the antinuclear antibody (ANA) was negative. No sexually transmitted infections (STI) were found. Regular monitoring of fasting and postprandial blood glucose revealed normal insulin levels and normoglycemia, respectively, and peptide-C levels were also within normal limits. The patient was observed in the hospital for the next 2 weeks while he received treatment for alcohol addiction. During the next 6 months, the patient experienced no further episodes of hypoglycemia or involuntary movements.
Case report 2. A 63-year-old woman presented with a history of alcohol dependence since the age of 43 years with an average consumption of 40-60 g of alcohol every day. The reason for current consultation was abnormal movements of the left upper limb for the last week and alcohol dependence. The patient reported that 10 days prior to the current consultation, her family members had found her unconscious and taken her to a hospital. The hospital records show that the patient had a low CBG (20 mg/dL) and recovered completely after dextrose infusion. The abnormal movements were noticed 2 days after this episode. Although, the intensity of the movements had decreased with no treatment in the last 7 days, the patient continued alcohol consumption and was brought in for treatment. Medical records revealed that she was diagnosed with diabetes mellitus (DM) 2 years back and was regularly taking 500 mg of metformin and 1 mg of glimepiride twice a day. Available medical records revealed acceptable control of fasting and postprandial blood glucose; HbA1c fraction was 6.5% and 6.3% in the preceding 3 months and 6 months, respectively.
On examination, the patient had choreiform movements of both upper limbs, more pronounced on the left side. The patient also had infrequent large amplitude movements of her left upper limb and facial grimacing. There were no focal neurologic or cognitive deficits. Investigations done at the time of presentation revealed the following: serum sodium, chloride, potassium, calcium, phosphate, creatinine, urea, albumin, bilirubin, AST, and ALT concentration were within normal limits while GGT was higher than normal (98 U/L). Fasting and postprandial blood glucose on 3 consecutive days were within normal limits with the mentioned antidiabetic medication. HbA1c was 6.5% at the time of presentation. Hemogram revealed hypochromic microcytic anemia (hemoglobin = 11 g/dL). Diagnostic evaluation for acute-onset chorea (ICD-10-CM) in adults, similar to Case 1, was undertaken.
