Effects of Milnacipran on Neurocognition, Pain, and Fatigue in Fibromyalgia: A 13-Week, Randomized, Placebo-Controlled, Crossover Trial

ABSTRACT
Objective: To investigate whether milnacipran is safe and effective in improving cognitive function in patients with fibromyalgia.
Method: Patients were randomly assigned to receive milnacipran or placebo for 6 weeks, followed by a 1-week washout and then crossover to the other arm for another 6 weeks. The overall trial lasted 13 weeks and was conducted between July 2011 and May 2013. Assessments were performed at each visit. Neurocognition was measured by the Brief Assessment of Cognition (BAC) and MATRICS. Pain was assessed by the visual analog scale (VAS) for pain. Global assessment of fibromyalgia symptoms was measured by the Fibromyalgia Impact Questionnaire (FIQ) and tender point examination. Depression was assessed by the Beck Depression Inventory (BDI). Fatigue was assessed by the Fatigue Severity Scale. Functional outcome was evaluated by the Health Assessment Questionnaire. The Clinical Global Impressions-Severity of Illness (CGI-S) and Improvement (CGI-I) scales and the Patients Clinical Global Impression of Change were used to measure the global impression of severity and improvement.
Results: 26 subjects were screened, and 20 subjects completed the trial. The change in verbal memory (P = .001) and the composite T score (P = .044) of the BAC and the change in the attention-vigilance domain T score (P = .042) were significantly improved, but there were no differences between the drug and placebo groups. The changes in the CGI-S scores were not significant, but the changes in the Clinical Impression-Improvement (CGI-I) scores showed worsening in the placebo group at week 1 (P = .032), week 2 (P = .024), week 4 (P = .024), and week 6 (P = .60) compared to baseline. The change in FIQ scores was not significant.
Conclusions: Milnacipran may have a potential role in the improvement of pain, disability, and mood. The effect of milnacipran on cognition in fibromyalgia needs further research.
Trial Registration: ClinicalTrials.gov identifier: NCT01829243
Prim Care Companion CNS Disord
2013;15(6):doi:10.4088/PCC.13m01555
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: July 10, 2013; accepted September 4, 2013.
Published online: December 26, 2013.
Corresponding author: Ashwin A. Patkar, MD, Department of Psychiatry, Duke University Medical Center, 2218 Elder St, Ste 127, Durham, NC 27705 ([email protected]).
It is known that patients with fibromyalgia often have memory and cognitive complaints in addition to widespread pain and fatigue. This is commonly termed fibro fog. Patients report that they have trouble remembering things, process information less efficiently, have trouble performing well in demanding jobs, and find complex mental tasks to be very tiring. Cognitive dysfunction is observed in fibromyalgia, especially for episodic memory, learning, and working memory.1 There is evidence for dysregulation of the attention system, from low-level sensory processes up to emotional processes, and increased sensitivity to distraction.1 Neuroimaging evidence shows differences between patients and healthy controls, with a pattern of increased cerebral activation in patients trying to attain the same performance level as controls.2 In patients with fibromyalgia, event-related potential studies show a reduction in focused cognitive effort (ie, reduced p300 amplitude). Symptoms central to each disorder are frequently correlated with the degree of cognitive dysfunction.2 Thus, higher fatigue in chronic fatigue syndrome and higher pain in fibromyalgia are related to cognitive function.
Cognitive dysfunction in fibromyalgia is associated with greater impairment in functioning and quality of life.3 An open-label, controlled study showed that milnacipran improved cognitive dysfunction and mood in poststroke patients compared to controls.4 In prior studies, milnacipran was effective at ameliorating impaired cognitive function in healthy volunteers aged > 65 years and depressed patients following traumatic brain injury.5 The pharmacologic action of milnacipran, normalizing both serotonin and norepinephrine systems, may be beneficial.6 Unlike venlafaxine and duloxetine, milnacipran is the only dual reuptake inhibitor with more norepinephrine than serotonin (5-HT) activity, and it is therefore considered to be part of a new class of agents known as norepinephrine-serotonin reuptake inhibitors. Milnacipran’s balance of norepinephrine to 5-HT is 3:1, similar to amitriptyline, a tricyclic antidepressant that has demonstrated efficacy in fibromyalgia, as compared to venlafaxine, which has a norepinephrine: 5-HT balance of 1:30, or duloxetine, which has a balance of 1:10.7 In addition, because of milnacipran’s effect on 5-HT, it should also be effective in treating other symptoms associated with fibromyalgia such as sleep disturbances and mood changes, as well as other functional somatic syndromes. It is worth noting that several medications used to treat fibromyalgia are sedating (eg, pregabalin, opioids, muscle relaxants) and impair neurocognition.
Pain and fatigue are consistently rated by patients as the most disabling symptoms of fibromyalgia.8 The pathophysiology of pain seems to be related to disturbances in central processing of pain and disturbances in neurotransmitter systems. Both pain and fatigue adversely impact the quality of life in fibromyalgia patients.
The efficacy of milnacipran is similar to amitriptyline and imipramine; however, milnacipran has less anticholinergic adverse effects than both of these tricyclic antidepressants. Results of meta-analysis indicate that milnacipran is more efficacious than the selective serotonin reuptake inhibitors (SSRIs) in terms of efficacy and that both are equally well tolerated for the treatment of major depression.9 Furthermore, milnacipran has minimal hepatic metabolism, does not inhibit any cytochrome P450 isoenzyme subtypes, and does not give rise to an active metabolite. These findings suggest that there is a lower risk of drug-drug interactions with milnacipran compared to conventional antidepressants or SSRIs. As many patients with fibromyalgia take other medications, such as analgesics that are metabolized through the CYP450 system, the characteristics of milnacipran suggest that it is likely to be advantageous for such patients.
This study was designed to investigate whether milnacipran is safe and effective in improving cognitive function in fibromyalgia. In addition, this study aimed to investigate whether improvement in neurocognitive status due to milnacipran correlates with improvements in pain and fatigue, whether treatment improves neurocognitive status, and whether improvement in pain and fatigue correlates with functional improvement.

- Milnacipran was well tolerated and showed beneficial improvement in pain and mood and thus can be effective in treatment of patients with fibromyalgia with depression, but further studies are needed.
- This milnacipran study was the first to use standardized and systematic cognitive batteries to evaluate the effect on cognition in fibromyalgia.
METHOD
Study Design
This was a single-site, block randomized (1:1 ratio), double-blind, placebo-controlled, prospective, crossover study. Patients were randomly assigned to receive milnacipran or placebo for 6 weeks, followed by a 1-week washout and then crossover to the other arm for another 6 weeks. The trial was conducted between July 2011 and May 2013, with patients being observed over a 13-week period (ClinicalTrials.gov identifier: NCT01829243).
Subjects
The study was approved by the Institutional Review Board of Duke University, Durham, North Carolina, and granted an investigational new drug exemption by the US Food and Drug Administration. The eligible subjects were men and women between the ages of 18 and 65 years who were diagnosed with fibromyalgia by their rheumatologist or physician, with confirmation of the diagnosis by American College of Rheumatology Criteria10 and a physical tender point examination. All participants provided informed consent.
Approved methods of contraception (ie, oral contraceptives, barrier protection, or prior tubal ligation) were required for all women of child-bearing age who participated in the study. Patients with depression were not excluded from the study given that depression could be a secondary diagnosis to fibromyalgia.
Exclusion criteria included subjects with (1) bipolar disorders or any psychotic disorder; (2) the existence of concomitant rheumatologic disorders, including rheumatoid arthritis, systemic lupus erythematosus, Hashimoto’s disease, Sjogren’s syndrome, or scleroderma; (3) substance dependence (except nicotine dependence) in the previous 3 months; (4) currently suicidal or high suicide risk; (5) serious or unstable medical disorders; (6) any psychotropic drug treatment in the previous 2 weeks before screening; (7) a positive urine pregnancy test; (8) screening laboratory values 3 times the limits of normal or judged clinically significant by the investigator; (9) history of hypersensitivity to milnacipran; (10) seizure disorder, traumatic brain injury, or any central nervous system disorder that affects cognitive status; and (11) taking concomitant medications (a minimum of 30 days on stable dose of analgesics and a minimum of 4-week washout from antidepressants and fibromyalgia-specific medication [eg, pregabalin, neurontin] and supplements [St John’s wort, SAM-E]).
Study Medication
Milnacipran was given to the maximum tolerated dose starting with 12.5 mg once daily on the first day, 12.5 mg twice daily for the next 2 days, 25 mg twice daily for the next 4 days, 50 mg twice daily for the next 7 days, and 100 mg twice daily thereafter. Patients who could not tolerate higher doses had a step-wise reduction in doses (eg, 200-mg/d dose would be reduced to 100 mg/d, 100 mg/d would be reduced to 50 mg/d). Milnacipran was discontinued at the end of the study. Subjects were referred to their treating psychiatrist for follow-up.
Efficacy and Safety Measures
Assessments were performed at each visit. Neurocognition was measured with the Brief Assessment of Cognition (BAC)11 and selected tests from the MATRICS Consensus Cognitive Battery (MCCB).12 Pain was assessed by the visual analog scale (VAS) for pain. Overall fibromyalgia symptoms were measured by the Fibromyalgia Impact Questionnaire (FIQ)13 and tender point examination. Depression was assessed by scores on the Beck Depression Inventory (BDI).14 Fatigue was assessed by the Fatigue Severity Scale (FSS).15 Functional outcome was evaluated by the Health Assessment Questionnaire (HAQ).16 The Clinical Global Impressions-Improvement (CGI-I) and -Severity of Illness (CGI-S) scales17 and the Patients Clinical Global Impression of Change (PCGIC)18 were used to measure global impression of severity and improvement.
The primary efficacy measure was defined as a change in VAS for pain from baseline to end of treatment in each phase. The VAS for pain operationally is a 100-mm line anchored by word description at each end. The patient marks a point on the line that reflects his/her current pain state. The distance in millimeters from the left anchor point is the score.
Cognitive assessment was measured with the BAC and MCCB. The BAC was designed to assess longitudinal change in cognition in clinical trials. A description of the BAC and its reliability and validity has been published previously.19,20 The BAC measures cognition in 4 of the major domains of cognition: reasoning and problem-solving, processing speed, verbal memory, and working memory. The BAC composite score is calculated by summing the z scores for each of the 6 measures (obtained by comparing each measure with a normative sample of 400 controls matched to the 2005 US Census) and dividing by the healthy control SD. This composite score has high test-retest reliability in clinical populations and healthy controls (intraclass correlations > 0.80).21 The advantages of the BAC are that it is relatively quick and easy to complete (35 minutes vs 75-90 minutes for the MCCB), can be used in people with limited education (5th grade level), is less influenced by fatigue due to its brevity, and has not been shown to be significantly affected by practice effects, thus permitting repeated testing.22
The BAC includes 6 tests from 4 of the MATRICS domains. We supplemented the BAC to include tests from the 3 additional MATRICS domains: the Continuous Performance Test (vigilance), the Brief Visuospatial Memory Test (visual learning), and the Managing Emotions tests (social cognition). This BAC/MCCB hybrid assessed all 7 MATRICS domains in approximately 60 minutes of testing.
Secondary efficacy measures included the FSS, FIQ, CGI-I, CGI-S, HAQ, and PCGIC. The FSS clearly distinguished patients from controls, and it was moderately correlated with a single-item VAS for fatigue intensity. In all patients, clinical improvement in fatigue was associated with reductions in scores on the FSS.
Safety evaluations in all subjects were determined by spontaneously reported adverse events and vital signs recorded at every visit and laboratory evaluations at screening, at the end of each arm, and at study termination. The Arizona Sexual Experience Scale23 was used to assess sexual dysfunction.
Study Visits
Study visits included screening at baseline, week 1, week 2, week 4, week 6, week 8, week 9, week 10, week 12, and week 13. Assessments were performed at each visit.
Data Analysis
The intent-to-treat group comprised all subjects who received at least 1 dose of the medication. The changes from baseline to each visit in VAS, FSS, FIQ, CGI-S, CGI-I, BDI, HAQ, and PCGIC scores were analyzed by repeatedly measuring analysis of variance at the .05 level of significance. For neurocognition (BAC/MCCB hybrid), the changes from baseline to end of treatment were analyzed. All analyses employed intent to treat with last observation carried forward (n = 31).
RESULTS
Subjects
Twenty-six subjects were screened. Subjects were first given milnacipran in phase 1 and then given placebo in phase 2. Altogether, 20 subjects completed the study, and 6 subjects were screen fails. Hence, 20 subjects completed phase 1 and the same subjects completed phase 2. The mean ± SD age of the subjects was 47.6. ± 9.1 years; 18 subjects were women.
