Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Osmotic demyelination syndrome is a feared complication of rapid correction of hyponatremia. While central pontine myelinosis is a well-known complication of rapid correction of chronic hyponatremia, physicians should be aware that myelinosis can occur outside the pons as well. The presence of extrapontine myelinosis resulting from osmotic demyelination has been detected during autopsy in up to 80% of demyelination cases.
Rapid Correction of Chronic Hyponatremia Secondary to Psychogenic Polydipsia: Hypoxic Injury or Extrapontine Myelinolysis?
To the Editor: Osmotic demyelination syndrome is a feared complication of rapid correction of hyponatremia. While central pontine myelinosis is a well-known complication of rapid correction of chronic hyponatremia, physicians should be aware that myelinosis can occur outside the pons as well. The presence of extrapontine myelinosis resulting from osmotic demyelination has been detected during autopsy in up to 80% of demyelination cases.1
Case report. Ms A is a 43-year-old single white woman on social security disability with a court-appointed guardian and a past medical history significant for schizoaffective disorder, panic disorder, and borderline personality disorder. She had been maintained on quetiapine and lamotrigine for her psychiatric conditions for several years. The reasoning behind lamotrigine use was unclear, since she did not have a diagnosed mood disorder. She was moved to a group home 5 months before her presentation to our hospital so that she could be monitored closely, as she had been reported to suffer from psychogenic polydipsia chronically and had poor medication compliance. At that time, her medications were changed for unclear reasons to paliperidone palmitate 156 mg intramuscular injection every 28 days, tegretol 200 mg 3 times a day, and aripiprazole of an unreported dosage, which was never ascertained throughout her stay in our hospital. She was compliant with her medications according to the group home staff that monitored medications and intake. She was caught, however, violating her prescribed fluid restriction regimen several times by drinking from bathroom toilets and faucets. Employees at the group home reported that she was often not oriented to place or time but did not seem to respond to internal stimuli. They additionally reported that Ms A typically avoided social contact, would often quote biblical passages in conversation, and had frequent sexual delusions. She was taken to an emergency department at a rural facility after her family witnessed her have a seizure and hit her face on the sidewalk.
When Ms A presented to the rural emergency department, she was found to be in status epilepticus. Her sodium level was 107 mmol/L. A head computed tomography scan (CT) was performed, and no intracranial pathology or evidence of hemorrhage was found. There was no evidence of cerebral edema at that time. Due to her status epilepticus, a magnetic resonance image (MRI) scan was delayed and not obtained. There was a high suspicion that this hyponatremia was chronic due to her reported psychogenic polydipsia and previous laboratory values indicating borderline hyponatremia over the past several years. Her sodium level was rapidly corrected to 126 mmol/L after a 100-mm bolus of 3% hypertonic saline was administered. Due to her status epilepticus, she was sedated and intubated with propofol, midazolam, and fentanyl and was transported to our facility. Before she arrived, a second CT scan was obtained, and no signs of cerebral edema were noted. This CT scan was estimated by emergency medical service staff to have been completed between 7.5 and 8.5 hours after arrival at the rural facility and before transportation to our facility.
Upon arrival at our hospital, Ms A’s sodium level was 134 mmol/L. Her sodium level, therefore, had been corrected from 107 mmol/L to 134 mmol/L in a span of 10.5 hours at the rural facility. A point-of-care venous blood gas level was drawn that showed a venous pH level of 7.38, with the venous pCO2 level low at 34.5 mm Hg and the venous pO2 level in the normal range at 42 mm Hg. An arterial blood gas (ABG) level was obtained 4 hours after her arrival, after her intubation, that showed her arterial pO2 level to be 162 mm Hg and her pCO2 level low at 31 mm Hg. No arterial blood gas laboratory values were available from the rural facility. She was started on 1 g of levetiracetam twice a day. To prevent osmotic demyelination syndrome and promote free water retention, she was also started on 2-4 μg of desmopressin every 6 hours as needed. Her intravenous fluids were adjusted to bring the serum sodium to a goal level of 118-120 mmol/L. The decision was made to not repeat a CT scan or to obtain an MRI at that time and to wait 2-6 days in the belief that evidence of osmotic demyelination would not be immediately apparent.
An electroencephalogram (EEG) was performed on Ms A’s third day of hospitalization and revealed generalized slow activity intermixed with spindle activity, which was consistent with moderate, generalized, nonspecific, cerebral dysfunction. Throughout this time, Ms A was unable to follow commands or track verbal or visual stimuli with eye movements; this behavior continued even when the patient was off sedation but still intubated on hospital day 6. After hospital day 6, Ms A began to show signs of slight improvement by exhibiting ability to follow 1-step commands and was oriented to person, time, and, sometimes, place. At this time, the first and only MRI performed at our institution was obtained with and without contrast. It showed multifocal, nonspecific, symmetric cortical T2 hyperintensity in the cerebellum, possibly related to posterior reversible encephalopathy syndrome, cerebellitis, toxic or metabolic insult, or other remote insult. The diffusion tensor MRI showed increased bilateral signal in the cerebellum adjacent to the brain stem. In the diffusion sequence, the MRI was reported as abnormal, with hyperintensity and restricted diffusion in the cerebellar hemispheres and in the pons and medulla. No abnormal contrast enhancement and no other abnormalities were reported. No previous imaging or EEGs were available from outside facilities for comparison. At this time, it was decided by the multidisciplinary team in charge of Ms A’s care that this MRI abnormality was most likely due to either suspected hypoxic injury secondary to status epilepticus or extrapontine demyelination syndrome. While no blood gas levels were available during the seizure that she experienced in the rural emergency department, the prolonged seizure of greater than a reported 10 minutes led to the belief that hypoxia was a possible secondary consequence. Neither diagnosis could be excluded at that time, thus it was decided that subsequent narrowing of the differential diagnosis would depend on Ms A’s clinical progression and outcome. After the MRI, no further cranial imaging was performed during her stay at our hospital. On hospital day 10, Ms A was extubated and continued to have moderate, diffuse cerebral dysfunction on the EEG. When assessed, the patient was uncooperative with the mental status examination and was not oriented to time, person, or place. Her sensorium was clouded, and her affect was noticeably flat. On hospital day 12, Ms A began to progressively deteriorate by having only a fixed blinking stare, not tracking verbal and visual stimuli, and not following commands. She was reintubated due to respiratory failure and inability to control oral secretions and increased risk of aspiration. Her ABG level at that time showed a basic pH of 7.47 and a low arterial pO2 of 65 mm Hg. She was reextubated once again on hospital day 13.
On hospital day 16, Ms A continued to not be oriented to time, place, or person but finally was able to begin to follow 2-step commands. By the 20th day of her hospitalization, she was able to track verbal stimuli with her eye movements and showed improvement by being oriented to person, place, and time. Additionally, she denied current hallucinations and paranoia. There was, however, significant thought blocking still present. Her affect continued to be flat with a dysphoric mood, but she demonstrated goal-directed thought processing. This finding correlates with her endorsed baseline level of functioning as reported by her group home caregivers. Psychiatrists were still unable to talk with Ms A extensively due to her significant dysarthria, which was not present at baseline (at the group home). In addition, she was experiencing significant dysarthria that was prolonging her hospital course and stay. Ms A was scheduled for outpatient gastric tube placement due to continued aspiration risk. She endorsed "learning her lesson" to not drink excessive amounts of water. At this time, Ms A was reinitiated on aripiprazole 5 mg orally since she was able to finally handle oral intake. It was recommended to the group home staff and her future psychiatrist to consider clozapine for long-term management. It was also recommended that Ms A receive new placement to a facility with 24-hour monitoring of patients so that she could receive a higher level of care due to her recent psychiatric issues being beyond the scope and capabilities of her current group home.
Ms A followed up with outside facilities after her discharge, and her records were unable to be obtained. Subsequent intracranial imaging studies that may have been performed since her discharge were not available.
