Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Valproate is indicated for treatment of seizure disorders and mania in bipolar disorder and is prescribed off-label to treat impulsivity, aggression, irritability, and agitation in other disorders. Prescribing information for valproate includes warnings for hyperammonemic encephalopathy in individuals with urea cycle disorders and for hyperammonemia despite normal liver function tests. A cohort study of 123 valproate-treated psychiatric inpatients (mean age of 45.1 years) reported a valproate-related hyperammonemia rate of 51.2%.
Valproate-Related Hyperammonemia in Older Adult Psychiatric Inpatients
To the Editor: Valproate is indicated for treatment of seizure disorders and mania in bipolar disorder and is prescribed off-label to treat impulsivity, aggression, irritability, and agitation in other disorders. Prescribing information for valproate includes warnings for hyperammonemic encephalopathy in individuals with urea cycle disorders and for hyperammonemia despite normal liver function tests. A cohort study of 123 valproate-treated psychiatric inpatients (mean age of 45.1 years) reported a valproate-related hyperammonemia rate of 51.2%.1 Neuropsychiatric manifestations of valproate-related hyperammonemia can include lethargy, tremor, ataxia, and mental status changes, although patients may be asymptomatic.1-3 In older adult patients, age-related metabolic changes and greater vulnerability of the aged brain may increase risk for mental status changes.
Published literature on valproate-related hyperammonemia in older adults is limited to case reports without comparison to patients not taking valproate.4 This study aimed to ascertain the clinical significance of valproate-related hyperammonemia in older adult psychiatric inpatients by determining (1) whether, in the absence of liver disease, valproate-related hyperammonemia is more frequent in older adult psychiatric inpatients taking valproate compared to those not taking valproate and (2) the frequency of clinically significant adverse effects of valproate-related hyperammonemia in older adult psychiatric inpatients.
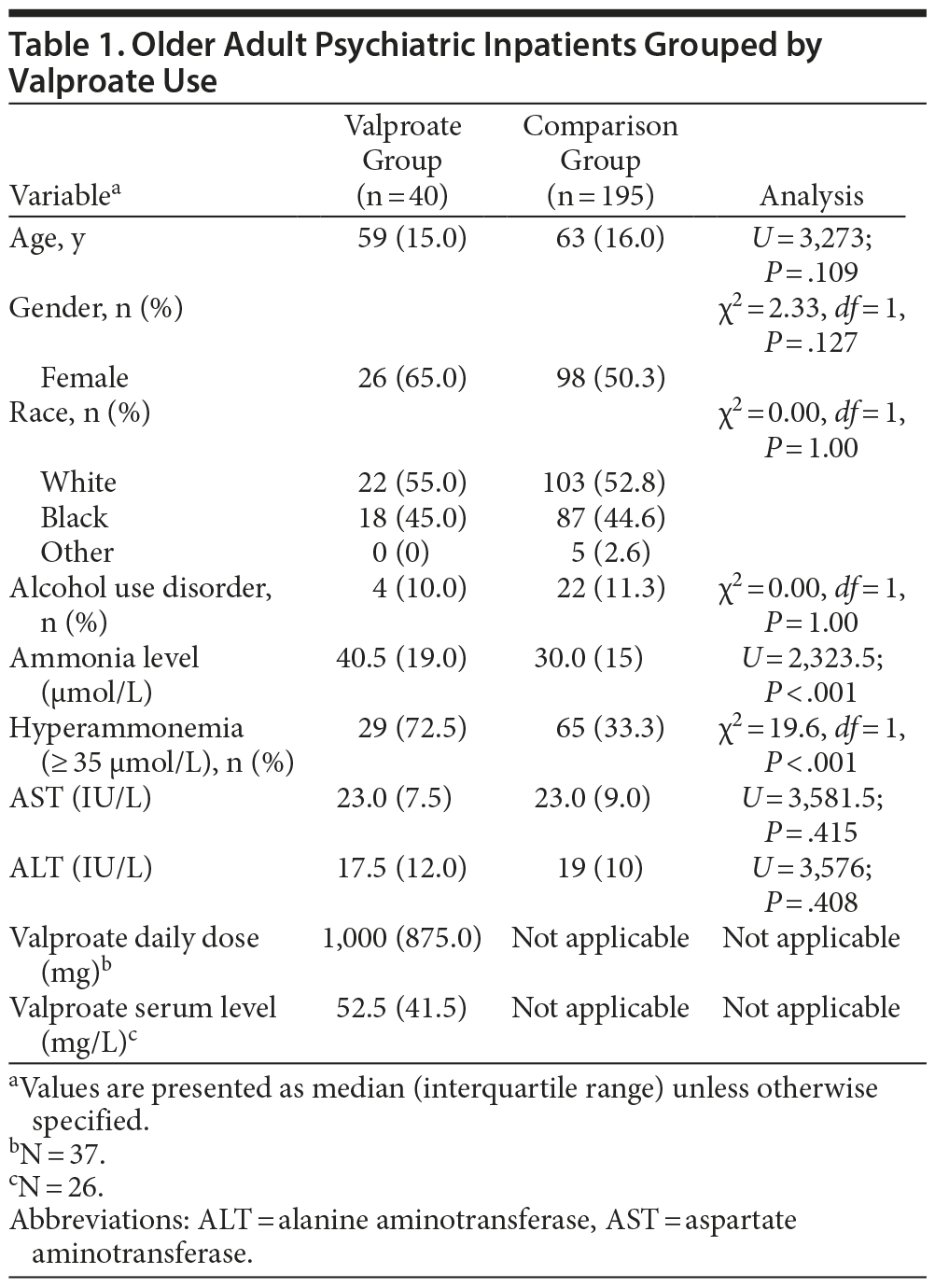
Method. This retrospective chart review study, approved by the University of Maryland Institutional Review Board, examined the medical records of all admissions to the Older Adult Psychiatric Inpatient Unit at the University of Maryland Medical Center, Baltimore, Maryland, from October 2007 to April 2010. A total of 235 patients met the following inclusion criteria: (1) age ≥ 50 years, (2) available routine admission screening levels of ammonia, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), (3) AST and ALT levels less than twice the upper normal limits, and (4) no history of active liver disease. