Eszopiclone Treatment for Insomnia: Effect Size Comparisons in Patients With Primary Insomnia and Insomnia With Medical and Psychiatric Comorbidity

ABSTRACT
Objective: The purpose of this post hoc analysis was to compare the treatment effect size of eszopiclone 3 mg for insomnia in patients with a diagnosis of primary insomnia and in several of the psychiatric and medical conditions that are most commonly comorbid with insomnia.
Method: Data were analyzed from 5 large, multicenter, randomized, double-blind, placebo-controlled studies of adult outpatients of at least 1 month duration published between 2006 and 2009. Diary-derived indices of sleep and daytime functioning and the Insomnia Severity Index were compared for patients with primary insomnia (DSM-IV-TR criteria, n = 828) and for those with insomnia comorbid with major depressive disorder (MDD, DSM-IV-TR criteria, n = 545), generalized anxiety disorder (GAD, DSM-IV-TR criteria, n = 595), perimenopause/postmenopause (Stages of Reproductive Aging Workshop criteria, n = 410), and rheumatoid arthritis (American College of Rheumatology criteria, n = 153). Cohen d effect sizes were calculated for each individual study as the between-treatment difference score divided by the pooled standard deviation.
Results: Effect sizes ranged from 0.40 to 0.69 (small-medium) as early as week 1 and were maintained at 0.26-0.63 at week 4 for sleep latency, wake time after sleep onset, and total sleep time. Sleep latency and total sleep time effect sizes increased from week 1 to week 4 in the primary insomnia group. At week 4, effect sizes on all 3 parameters and the Insomnia Severity Index tended to be highest for the primary insomnia patients and tended to be lowest for patients with comorbid GAD and MDD. The effect sizes for daytime functioning were small for all insomnia patient groups.
Conclusions: Eszopiclone 3 mg is an effective treatment for insomnia across 5 clinically diverse patient populations; however, magnitude of effect is mediated by underlying comorbidity and their treatments, with largest measures of effect seen in primary insomnia and lowest in MDD and GAD. These consistent results, and the fact that clinical trials were conducted in patients being treated as appropriate for their comorbid clinical conditions, support the results’ real-world generalizability and utility to clinical practice.
Prim Care Companion CNS Disord 2012;14(4):doi:10.4088/PCC.11m01296
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: September 19, 2011; accepted December 6, 2011.
Published online: July 5, 2012.
Corresponding author: Andrew D. Krystal, MD, MS, DUMC 3309, Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Durham, NC 27710 ([email protected]).
The prevalence of chronic insomnia in the general population ranges from as high as 22.1% using DSM-IV-TR criteria1 to as low as 3.9% to 10.2% when additional ICD-10 or Research Diagnostic Criteria are used.1 Patients with insomnia have increased rates of medical and psychiatric illness compared with the general population2; likewise, patients with serious medical and psychiatric disorders have extremely high rates of insomnia.3-6 For example, insomnia is reported at rates as high as 90% in depressed patients,7,8 and at least two-thirds of patients with generalized anxiety disorder (GAD) have 1 or more types of comorbid sleep disturbance.9,10 Markedly higher rates of insomnia are also reported in patients with painful arthritis11,12 and in perimenopausal and postmenopausal women.13,14 At the same time, it has been reported that the incidence and prevalence of major depressive disorder (MDD)15-17 or an anxiety disorder15,18,19 are higher in patients diagnosed with insomnia.
The consequences of insomnia comorbidity are significant. The presence of insomnia in patients with a primary medical or psychiatric diagnosis is associated with reduced quality of life,20 increased functional impairment,8 and higher illness-related symptom severity compared to patients with a similar medical history and no insomnia. For example, high pain severity ratings have been reported in patients with insomnia and arthritis,21 and high levels of resistance to antidepressant treatment22 and suicidal ideation23 have been reported in patients with insomnia and MDD. Insomnia has also been found to be a common residual symptom and a significant risk factor for recurrence of MDD.24
Most medications indicated for insomnia are approved on the basis of double-blind, placebo-controlled, randomized clinical trials in patients with primary insomnia (PI), excluding patients with clinically significant medical or psychiatric comorbidity. However, in most clinical practice settings, comorbidity is the rule rather than the exception. Unfortunately, there are relatively few well-designed randomized clinical trials8,21,25-31 that have evaluated the more clinically relevant questions of efficacy in patients with comorbid insomnia or reported data on effect sizes to better estimate the clinical effectiveness of treatment.
Eszopiclone, a γ-aminobutyric acid (GABA)-ergic sedative hypnotic, is thought to induce sleep via allosteric modulation of GABAA receptors enhancing inhibition of wake-promoting brain regions.32 Eszopiclone has been shown to significantly improve sleep onset and maintenance in PI,33,34 as well as in several other psychiatric8,28 and medical populations.21,27 The pathophysiology of insomnia in each of these populations is not known but may differ,35 raising questions about the relative effectiveness of hypnotics to treat the comorbid insomnia occurring in these different comorbid populations. In addition, insomnia might be caused by medications used to treat the comorbid conditions (eg, selective serotonin reuptake inhibitors [SSRIs] causing activation and sleep fragmentation).36,37
The objective of this post hoc analysis was to compare the magnitude and pattern of the insomnia response to eszopiclone in PI and across several of the most common insomnia comorbidities in the presence of medications coadministered to treat those comorbid conditions.