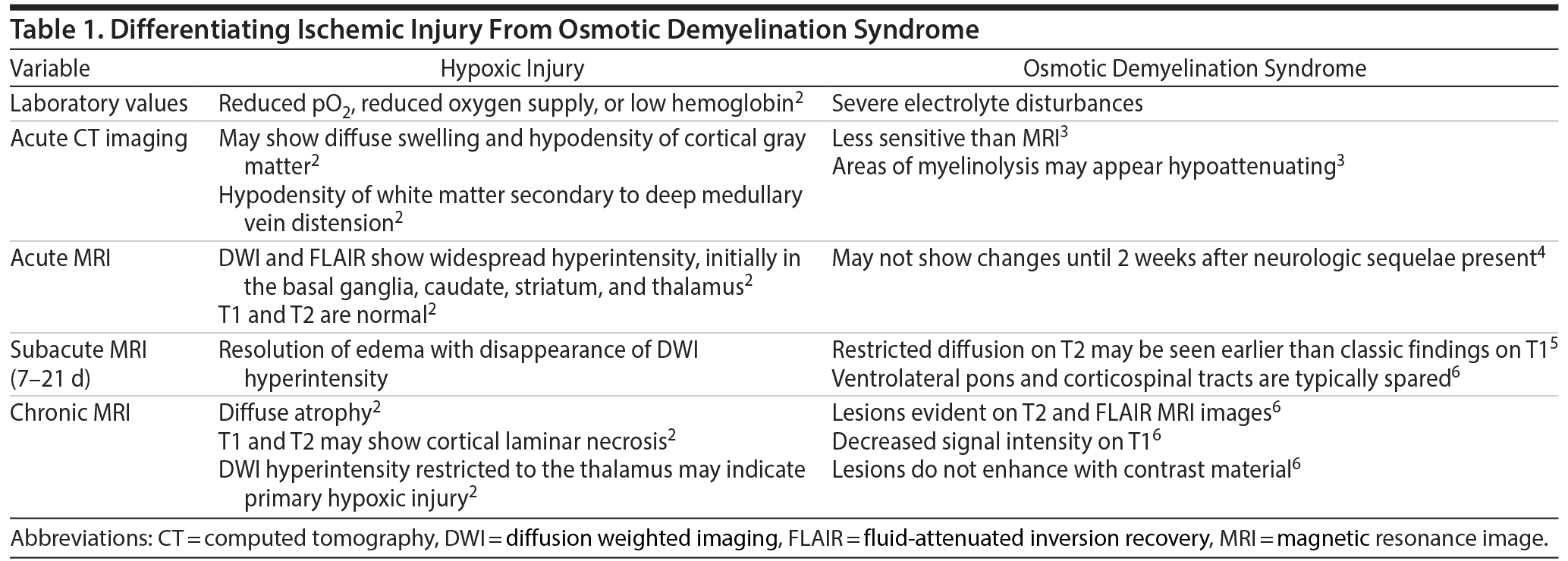
The constellation of Ms A’s symptoms brought several theories to the table throughout her hospital stay, including ischemia due to her status epilepticus and osmotic demyelination syndrome. In fact, the general theme for much of her hospital stay, especially at the beginning, was to prevent osmotic demyelination syndrome. While at the end of her hospital stay it was decided by the neurology team that the MRI findings found at our facility were most likely representative of hypoxic injury secondary to her experienced status epilepticus, it is important to recognize and entertain the possibility of extrapontine osmotic demyelination and that MRI findings can be delayed and may have not been evident yet (Table 1). Dysphagia, disorientation, and dysarthria, all of which Ms A experienced, have been reported to be sequelae of extrapontine osmotic demyelination.