Primary Measures
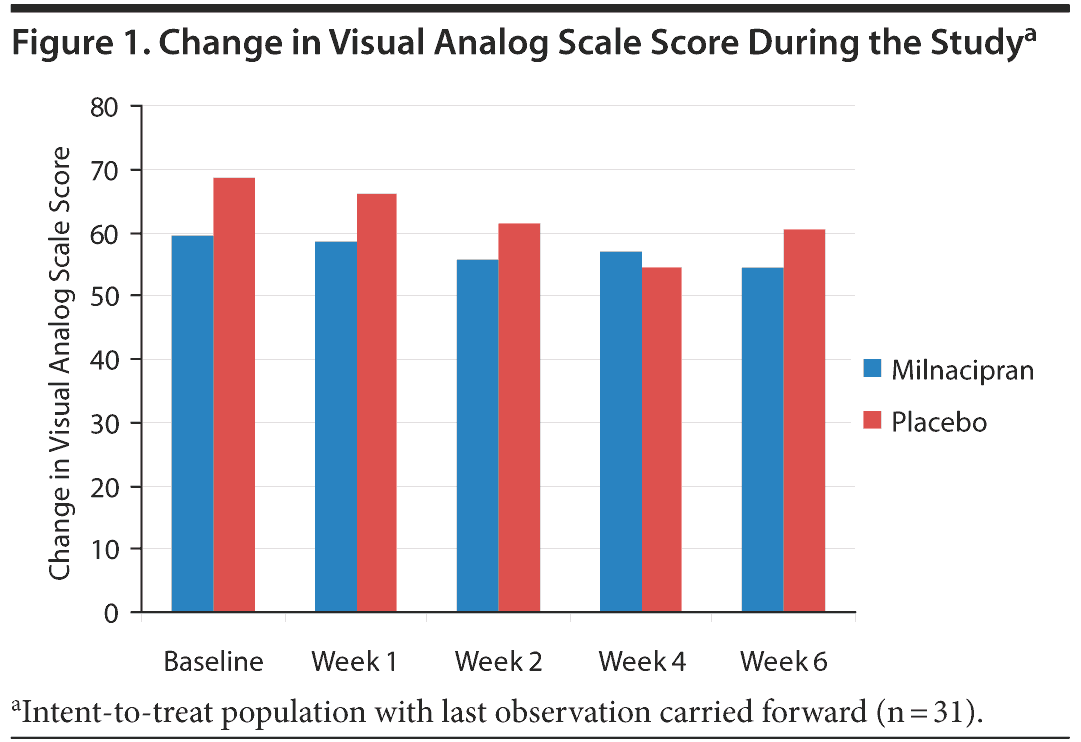
The change in VAS score was not significant from baseline to end of treatment; there was no difference between the milnacipran and placebo groups. Figure 1 summarizes the mean VAS scores at each week.
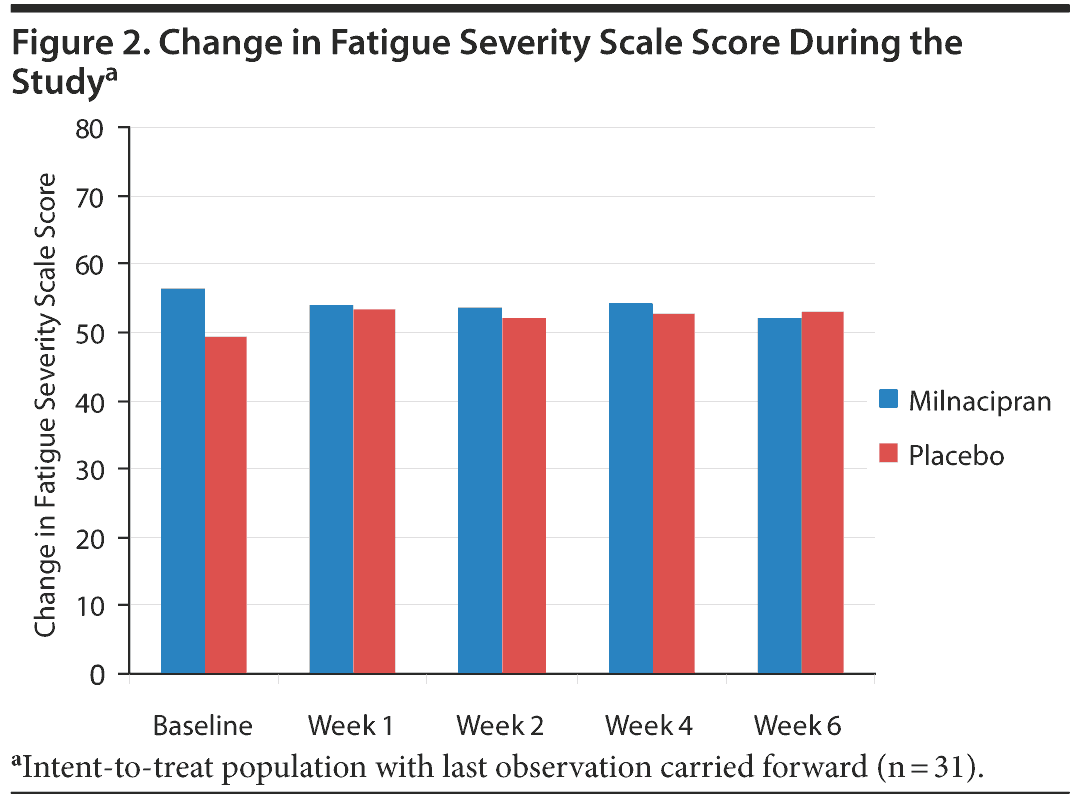
The change in FSS score was not significant over the time period, and there were no differences between the 2 groups. Figure 2 summarizes the mean FSS scores at each visit.
Cognitive Measures
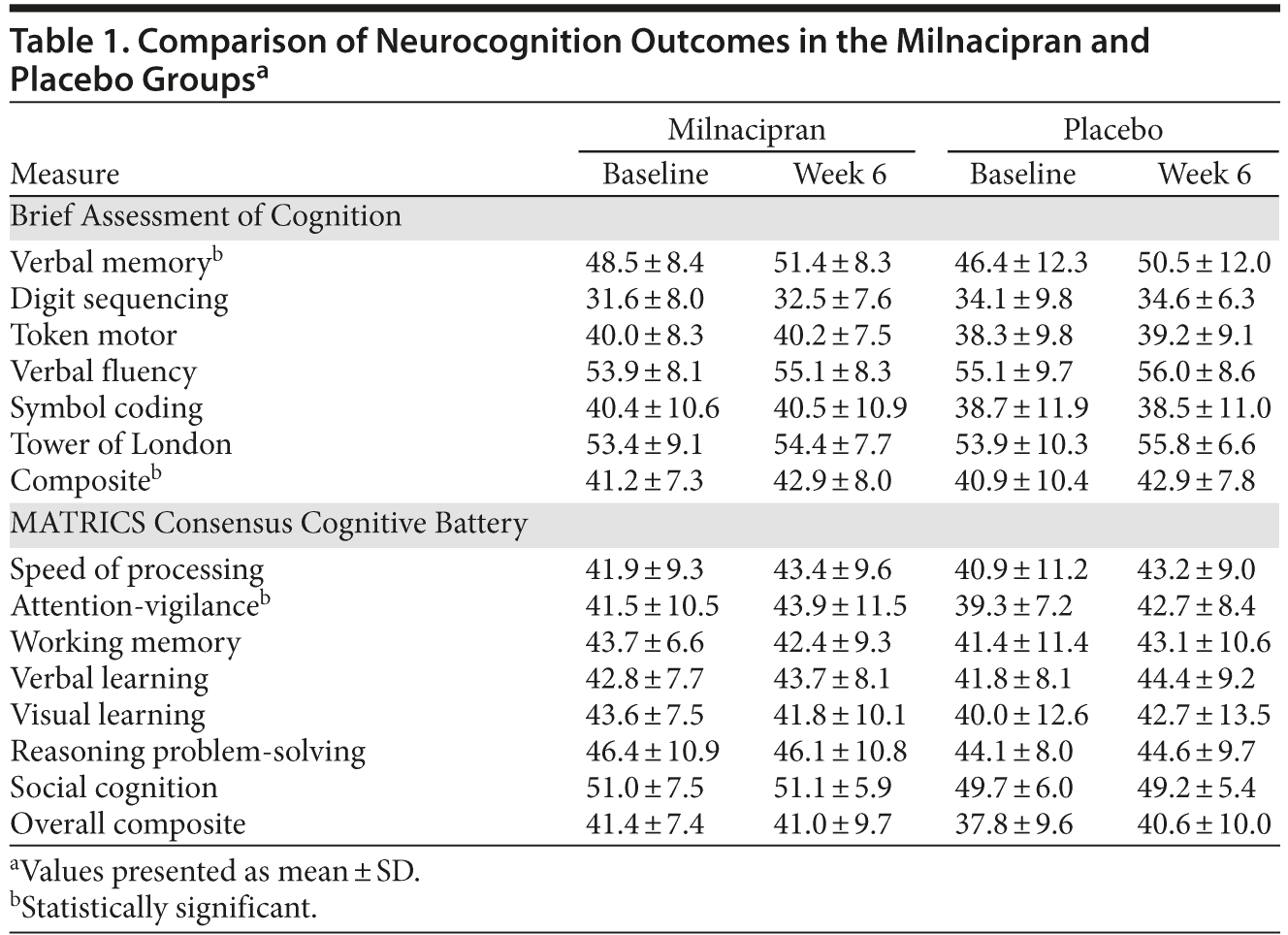
There were no statistically significant between-group differences in cognitive change with treatment. For the BAC, change in verbal memory (P = .001) and composite T score (P = .044) was significantly different from baseline to end of treatment in both groups. For the MCCB tests, the change in attention-vigilance domain T score (P = .042) was significantly different from baseline to end of treatment in both groups. Table 1 shows all domains of neurocognition.