METHOD
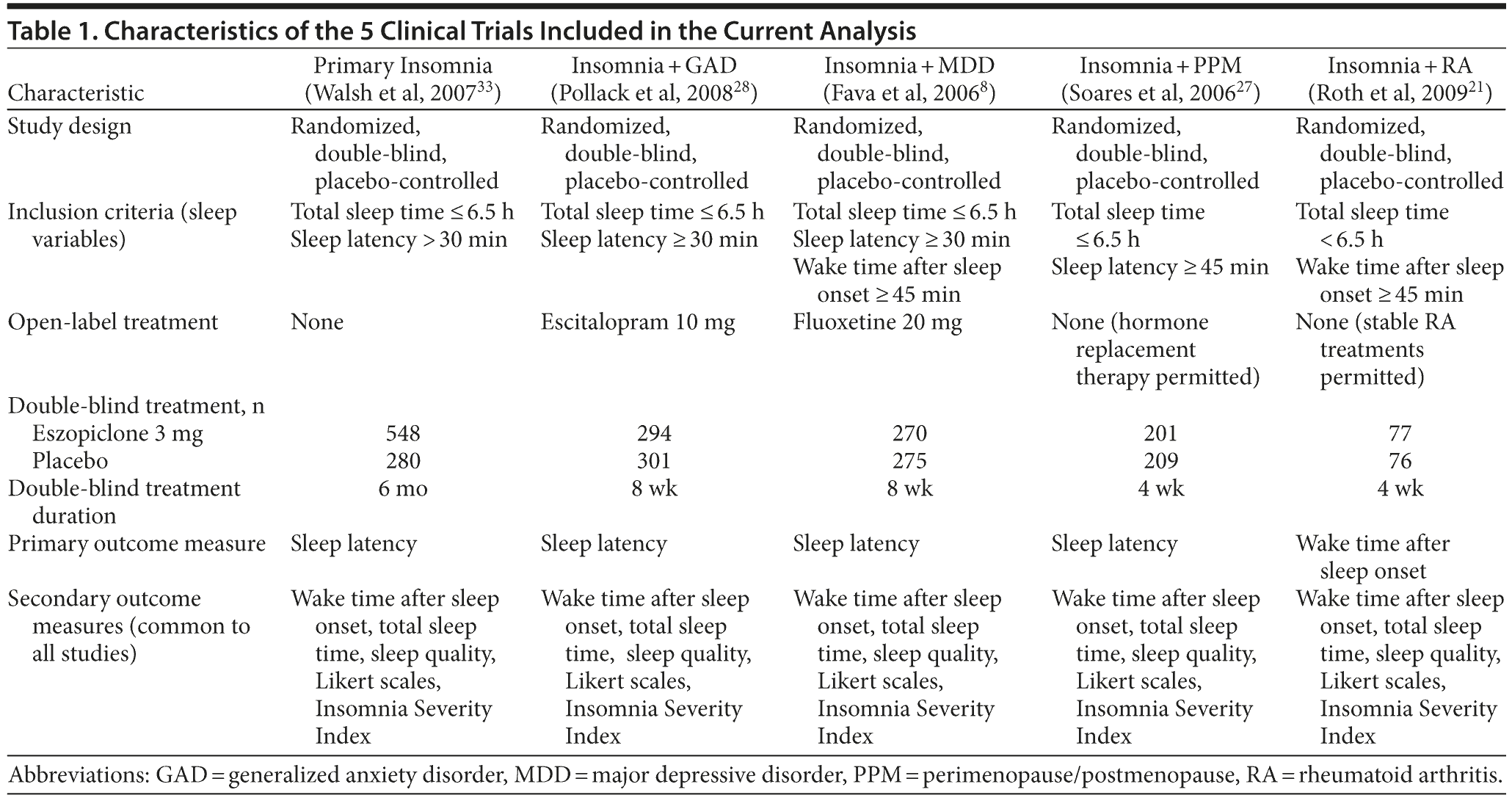
Data were analyzed separately from 5 large, multisite, randomized, double-blind, placebo-controlled studies published between 2006 and 2009 of adult outpatients of at least 1 month duration with the following: (1) PI (DSM-IV-TR criteria, n = 828),33 (2) insomnia comorbid with MDD (DSM-IV-TR criteria, n = 545),8 (3) insomnia comorbid with GAD (DSM-IV-TR criteria, n = 595),28 (4) insomnia comorbid with rheumatoid arthritis (RA, n = 410),21 and (5) insomnia comorbid with perimenopause/postmenopause (PPM, n = 410).27 Detailed information on entry criteria, study design, sample characteristics, patient disposition, and efficacy results was previously reported in the primary publication of each trial.8,21,27,28,33 Key entry criteria for each of the studies were as follows: (1) insomnia: total sleep time ≤ 6.5 hours at least 3 times per week for the past month and sleep latency > 30 minutes (PI study), ≥ 30 minutes (MDD, GAD, and RA studies), and ≥ 45 minutes (PPM study); (2) MDD: Hamilton Depression Rating Scale (HDRS) total score ≥ 14 (the 17-item HDRS minus the 3 sleep disturbance items); (3) GAD: Hamilton Anxiety Rating Scale total score ≥ 20; (4) RA: American College of Rheumatology criteria and taking a stable dose of an RA medication for ≥ 90 days prior to randomization; and (5) PPM staging: Stages of Reproductive Aging Workshop criteria.38

- Eszopiclone 3 mg is an effective treatment for insomnia across 5 clinically diverse patient populations including those with primary insomnia, major depressive disorder, generalized anxiety disorder, perimenopause/
postmenopause, and rheumatoid arthritis. - For those with insomnia and major depressive disorder, eszopiclone was studied in conjunction with concomitant therapy with a selective serotonin reuptake inhibitor, which is how eszopiclone should be used in clinical practice in patients with these insomnia comorbidities.
The onset of insomnia was required as an inclusion criterion to be temporally related to the comorbid MDD, RA, and PPM in those 3 studies. For example, in the MDD study, onset of insomnia must not have predated onset of MDD by more than 10 weeks; in the PPM trial, the menopausal transition must have predated insomnia onset, with no other secondary causes of the insomnia. No temporal relationship was required in those patients with comorbid insomnia and GAD. Additional study design characteristics are summarized in Table 1.
Assessments
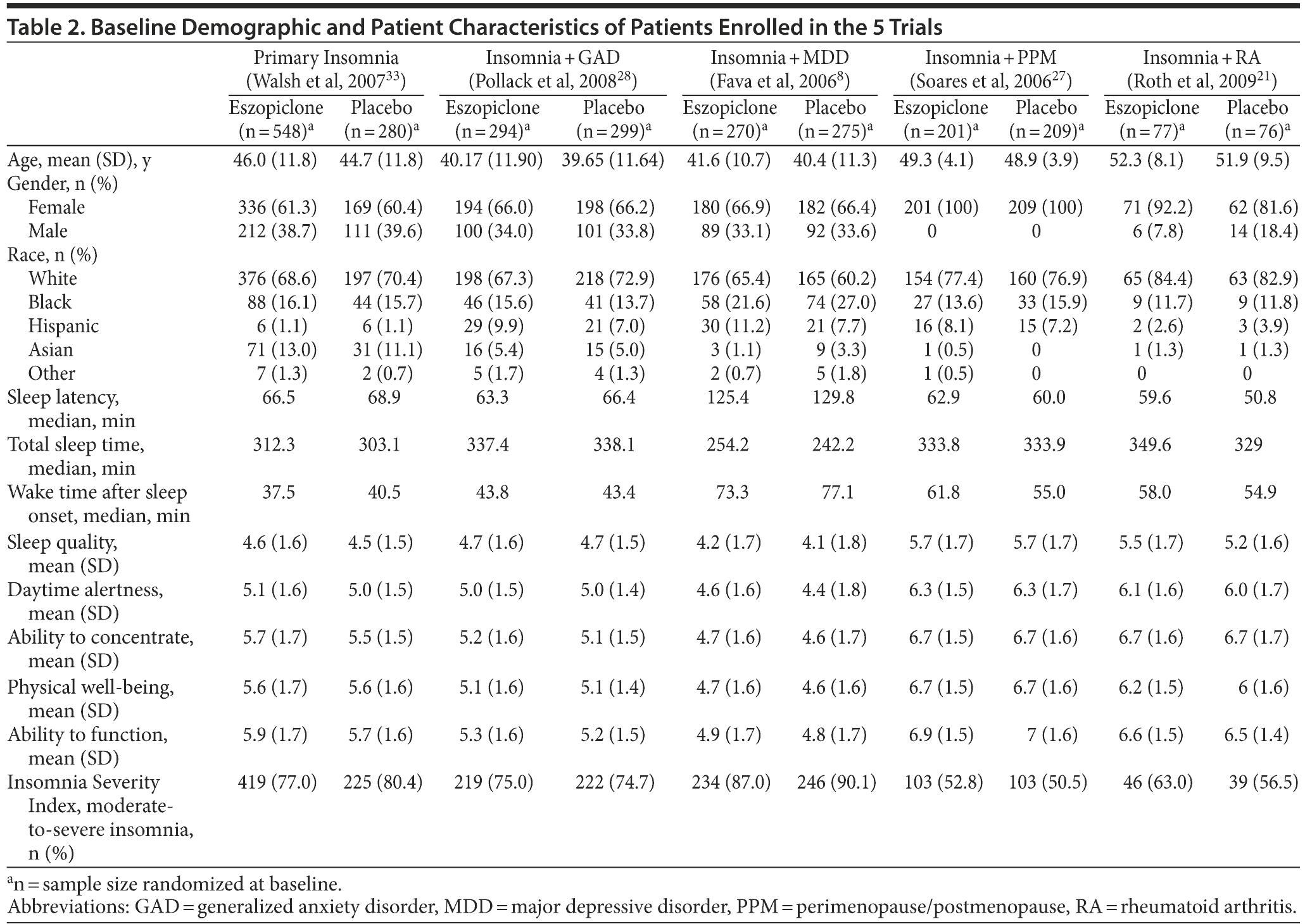
The following patient self-reported sleep parameters were collected in the morning via an interactive voice response system for the PI, MDD, RA, and PPM trials and via electronic diary for the GAD trial: sleep latency, defined as the number of minutes taken to fall asleep after bedtime; wake time after sleep onset, defined as the total number of minutes of wake time after initial sleep onset; and total sleep time, defined as the total number of minutes of sleep during the night. Sleep quality, daytime alertness, ability to concentrate, physical well-being, and ability to function were also evaluated using individual 0-10 Likert scales, with higher scores indicating better quality of sleep or functioning. Patients also completed the Insomnia Severity Index39 in order to assess the overall severity of insomnia.
Statistical Methods
The analyses of all efficacy endpoints were performed using the modified intent-to-treat population, consisting of all patients who were randomized and received at least 1 dose of double-blind study medication. However, for the insomnia comorbid with PPM and insomnia comorbid with RA studies, observed cases was the unit of analysis. Change from baseline to week 1 and week 4 were analyzed for each of the subjective sleep and daytime efficacy parameters using an analysis of covariance model, with treatment and site as fixed effects and baseline as the covariate. Values for wake time after sleep onset and sleep latency were log transformed prior to the analysis as in prior work.8,21,27,28,33 Because a number of key sleep parameters are not normally distributed in the general population,40 the median of these variables is a more appropriate measure of central tendency than the mean and is therefore the central tendency statistic reported. No adjustment for multiple comparisons was carried out since this was an exploratory post hoc comparison. Effect size calculations from the PI, MDD, and GAD studies were based on the last observation carried forward method with change from baseline values, while effect size calculations from the PPM and RA studies were based on the completer analysis with change from baseline values. These approaches were based on the prespecified analysis method in the study protocols.
Effect sizes based on Cohen d for each individual study at week 1 and week 4 are provided. The Cohen d effect size was calculated for weekly efficacy measures as the between-treatment difference score divided by the pooled standard deviation.41 An effect size of 0.2 to < 0.5 and ≥ 0.5 to < 0.8 has been suggested to represent a small and medium treatment effect, respectively, while an effect size ≥ 0.8 represents a large treatment effect.41 A timeframe of 4 weeks was studied because the shortest of the studies included in this post hoc analysis was 4 weeks in duration.
RESULTS
The baseline demographic and clinical insomnia characteristics of patients enrolled in the 5 trials are summarized in Table 2. Between-study differences were consistent with the comorbid diagnosis. For example, patients in the RA trial were the oldest (mean age of 52.1 years), followed by the patients in the PPM trial (49.1 years), while the youngest patients were those in the GAD and MDD trials (mean age of 39.9 years and 41.0 years, respectively). The majority of patients in all 5 of the trials were women. The most notable between-group difference was greater overall insomnia severity in the MDD patients compared with those in the other studies, as shown by markedly higher sleep latency and wake time after sleep onset, markedly shorter total sleep time, and higher Insomnia Severity Index scores.