Osmotic demyelination is caused by damage to the myelin sheath of brain cells that typically presents in the central pontine demyelination, but extrapontine involvement is also common.7 Osmotic demyelination syndrome has been associated with chronic hyponatremia correction, alcoholism, and liver transplant.7 There is a general consensus that acute hyponatremia can be corrected rapidly, but chronic hyponatremia, like when brought on by psychogenic polydipsia, cannot. The general recommendation is to not correct the hyponatremia greater than 8 mmol/L/d.8 Central pontine and extrapontine myelinolysis has been described in the literature with a variety of physical manifestations including rapidly evolving flaccid quadriplegia, weakness of the face and tongue, and, of particular importance to the case of Ms A, inability to speak and swallow.9 Clinical manifestations of extrapontine myelinolysis include akinesias, ataxia, catatonia, cogwheel rigidity, disorientation, dysarthria, dystonia, extrapyramidal symptoms, emotional lability, mutism, myoclonus, and tremor.9 Of note, Ms A experienced significant disorientation and dysarthria once extubated. In particular, significant dysphagia that was not present before her hospitalization was observed following her extubation. At the time of Ms A’s discharge, she continued to have aspiration with nectar consistencies.
Clinical or radiographic evidence of osmotic demyelination can occur as early as the first hospital day after the insult but can be delayed by up to 16 days.1 Additionally, patients can often demonstrate a biphasic course in which original symptoms are due to nonlocalizing encephalopathy secondary to the initial hyponatremia followed by a period of improvement and then a subsequent development of osmotic demyelination syndrome.1 Extrapontine myelinolysis changes can occur with or without central pontine myelinolysis changes. In 1 study,8 two-fifths of the causes of central nervous system myelinolysis were isolated extrapontine myelinolysis without central pontine changes. MRI results typically indicative of osmotic demyelination show symmetric T2 signal abnormalities, which are typical of metabolic abnormalities.10 Extrapontine myelinolysis lesions typically occur in the cerebellum in 33% of cases.1,10 These changes are consistent with what was observed in Ms A’s MRI. It is important to note, however, that hypoxia during status epilepticus could also present with similar MRI findings. Additionally, extrapontine myelinolysis lesions can also occur in the external and extreme capsule, basal ganglia, thalamus, and hippocampus,10 none of which were observed in Ms A. When lesions are present and seen on CT, they are typically symmetric and hypodense.10
Of further note, hypokalemia has also been implicated as an additive cause and may predispose patients to develop osmotic demyelination when associated with hyponatremia.11,12 Koul et al11 hypothesized that "reduced endothelial cell membrane concentration of NaK-ATPase in hypokalemia may predispose the cell to injury by osmotic stress associated with the rapid rise in the serum sodium concentration."(p233) Due to this new implicating evidence, physicians in charge of Ms A acted appropriately by aggressively treating her hypokalemia to help prevent osmotic demyelination.
While osmotic demyelination syndrome has a poor prognosis, there has been evidence in animal models and human case reports that relowering the serum sodium level after the onset of neurologic symptoms can improve outcomes. In a rat model, it was demonstrated that relowering the sodium level after neurologic deficits appeared and relowering it sooner (within 4 hours vs 8-10 hours) resulted in better outcomes with regard to survival and resolution of neurologic deficits.13 Additionally, 2 case reports14,15 regarding human subjects reported that after initial onset of neurologic sequelae, rapid relowering of serum sodium level and slowly trending the sodium level back up resulted in full neurologic recovery. Additionally, evidence supporting plasmapheresis exists clinically in a case report16 of 3 hyponatremic patients treated with plasmapheresis after diagnosis of osmotic demyelination syndrome. While the patients’ MRIs remained unchanged, 2 of the patients markedly improved, while 1 improved but still had impairment in memory and gait.16
Research and recommendations regarding prevention, as well as clinical and radiologic sequelae of osmotic demyelination syndrome, is ongoing and ever evolving. Recommendations for rate of sodium correction, relowering the sodium level after rapid correction, the role of hypokalemia, and extrapontine manifestations are just a few of the most recently discussed and debated topics regarding osmotic demyelination syndrome. It is important for physicians to recognize the distinct population of patients suffering from psychological illnesses who may be at increased risk for psychogenic polydipsia. Physicians need to consider this risk in the differential diagnosis of patients with a history of psychological illness and recognize that psychogenic polydipsia creates a chronic hyponatremia, not acute, and thus must be corrected slowly. It is also important for physicians to be aware of the ongoing mounting evidence that extrapontine myelinolysis can occur and result in clinical manifestations. While this diagnosis was difficult to make in the case of Ms A due to her concomitant witnessed hypoxia and status epilepticus, it is important to recognize that she did experience symptoms consistent with the diagnosis, and, therefore, the diagnosis should be and was correctly considered.