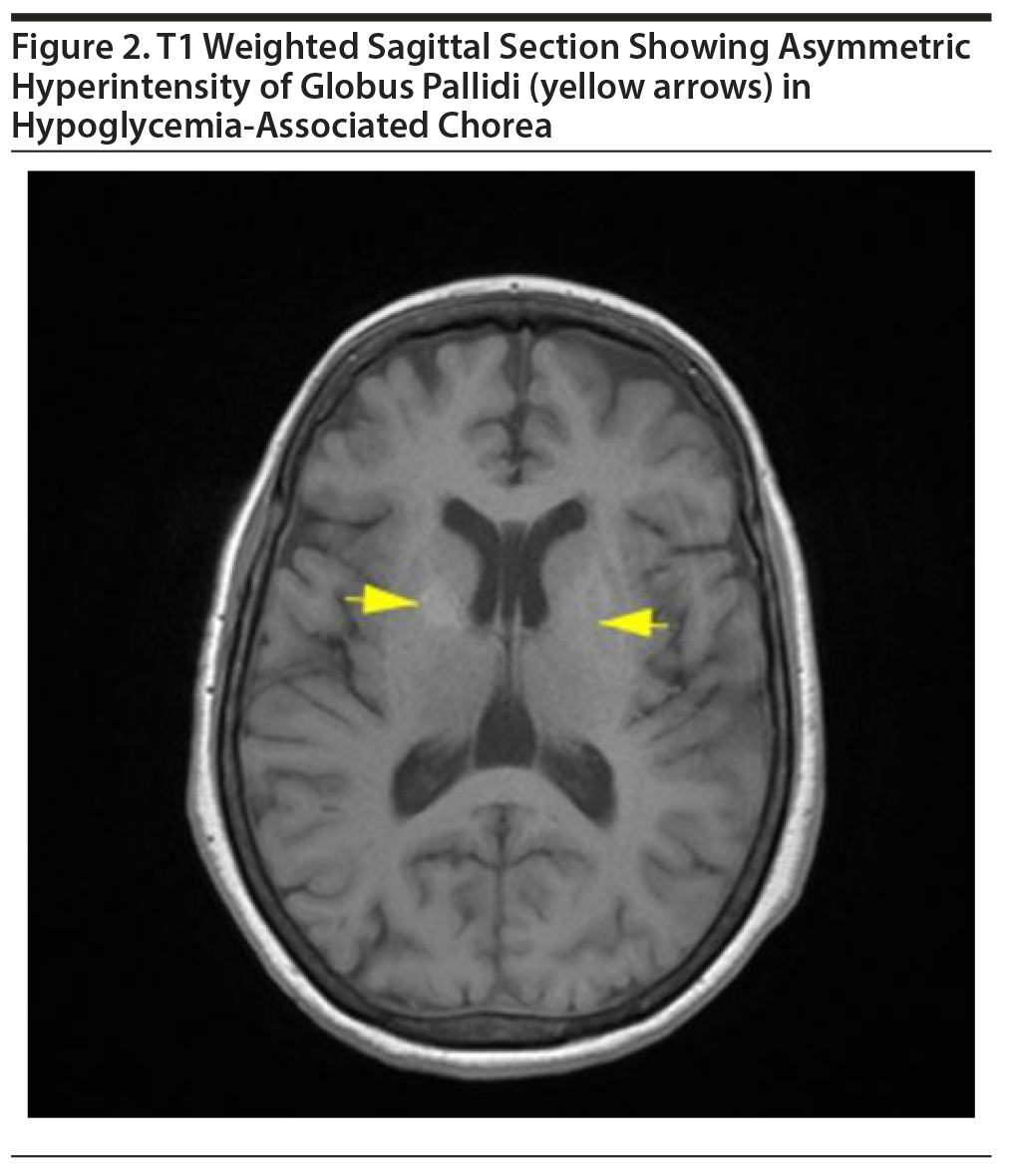
MRI of the brain revealed asymmetric (right more than left) T1 hyperintensities in globus pallidi and minimal involvement of putamen with normal intensity in T2 and diffusion-weighted imaging (Figure 2). Also, a gliotic area was noted in right middle frontal gyrus along with T2 and FLAIR hyperintensities in the subcortical white matter. ANA and STI results were negative, and ESR was within normal limits. The patient received a tapering dose of diazepam (dose on first day = 40 mg) for 6 days. She was observed as an inpatient for the next 2 weeks, and the abnormal movements disappeared completely by the 5th day. This woman remained under follow-up for the next 3 months, and there has been no recurrence.
We have presented 2 patients suffering from alcohol dependence syndrome (ICD-10-CM) who developed an acute-onset, transient hyperkinetic movement disorder after resuscitation from a neuroglycopenic episode. The purpose of discussing these cases is 2-fold. First, they illustrate the diagnostic process of acute-onset transient movement disorder. Second, they may provide pathophysiologic insights in the link between severe perturbations of blood glucose and basal ganglia lesions.
Chorea is associated with a dauntingly large number of medical, neurologic, and psychogenic etiologies.2 However, we could narrow this list based on a key factor: both patients had an acute-onset nonprogressive chorea that favors an acquired over genetic (hereditary or sporadic) cause.2 Among the acquired causes of chorea in adults, stroke, metabolic causes, and autoimmune illness are associated with an acute onset.3 We excluded autoimmune illness, Wilson disease, neuroacanthocytosis, polycythemia, human immunodeficiency virus (HIV), and syphilis in both of the cases. Stroke was ruled out based on MRI findings and lack of focal neurologic deficits. Medical history and urine drug testing ruled out tardive dyskinesia and stimulant-induced movement disorder, respectively, which can present with chorea. Finally, temporal correlation with neuroglycopenia in these cases points toward a metabolic cause.