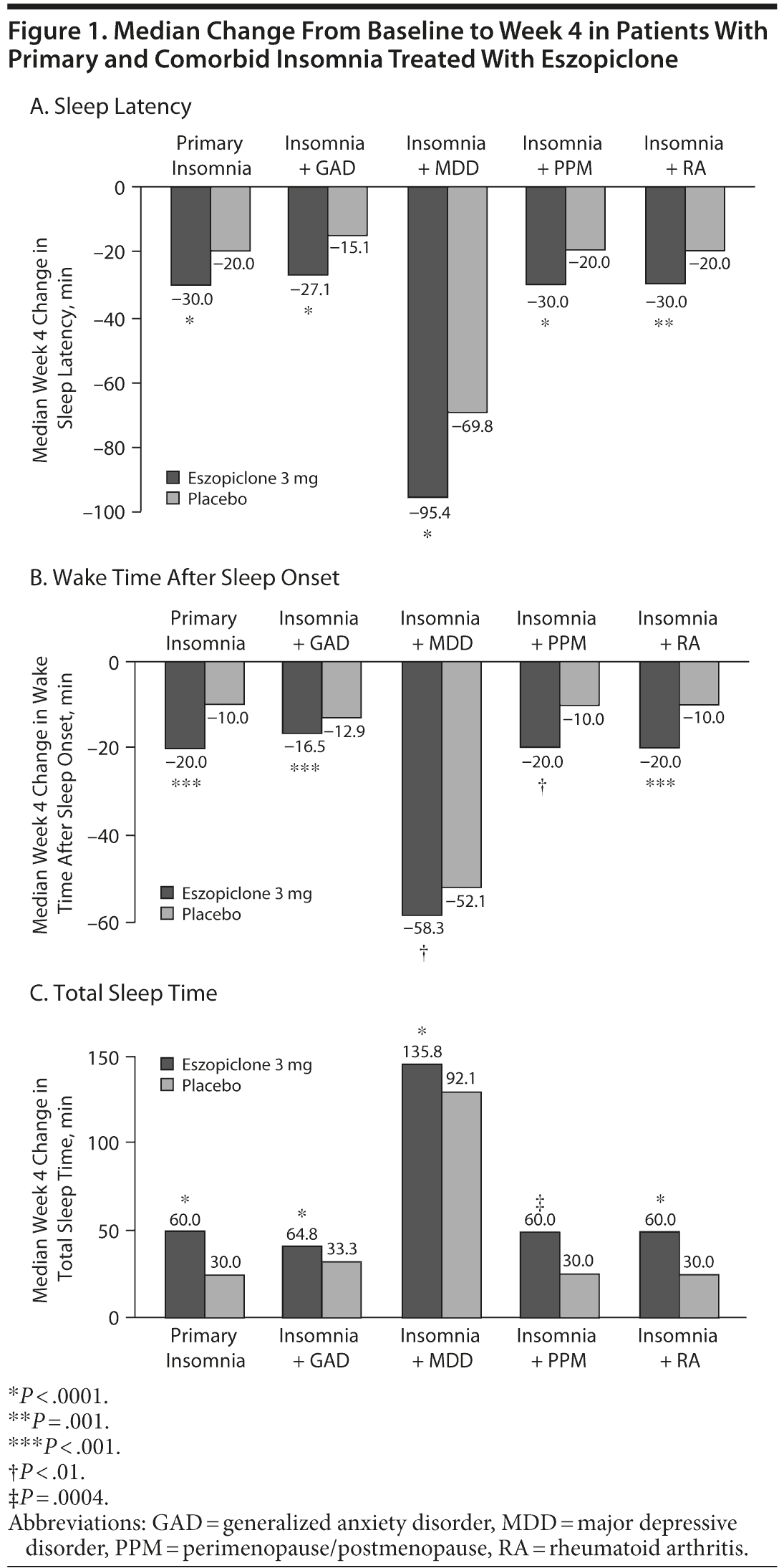
Treatment with eszopiclone 3 mg was associated with significant reduction in sleep latency and wake time after sleep onset and increase in total sleep time at week 4 in all 5 trials (Figure 1A-C). Across groups, the greatest reduction from baseline in sleep latency and wake time after sleep onset and greatest increase in total sleep time was observed in patients with comorbid MDD.
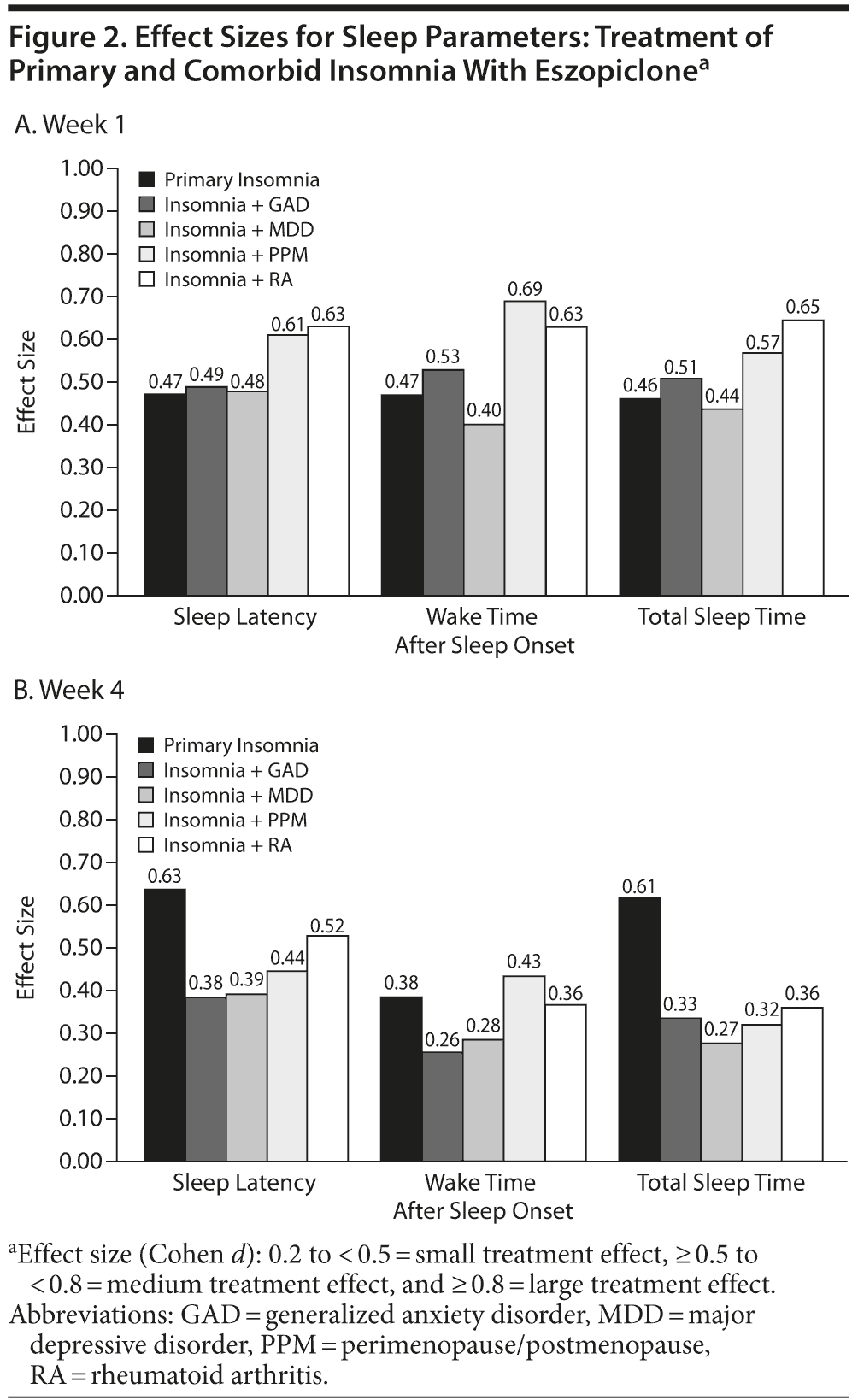
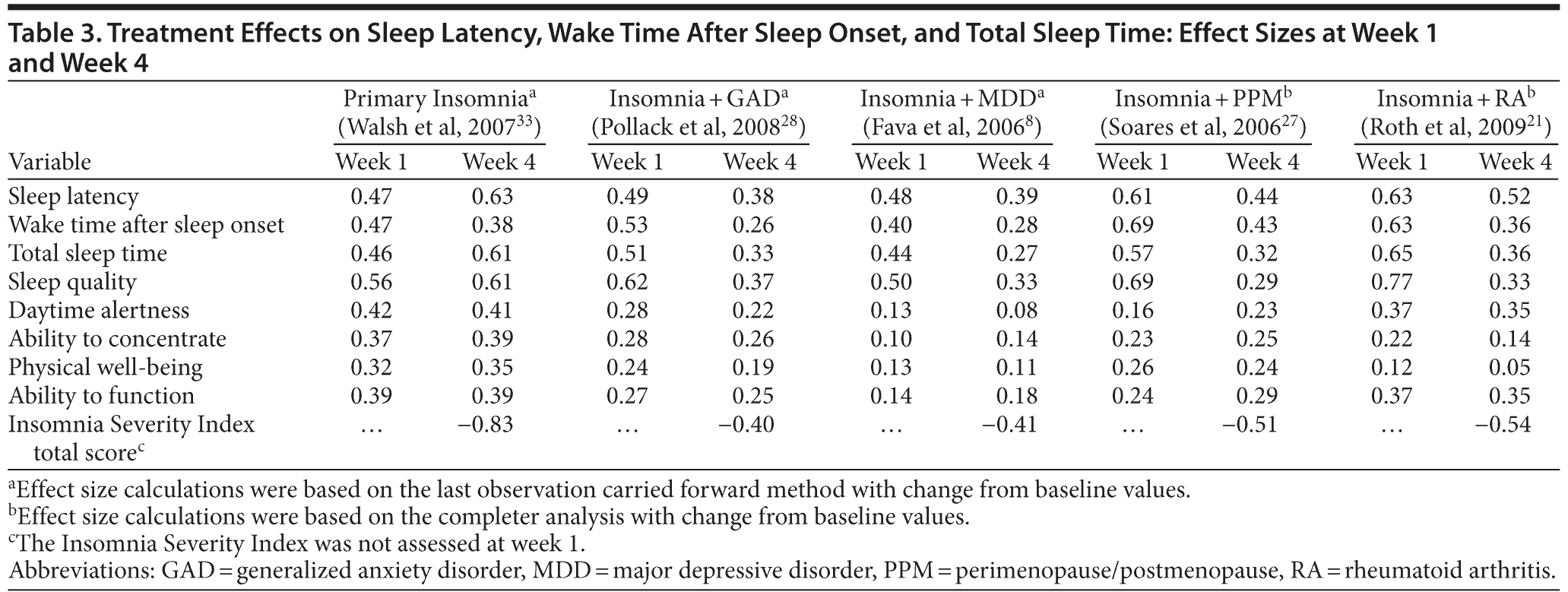
Following treatment with eszopiclone 3 mg, effect sizes calculated at week 1 were in the 0.40-0.69 range (small-medium effect) for sleep latency, wake time after sleep onset, and total sleep time (Figure 2A-B). Effect sizes at week 4 for sleep latency, wake time after sleep onset, and total sleep time were also in the small-medium range (0.26-0.63), but were somewhat more divergent. Effect