Secondary Measures
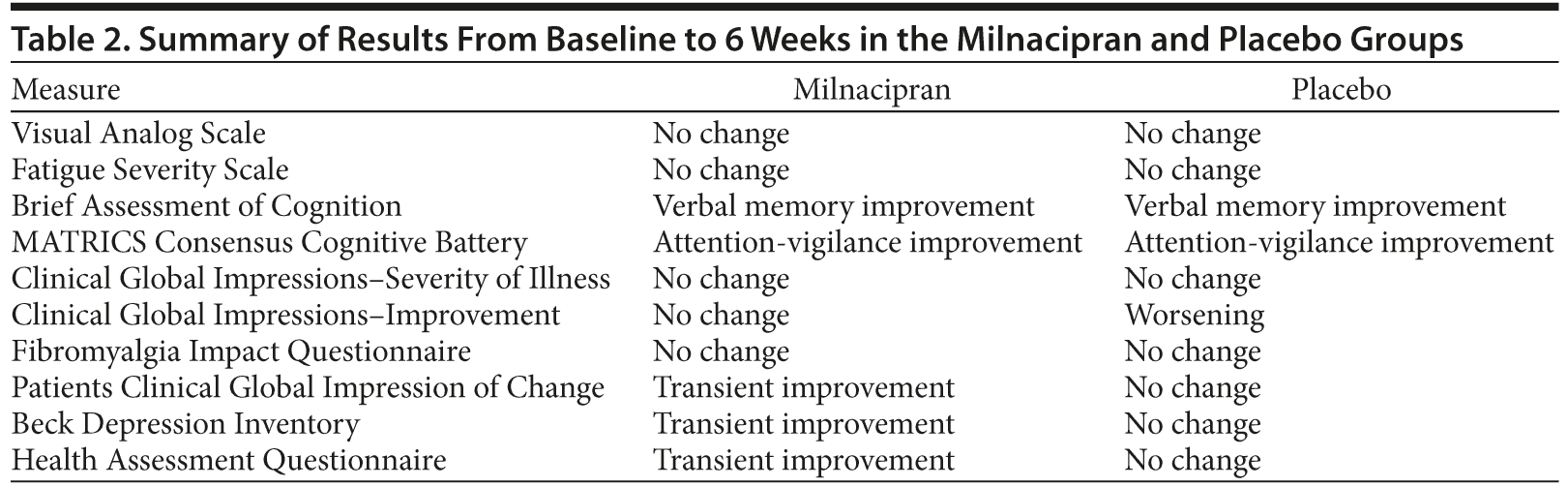
The changes in CGI-S score were not significant, but the changes in the CGI-I score showed worsening in the placebo group at week 1 (P = .032), week 2 (P = .024), week 4 (P = .024), and week 6 (P = .60) compared to baseline. The changes in FIQ scores were not significant. The PCGIC scores decreased significantly at week 1 (P = .34 to > .034) in the milnacipran group. The mean ± SD BDI scores also decreased significantly at week 1 (23.1 ± 13.5 vs 26.5 ± 14.8, P = .007) in the milnacipran group. Among 3 subscales of the HAQ, the disability index score improved at week 1 (P = .012) and week 2 (P = .041) in the milnacipran group. Table 2 shows a summary of the study results for all assessments.
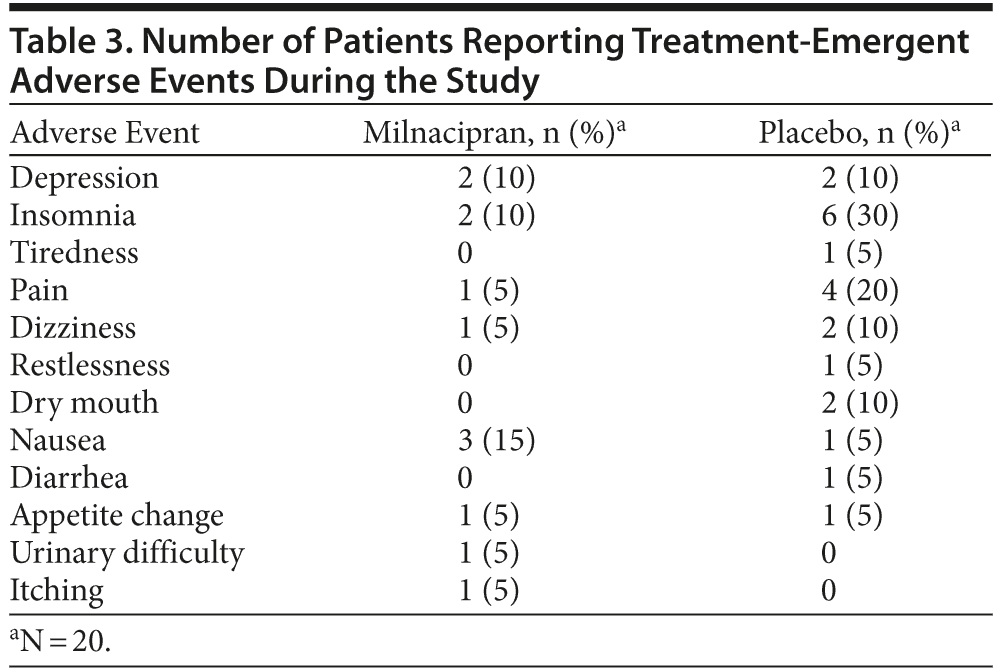
Adverse Events
Table 3 shows the adverse events reported by patients. Most of the treatment-emergent adverse events were mild to moderate in severity. No patients experienced any serious adverse events. Nausea was the most frequent adverse events in the milnacipran group. Insomnia and pain were the most frequent adverse events in the placebo group.
DISCUSSION
This randomized, placebo-controlled, crossover study investigated the safety and effectiveness of milnacipran in improving cognitive function in fibromyalgia. On the primary measure, the change in VAS score, the results did not show a significant change from baseline to end of treatment, and, hence, did not shown any difference between the milnacipran and placebo groups. The CGI-I showed significant improvement in the milnacipran group but worsening in the placebo group, the CGI-S was not significant in either group, and the PCGIC decreased significantly in the milnacipran group. The total BDI score decreased, and the disability index score of the HAQ improved during the early period of milnacipran treatment.
In addition, this study also aimed to investigate whether improvement in neurocognitive status due to milnacipran correlates with improvements in pain, whether improvement in neurocognitive status due to milnacipran correlates with improvements in fatigue, and whether treatment with improvement in neurocognitive status, pain, and fatigue correlates with functional improvement.
For neurocognitive measures, the verbal memory and composite scores of the BAC and the attention-vigilance domain T score significantly improved, but there was no difference between the 2 groups. There was no change in other cognitive domains between baseline and end of treatment. In a review of cognitive impairment in fibromyalgia,24 the most impairment was seen on measures of working memory, followed by episodic memory and access to semantic memory, all of which were assessed in our study. In addition, a new focus has emerged that points toward a particular difficulty in dealing with distracting information. This was the first study using standardized and systematic cognitive batteries. Although our results showed improvement in the verbal memory and attention domains in the milnacipran group, there was no difference between the milnacipran and placebo groups. Our results do not rule out the possibility that treatment differences with milnacipran would be found with large sample sizes.
In conclusion, milnacipran did not show superiority over placebo in improvement of neurocognitive symptoms. Milnacipran was well tolerated and showed beneficial improvement in pain and mood. The effect of milnacipran on neurocognition in fibromyalgia deserves further research.
Drug names: duloxetine (Cymbalta), gabapentin (Neurontin), imipramine (Tofranil and others), milnacipran (Savella), pregabalin (Lyrica), venlafaxine (Effexor and others).
Author affiliations: Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, North Carolina (all authors); Department of Psychiatry, School of Medicine, Chungnam National University, Daejeon, South Korea (Dr Kim); Global Medical Education, New York, New York (Dr Masand); and Carolina Behavioral Care, Durham, North Carolina (Dr Millet).
Potential conflicts of interest: Dr Marks has served as a consultant to Forest, Dey, Gilead, and TTK; has received grant/research support from Bristol-Myers Squibb, Dov, Eli Lilly, Endo, GlaxoSmithKline, Janssen, Johnson & Johnson, Pfizer, Saegis, Sepracor, and Somaxon; and has served on the speakers or advisory boards of Alkermes, Bristol-Myers Squibb, Dey, Pfizer, and Sunovion. Dr Masand has served as a consultant to Forest, Lundsbeck, Merck, Pfizer, and Sunovion; has received grant/research support from Forest; has received honoraria from or served on the speakers or advisory boards of Forest, GlaxoSmithKline, Merck, Pfizer, and Sunovion; and is a stock shareholder in Global Medical Education. Dr Millet has received grant/research support from Forest. Dr Keefe has served as a consultant to Abbvie, Akebia, Amgen, Asubio, BiolineRx, Biomarin, Boehringer-Ingelheim, Eli Lilly, EnVivo, Lundbeck, Merck, Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, and Targacept; has received grant/research support from Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, PsychoGenics, Research Foundation for Mental Hygiene, and Singapore Medical Research Council; is a stock shareholder in NeuroCog Trials; and has received royalties from the Brief Assessment of Cognition in Schizophrenia (BACS) and MATRICS Battery (BACS Symbol Coding). Dr Patkar has served as a consultant to Dey, Forest, Gilead, and TTK; has received grant/research support from Dey, Duke Endowment, Envivo, Forest, Janssen, Lundbeck, National Institutes of Health (National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism), Pfizer, Shire, Sunovion, and Titan; and has served on the speakers or advisory boards of Alkermes, Bristol-Myers Squibb, Dey, Pfizer, and Sunovion. Dr Kim and Mss Rele and Yerramsetty report no conflicts of interest related to the subject of this article.