Oh et al1 have suggested a syndrome of chorea, hyperglycemia, and basal ganglia lesions (CHBG). A typical patient is described as an elderly (mean age = 71.7 years) woman (female-to-male ratio = 30/17) of Asian descent with poorly controlled DM (mean HbA1c = 14.4%), hyperglycemia (mean blood glucose = 481.5 mg/dL), and hyperosmolar state (mean serum osmolarity = 305.9 mOsm/L) at the time of presentation.1 In our first case, no biochemical evidence of DM or hyperglycemia was found. The second case is similar to the profile of CHBG, but evidence of hyperglycemia severe enough to cause hyperosmolarity is absent. We infer this on the basis of HbA1c values in the past year in which the highest value of 6.5% corresponds to a mean blood glucose of 140 mg/dL.4 Notably, there are previous reports1 of chorea that occurs in the wake of hypoglycemia and rapid correction of hypoglycemia. It is possible that in case 2, hypoglycemia was overcorrected to the extent of causing brief lasting hyperosmolar hyperglycemia.
Basal ganglia are particularly susceptible to glucose deprivation due to high metabolic activity.5 During neuroglycopenia, brain cells rapidly switch to alternate substrates like lactate and glutamate.6 Glutamate, in particular, is also a precursor of γ-aminobutyric acid (GABA), and during neuroglycopenia, glutamate may be preferentially shunted to the Krebs cycle.7 It is proposed that decreased GABA-mediated inhibition of the thalamus leads to hyperkinetic movements.8 It is worth noting that chronic exposure to alcohol alters GABA and glutamate neurotransmission and may thus increase the susceptibility to hypoglycemia-related basal ganglia dysfunction,which may also explain the prompt resolution of symptoms with diazepam in both of our patients. To summarize, we propose that chronic alcohol exposure predisposed these 2 patients to a reversible metabolic lesion of basal ganglia due to neuroglycopenia.
References
1. Oh SH, Lee KY, Im JH, et al. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200(1-2):57-62. PubMed CrossRef
2. Wild EJ, Tabrizi SJ. The differential diagnosis of chorea. Pract Neurol. 2007;7(6):360-373. PubMed CrossRef
3. Walker RH. An approach to the patient with chorea. International Parkinson and Movement Disorder Society website. https://www.movementdisorders.org/MDS/News/Online-Web-Edition/Archived-Editions/An-approach-to-the-patient-with-chorea.htm. Aug/Sep 2010. Accessed October 24, 2018.
4. Nathan DM, Kuenen J, Borg R, et al; A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478. PubMed CrossRef
5. Fujioka M, Okuchi K, Hiramatsu KI, et al. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28(3):584-587. PubMed CrossRef
6. Rooijackers HMM, Wiegers EC, Tack CJ, et al. Brain glucose metabolism during hypoglycemia in type 1 diabetes: insights from functional and metabolic neuroimaging studies. Cell Mol Life Sci. 2016;73(4):705-722. PubMed CrossRef
7. McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85(15):3347-3358. PubMed CrossRef
8. Kim JS, Lee KS, Lee KH, et al. Evidence of thalamic disinhibition in patients with hemichorea: semiquantitative analysis using SPECT. J Neurol Neurosurg Psychiatry. 2002;72(3):329-333. PubMed CrossRef
aCentre for Addiction Medicine, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
bDepartment of Psychiatry, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: The patient provided consent to publish this case, and information has been de-identified to protect anonymity.
Published online: January 3, 2019.
Prim Care Companion CNS Disord 2019;21(1):18l02308
To cite: Shukla L, Reddy S, Kulkarni G, et al. Alcohol-dependence, hypoglycemia, and transient movement disorders. Prim Care Companion CNS Disord. 2019;21(1):18l02308.
To share: https://doi.org/10.4088/PCC.18l02308
© Copyright 2019 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.