sizes for sleep latency and total sleep time increased further in the PI group from weeks 1-4 but tended to decrease from weeks 1-4 in the other trials. At week 4, effect sizes on all measures of sleep latency, wake time after sleep onset, total sleep time, and the Insomnia Severity Index tended to be highest for the PI patients and tended to be lowest for patients with comorbid GAD and MDD (Table 3).
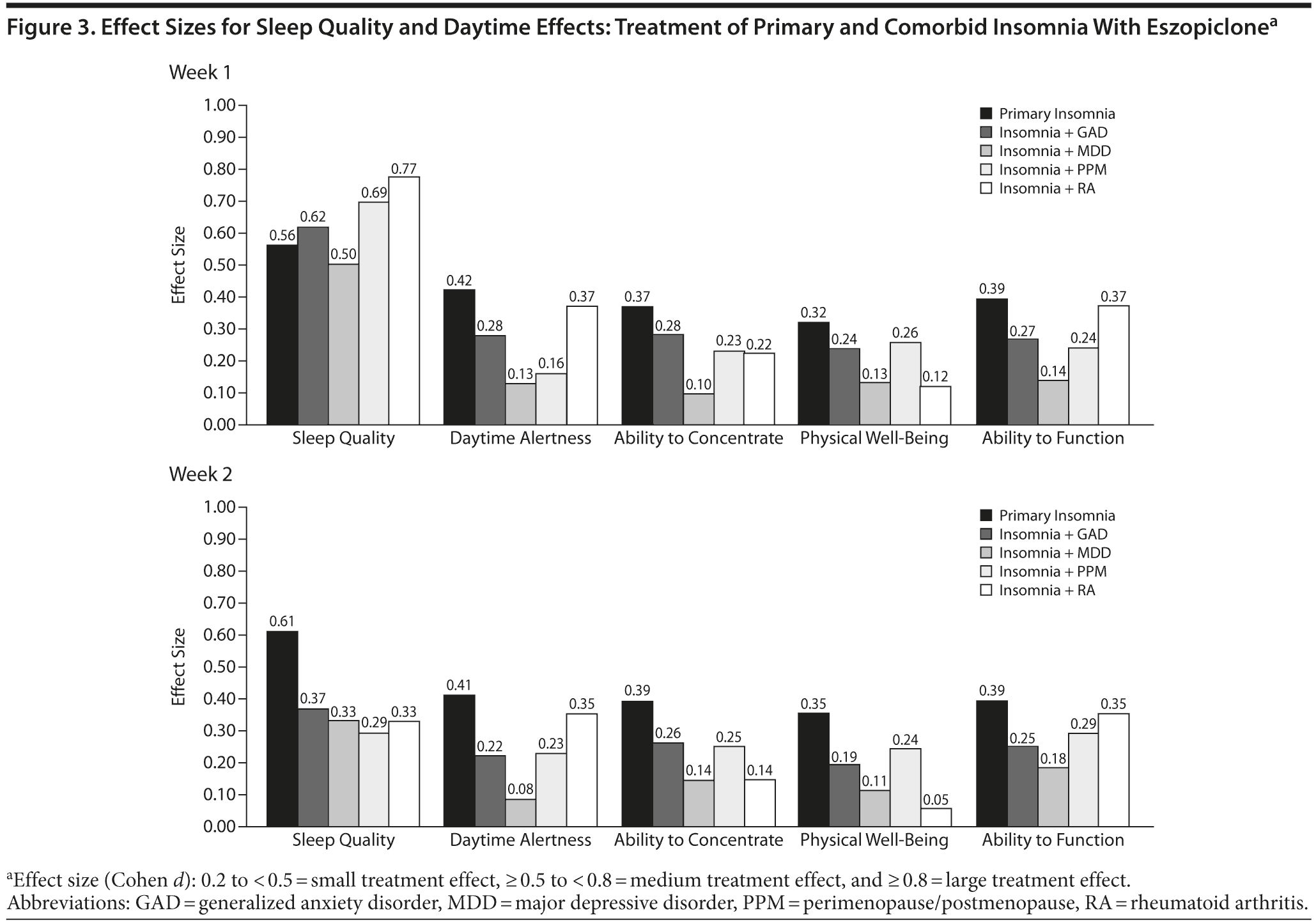
The effect sizes for daytime functioning (Likert ratings of daytime alertness, ability to concentrate, physical well-being, ability to function) were small for all insomnia patient groups (Table 3). Figure 3A-B shows the effect sizes for sleep quality and for daytime functioning for each insomnia group for weeks 1 and 4. The effect sizes were consistently highest in the PI group and tended to be lowest in patients with comorbid GAD and MDD.