Funding/support: This study was supported by Forest Laboratories through an Investigator-Initiated Award.
Role of the sponsor: The sponsor provided funding support for an investigator-initiated grant (principal investigator: Dr Patkar). The sponsor played no role in protocol design, conduct of the trial, data analysis and interpretation, or manuscript production.
REFERENCES
1. Glass JM. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: new trends and future directions. Curr Rheumatol Rep. 2006;8(6):425-429. PubMed doi:10.1007/s11926-006-0036-0
2. Yunus MB, Celiker R, Aldag JC. Fibromyalgia in men: comparison of psychological features with women. J Rheumatol. 2004;31(12):2464-2467. PubMed
3. Dick BD, Verrier MJ, Harker KT, et al. Disruption of cognitive function in fibromyalgia syndrome. Pain. 2008;139(3):610-616. PubMed doi:10.1016/j.pain.2008.06.017
4. Sato S, Yamakawa Y, Terashima Y, et al. Efficacy of milnacipran on cognitive dysfunction with post-stroke depression: preliminary open-label study. Psychiatry Clin Neurosci. 2006;60(5):584-589. PubMed doi:10.1111/j.1440-1819.2006.01562.x
5. Hindmarch I, Rigney U, Stanley N, et al. Pharmacodynamics of milnacipran in young and elderly volunteers. Br J Clin Pharmacol. 2000;49(2):118-125. PubMed doi:10.1046/j.1365-2125.2000.00124.x
6. Kanetani K, Kimura M, Endo S. Therapeutic effects of milnacipran (serotonin noradrenalin reuptake inhibitor) on depression following mild and moderate traumatic brain injury. J Nippon Med Sch. 2003;70(4):313-320. PubMed doi:10.1272/jnms.70.313
7. Stahl SM, Grady MM, Moret C, et al. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10(9):732-747. PubMed
8. Bennett RM. Clinical manifestations and diagnosis of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):215-232. PubMed doi:10.1016/j.rdc.2009.05.009
9. Lopez-Ibor J, Guelfi JD, Pletan Y, et al. Milnacipran and selective serotonin reuptake inhibitors in major depression. Int Clin Psychopharmacol. 1996;11(suppl 4):41-46. PubMed doi:10.1097/00004850-199609004-00006
10. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600-610. PubMed doi:10.1002/acr.20140
11. Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297. PubMed doi:10.1016/j.schres.2003.09.011
12. Keefe RS, Fox KH, Harvey PD, et al. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125(2-3):161-168. PubMed doi:10.1016/j.schres.2010.09.015
13. Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol. 1991;18(5):728-733. PubMed
14. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561-571. PubMed doi:10.1001/archpsyc.1961.01710120031004
15. Krupp LB, LaRocca NG, Muir-Nash J, et al. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121-1123. PubMed doi:10.1001/archneur.1989.00520460115022
16. Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol. 2005;23(suppl 39):S14-S18. PubMed
17. Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health. 1976.
18. Geisser ME, Clauw DJ, Strand V, et al. Contributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among persons with fibromyalgia treated with milnacipran. Pain. 2010 May;149(2):373-378. doi: 10.1016/j.pain.2010.02.043 PubMed. >
19. Keefe RS, Poe M, Walker TM, et al. The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. J Clin Exp Neuropsychol. 2006;28(2):260-269. PubMed doi:10.1080/13803390500360539
20. Keefe RS, Sweeney JA, Gu H, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison.. Am J Psychiatry. 2007;164(7):1061-1071. PubMed doi:10.1176/appi.ajp.164.7.1061
21. Keefe RSE, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297. PubMed doi:10.1016/j.schres.2003.09.011
22. Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102(1-3):108-115. PubMed doi:10.1016/j.schres.2008.03.024
23. McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25-40. PubMed doi:10.1080/009262300278623
24. Glass JM. Review of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairments. Rheum Dis Clin North Am. 2009;35(2):299-311. PubMed doi:10.1016/j.rdc.2009.06.002