References
1. de Souza A, Desai PK. More often striatal myelinolysis than pontine? a consecutive series of patients with osmotic demyelination syndrome. Neurol Res. 2012;34(3):262-271. PubMed doi:10.1179/1743132812Y.0000000009
2. Howard RS, Holmes PA, Koutroumanidis MA. Hypoxic-ischaemic brain injury. Pract Neurol. 2011;11(1):4-18. PubMed doi:10.1136/jnnp.2010.235218
3. Miller GM, Baker HL Jr, Okazaki H, et al. Central pontine myelinolysis and its imitators: MR findings. Radiology. 1988;168(3):795-802. PubMed doi:10.1148/radiology.168.3.3406409
4. Kumar SR, Mone AP, Gray LC, et al. Central pontine myelinolysis: delayed changes on neuroimaging. J Neuroimaging. 2000;10(3):169-172. PubMed doi:10.1111/jon2000103169
5. Cramer SC, Stegbauer KC, Schneider A, et al. Decreased diffusion in central pontine myelinolysis. AJNR Am J Neuroradiol. 2001;22(8):1476-1479. PubMed
6. Osborn AG, Cooper JA, Castillo M, et al. Diagnostic Imaging: Brain. Salt Lake City, Utah: Amirsys; 2004.
7. Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. 2014;21(12):1443-1450. PubMed doi:10.1111/ene.12571
8. Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004;75(suppl 3):iii22-iii28. PubMed doi:10.1136/jnnp.2004.045906
9. Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol. 2000;13(6):691-697. PubMed doi:10.1097/00019052-200012000-00014
10. Alleman AM. Osmotic demyelination syndrome: central pontine myelinolysis and extrapontine myelinolysis. Semin Ultrasound, CT MRI. 2014;35(2):153-159.
11. Koul PA, Khan UH, Jan RA, et al. Osmotic demyelination syndrome following slow correction of hyponatremia: possible role of hypokalemia. Indian J Crit Care Med. 2013;17(4):231-233. PubMed doi:10.4103/0972-5229.118433
12. Lohr JW. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994;96(5):408-413. PubMed doi:10.1016/0002-9343(94)90166-X
13. Soupart A, Penninckx R, Stenuit A, et al. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol. 1996;55(5):594-601. PubMed doi:10.1097/00005072-199605000-00011
14. Soupart A, Ngassa M, Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51(6):383-386. PubMed
15. Soupart A, Penninckx R, Stenuit A, et al. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol. 1996;55(5):594-601. PubMed doi:10.1097/00005072-199605000-00011
16. Bibl D, Lampl C, Gabriel C, et al. Treatment of central pontine myelinolysis with therapeutic plasmapheresis. Lancet. 1999;353(9159):1155. PubMed doi:10.1016/S0140-6736(99)01145-9
aDepartment of Neurology, University of Utah, Salt Lake City
bDepartment of Psychiatry, Banner Thunderbird Medical Center, Glendale, Arizona
cDepartment of Psychiatry, University of Nebraska Medical Center, Omaha
dDepartment of Pediatrics, University of North Carolina Medical Center, Chapel Hill
Potential conflicts of interest: None.
Funding/support: None.
Published online: October 20, 2016.
Prim Care Companion CNS Disord 2016;18(5):doi:10.4088/PCC.15l01872
© Copyright 2016 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!