DISCUSSION
Together, these studies provide findings that are relevant to a substantial proportion of insomnia sufferers treated by physicians. It is estimated that only 25% of persons with insomnia have no associated comorbid condition, leaving the majority of patients with associated psychiatric and medical conditions that contribute to sleep disturbance in diverse ways.35 To date, however, there are no published epidemiologic studies reporting the specific prevalence of the major comorbid conditions observed within this population.
Multiple studies have found that anxiety and depressive disorders are the most common psychiatric comorbidities in patients with chronic insomnia. For example, 1 large study reported that, among persons with difficulty falling asleep, 37% had an anxiety disorder and 16.5% had MDD.42 Insomnia is also a common complaint in a variety of chronic somatic illnesses, including but not limited to chronic pain disorders such as back pain, fibromyalgia, osteoarthritis, and RA. In fact, a large national representative health survey (N = 49,000) in Norway found that approximately 25% of those with RA also complain of insomnia.43 Endocrine disorders such as diabetes mellitus are also commonly associated with insomnia. In an epidemiologic study of over 3,200 primary care patients, 17% of those with insomnia reported comorbid endocrine disorders.44 Insomnia complaints are common during periods of life associated with somatic and psychological discomforts such as menopausal transition.45 Perimenopausal and postmenopausal women typically have more sleep complaints than younger women,46 and sleep problems are commonly seen in perimenopausal and postmenopausal women, with prevalence rates ranging from 15% to 60%.13,47
The pathophysiology of chronic insomnia is just beginning to be understood. To date, a substantial body of research into causal models of insomnia has been conducted in patients with PI, healthy normal volunteers, and animal models. However, such studies are lacking for conditions wherein insomnia is a core symptom of other illnesses such as mood disorders, GAD, and PPM.48-51 Hypnotics have been used for decades to treat insomnia in a wide range of patients, regardless of comorbid condition and despite the fact that the efficacy and tolerability of these medications were established only in patients with PI.52,53 Our analyses suggest that eszopiclone is effective for the treatment of insomnia across 5 different patient subgroups, with mixed small-to-moderate effect sizes in each of them. The level of improvement observed across all 5 conditions suggests that even though the disrupted sleep seen in diverse conditions may not have a common pathophysiologic pathway, each can be impacted to a similar extent via allosteric modulation of GABAA receptor inhibition of wake-promoting areas, the presumed mechanism of action of eszopiclone.32,54 Comparative assessments of the effects of other hypnotic agents across insomnia populations are needed to determine the extent to which the similarities in improvement seen across these insomnia subgroups are unique to eszopiclone, common to all agents that enhance gabaA receptor-mediated inhibition, or a general characteristic of pharmacologic promotion of sleep regardless of mechanism of action.
Although the relative effect size across the 5 conditions varies somewhat for the different insomnia parameters studied, there appears to be a somewhat larger effect size for PI, PPM, and RA compared with MDD and GAD. Also, in contrast to the PI group, the effect size on quantitative sleep parameters in the MDD and GAD groups was somewhat reduced by week 4. Differences between weeks 1 and 4 could be related to progressive improvement in the placebo group over time, a common phenomenon in central nervous system trials. Possible reasons for the effect size and pattern of response differences among the comorbid populations studied include the effects of the concomitant medications for the comorbid condition (eg, an SSRI) or physiologic differences between different subtypes of insomnia in the various conditions. An effect of concomitant medications seems likely, given that the 2 conditions for which an SSRI was coadministered with eszopiclone tended to have the lowest effect sizes, and that SSRIs are known to improve insomnia along with the total syndrome in both MDD and GAD.
It is important to note that, although the eszopiclone versus placebo effect size was found to be smaller in MDD and GAD compared with PI, PPM, and RA, the absolute degree of improvement in GAD and MDD patients from baseline with eszopiclone plus SSRI medication was as large or larger than the improvement seen in PI, PPM, and RA patients who received eszopiclone alone. As a result, the degree of improvement seen in clinical practice (where no comparison with placebo occurs) with eszopiclone in GAD and MDD patients, who are generally treated with medications such as SSRIs, would be expected to be at least as large as the improvement seen in the other insomnia subgroups. This is a crucial point because studies carried out for US Food and Drug Administration approval of hypnotic medications are conducted in PI, although most patients seen in clinical practice have comorbid anxiety and mood symptoms. It should therefore not be concluded that PI studies overestimate the results seen in clinical practice.
There are several important limitations to this study. Only 5 multicenter studies were available for inclusion in the analysis, limiting the generalizability of our conclusions. In the MDD study, both the baseline severity of insomnia and the degree of improvement were substantially greater than in the other conditions. Comparisons across multiple types of patient groups and trial designs (eg, monotherapy versus combination therapy) introduce considerable heterogeneity. This heterogeneity is partly balanced by examining the effect size of a single medication. The effect of eszopiclone alone in the MDD and GAD populations has not been studied because of the assumption that appropriate treatment of insomnia occurring with MDD and GAD in clinical practice will always include an intervention targeted to the MDD or GAD, along with insomnia therapy. The effect size of eszopiclone alone on insomnia in MDD and GAD, and the incremental improvement in insomnia occurring with eszopiclone above and beyond the effects of SSRI therapy, are of theoretical/mechanistic interest because they illustrate both the limitations of a typical monotherapy for these conditions for insomnia and the extent to which the combination of enhancing the inhibitory effects of GABA and serotonin reuptake inhibition can further benefit patients. A further limitation is that we did not evaluate or account for the potential influence of factors such as age, gender, baseline insomnia severity, or other covariates of interest on the eszopiclone effect sizes. This limitation occurred because our a priori plan was to evaluate effect size on the basis of a prespecified primary outcome analysis for each of the studies included.
In summary, this effect size comparison showed a relatively consistent effect of eszopiclone treatment of insomnia across 5 clinically diverse populations. Moreover, patients in these trials were also being treated with appropriate clinical therapies for their condition, including concomitant treatment with SSRIs (GAD and MDD), appropriate pain medications (in RA), and hormone supplementation (perimenopause), supporting the real-world generalizability of the present study to clinical practice and mitigating concerns that randomized clinical trials conducted in restricted PI populations for drug approval purposes do not show therapeutic responses representative of those attainable in typical clinical practice.
Drug names: escitalopram (Lexapro and others), eszopiclone (Lunesta), fluoxetine (Prozac and others).
Author affiliations: Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Durham, North Carolina (Dr Krystal); Department of Psychiatry and Behavioral Medicine, Wake Forest University Health Sciences, Winston-Salem, North Carolina (Dr McCall); Depression Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, Cambridge (Dr Fava); Center for Women’s Mental Health, Massachusetts General Hospital, Boston (Dr Joffe); Department of Psychiatry and Behavioral Neurosciences, McMaster University, Toronto, Ontario, Canada (Dr Soares); and Sunovion Pharmaceuticals Inc, Marlborough, Massachusetts (Drs Huang and Marshall, Ms Zummo, and Messrs Grinell and Spalding).
Potential conflicts of interest: Dr Krystal has received grant/research support from Abbott, Astellas, Cephalon, Evotec, GlaxoSmithKline, Merck, National Institutes of Health, Neurocrine, Neurogen, Neuronetics, Pfizer, Phillips-Respironics, Sanofi-Aventis, Somaxon, Sunovion/Sepracor, Takeda, and Transcept and has served as a consultant to Abbott, Actelion, Arena, Astellas, AstraZeneca, Axiom, Bristol-Myers Squibb, Cephalon, Eisai, Eli Lilly, GlaxoSmithKline, Jazz, Johnson & Johnson, King, Kingsdown Inc, Merck, Neurocrine, Neurogen, Neuronetics, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Somaxon, Somnus, Sunovion/Sepracor, Takeda, and Transcept. Dr McCall has served as a consultant to AstraZeneca, Merck, Sealy, and Sunovion and has received honoraria from Merck and Sunovion. Dr Fava has received research support from Abbott, Alkermes, Aspect Medical Systems, AstraZeneca, Bio Research, BrainCells, Bristol-Myers Squibb, Cephalon, Clinical Trial Solutions, Eli Lilly, Forest, Ganeden Biotech, GlaxoSmithKline, Johnson & Johnson, Lichtwer Pharma GmbH, Lorex, National Alliance for Research on Schizophrenia and Depression, National Center for Complementary and Alternative Medicine, National Institute on Drug and Alcohol Abuse, National Institute of Mental Health, Novartis, Organon, Pamlab, Pfizer, Pharmavite, Roche, Sanofi-Aventis, Shire, Solvay, Synthelabo, and Wyeth-Ayerst; has served on the advisory boards of or as a consultant to Abbott, Amarin, Aspect Medical Systems, AstraZeneca, Auspex, Bayer AG, Best Practice Project Management, BioMarin, Biovail, BrainCells, Bristol-Myers Squibb, Cephalon, Clinical Trials Solutions, CNS Response, Compellis, Cypress, Dov, Eli Lilly, EPIX, Euthymics Bioscience, Fabre-Kramer, Forest, GlaxoSmithKline, Grunenthal GmbH, Janssen, Jazz, Johnson & Johnson, Knoll, Labopharm, Lorex, Lundbeck, MedAvante, Merck, Methylation Sciences, Neuronetics, Novartis, Nutrition 21, Organon, Pamlab, Pfizer, PharmaStar, Pharmavite, Precision Human Biolaboratory, PsychoGenics, Roche, Sanofi-Aventis, Sepracor, Schering-Plough, Solvay, Somaxon, Somerset, Synthelabo, Takeda, Tetragenex, Transcept, TransForm, Vanda, and Wyeth-Ayerst; has received speaker and publishing fees from Advanced Meeting Partners, American Psychiatric Association, AstraZeneca, Belvoir, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Imedex, Massachusetts General Hospital Psychiatry Academy/Primedia, Massachusetts General Hospital Psychiatry Academy/Reed-Elsevier, Novartis, Organon, Pfizer, PharmaStar, UBC Pharma, and Wyeth-Ayerst; is a stock shareholder in Compellis; has patent applications for sequential parallel comparison of design and for a combination of azapirones and bupropion in major depressive disorder; and receives copyright royalties for the following Massachusetts General Hospital assessment tools: the Cognitive and Physical Functioning Questionnaire, the Sexual Functioning Inventory, the Antidepressant Treatment Response Questionnaire, the Discontinuation-Emergent Sign and Symptom scale, and SAFER. Dr Joffe has served as a consultant to Sunovion and has received grant/research support from Bayer, Forest, and GlaxoSmithKline. Dr Soares has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Lundbeck, and Pfizer and has received grant/research support from AstraZeneca, Lundbeck, and Pfizer. Drs Huang and Marshall, Ms Zummo, and Messrs Grinell and Spalding are employees of Sunovion.
Funding/support: The studies included in this post hoc analysis were sponsored by Sunovion Pharmaceuticals Inc, Marlborough, Massachusetts.
REFERENCES
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592-600. PubMed doi:10.1016/j.biopsych.2010.10.023
2. Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31(3):333-346. PubMed doi:10.1016/S0022-3956(97)00002-2
3. Thase ME. Correlates and consequences of chronic insomnia. Gen Hosp Psychiatry. 2005;27(2):100-112. PubMed doi:10.1016/j.genhosppsych.2004.09.006
4. Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14(1):35-46. PubMed doi:10.1016/j.smrv.2009.09.003
5. Bassetti CL. Nonmotor disturbances in Parkinson’s disease. Neurodegener Dis. 2011;8(3):95-108. PubMed doi:10.1159/000316613
6. Louie GH, Tektonidou MG, Caban-Martinez AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk: data from the 2007 National Health Interview Survey. Arthritis Care Res (Hoboken). 2011;63(2):247-260. PubMed doi:10.1002/acr.20362
7. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry. 1999;60(suppl 17):28-31, discussion 46-48. PubMed
8. Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59(11):1052-1060. PubMed doi:10.1016/j.biopsych.2006.01.016
9. Papadimitriou GN, Kerkhofs M, Kempenaers C, et al. EEG sleep studies in patients with generalized anxiety disorder. Psychiatry Res. 1988;26(2):183-190. PubMed doi:10.1016/0165-1781(88)90073-X
10. Stein MB, Roy-Byrne PP, Craske MG, et al. Functional impact and health utility of anxiety disorders in primary care outpatients. Med Care. 2005;43(12):1164-1170. PubMed doi:10.1097/01.mlr.0000185750.18119.fd
11. Drewes AM, Nielsen KD, Hansen B, et al. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology (Oxford). 2000;39(11):1287-1289. PubMed doi:10.1093/rheumatology/39.11.1287
12. Kirwan J, Heiberg T, Hewlett S, et al. Outcomes from the Patient Perspective Workshop at OMERACT 6. J Rheumatol. 2003;30(4):868-872. PubMed
13. Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351-358. PubMed doi:10.1016/S0029-7844(00)00930-3
14. Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262-1268. PubMed doi:10.1001/archinte.166.12.1262
15. Taylor DJ, Lichstein KL, Durrence HH, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457-1464. PubMed
16. Kaneita Y, Ohida T, Uchiyama M, et al. The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J Clin Psychiatry. 2006;67(2):196-203. PubMed doi:10.4088/JCP.v67n0204
17. Ohayon MM, Hong SC. Prevalence of major depressive disorder in the general population of South Korea. J Psychiatr Res. 2006;40(1):30-36. PubMed doi:10.1016/j.jpsychires.2005.02.003
18. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262(11):1479-1484. PubMed doi:10.1001/jama.1989.03430110069030
19. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411-418. PubMed doi:10.1016/0006-3223(95)00188-3
20. McCall WV, Reboussin BA, Cohen W. Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res. 2000;9(1):43-48. PubMed doi:10.1046/j.1365-2869.2000.00186.x
21. Roth T, Price JM, Amato DA, et al. The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis: a pilot study. Prim Care Companion J Clin Psychiatry. 2009;11(6):292-301. PubMed doi:10.4088/PCC.08m00749bro
22. Casper RC, Katz MM, Bowden CL, et al. The pattern of physical symptom changes in major depressive disorder following treatment with amitriptyline or imipramine. J Affect Disord. 1994;31(3):151-164. PubMed doi:10.1016/0165-0327(94)90024-8
23. McCall WV, Blocker JN, D’ Agostino R Jr, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11(9):822-827. PubMed doi:10.1016/j.sleep.2010.04.004
24. Nierenberg AA, Petersen TJ, Alpert JE. Prevention of relapse and recurrence in depression: the role of long-term pharmacotherapy and psychotherapy. J Clin Psychiatry. 2003;64(suppl 15):13-17. PubMed
25. Ferguson JM, Bielski RJ, Houston J, et al. Comparison of estazolam and placebo in the outpatient treatment of insomnia associated with major depression. Curr Ther Res Clin Exp. 1991;49(5):898-907.
26. Joffe H, Petrillo L, Viguera A, et al. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: a randomized, double-blinded, placebo-controlled crossover trial. Am J Obstet Gynecol. 2010;202(2):171.e1-171.e11. PubMed doi:10.1016/j.ajog.2009.10.868
27. Soares CN, Joffe H, Rubens R, et al. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1402-1410. PubMed doi:10.1097/01.AOG.0000245449.97365.97
28. Pollack M, Kinrys G, Krystal A, et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):551-562. PubMed doi:10.1001/archpsyc.65.5.551
29. Fava M, Asnis GM, Shrivastava R, et al. Zolpidem extended-release improves sleep and next-day symptoms in comorbid insomnia and generalized anxiety disorder. J Clin Psychopharmacol. 2009;29(3):222-230. PubMed doi:10.1097/JCP.0b013e3181a390ba
30. Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60(10):668-676. PubMed doi:10.4088/JCP.v60n1005
31. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928. PubMed doi:10.4088/JCP.09m05571gry
32. Scharf M. Eszopiclone for the treatment of insomnia. Expert Opin Pharmacother. 2006;7(3):345-356. PubMed doi:10.1517/14656566.7.3.345
33. Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30(8):959-968. PubMed
34. Zammit GK, McNabb LJ, Caron J, et al. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20(12):1979-1991. PubMed doi:10.1185/174234304X15174
35. Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158(10):1099-1107. PubMed doi:10.1001/archinte.158.10.1099
36. Feige B, Voderholzer U, Riemann D, et al. Fluoxetine and sleep EEG: effects of a single dose, subchronic treatment, and discontinuation in healthy subjects. Neuropsychopharmacology. 2002;26(2):246-258. PubMed doi:10.1016/S0893-133X(01)00314-1
37. Silvestri R, Pace-Schott EF, Gersh T, et al. Effects of fluvoxamine and paroxetine on sleep structure in normal subjects: a home-based Nightcap evaluation during drug administration and withdrawal. J Clin Psychiatry. 2001;62(8):642-652. PubMed doi:10.4088/JCP.v62n0812
38. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4(4):267-272. PubMed
39. Bastien CH, Valliרres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. PubMed doi:10.1016/S1389-9457(00)00065-4
40. Groeger JA, Zijlstra FR, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res. 2004;13(4):359-371. PubMed doi:10.1111/j.1365-2869.2004.00418.x
41. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988
42. Roth T, Jaeger S, Jin R, et al. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60(12):1364-1371. PubMed doi:10.1016/j.biopsych.2006.05.039
43. Sivertsen B, Krokstad S, טverland S, et al. The epidemiology of insomnia: associations with physical and mental health: the HUNT-2 study. J Psychosom Res. 2009;67(2):109-116. PubMed doi:10.1016/j.jpsychores.2009.05.001
44. Terzano MG, Parrino L, Cirignotta F, et al; Studio Morfeo Committee. Studio Morfeo: insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004;5(1):67-75. PubMed doi:10.1016/j.sleep.2003.09.006
45. Budhiraja R, Roth T, Hudgel DW, et al. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859-867. PubMed
46. Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26(6):667-672. PubMed
47. Shin C, Lee S, Lee T, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59(4):395-402. PubMed doi:10.1111/j.1440-1819.2005.01391.x
48. Poulin J, Chouinard S, Pampoulova T, et al. Sleep habits in middle-aged, non-hospitalized men and women with schizophrenia: a comparison with healthy controls. Psychiatry Res. 2010;179(3):274-278. PubMed doi:10.1016/j.psychres.2009.08.009
49. Krystal AD, Erman M, Zammit GK, et al; ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31(1):79-90. PubMed
50. Xu M, Bélanger L, Ivers H, et al. Comparison of subjective and objective sleep quality in menopausal and non-menopausal women with insomnia. Sleep Med. 2011;12(1):65-69. PubMed doi:10.1016/j.sleep.2010.09.003
51. Bourey RE. Primary menopausal insomnia: definition, review, and practical approach. Endocr Pract. 2011;17(1):122-131. PubMed doi:10.4158/EP08121.RA
52. National Institutes of Health. National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28(9):1049-1057. PubMed
53. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504. PubMed
54. Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med. 2006;2(2):S19-S26. PubMed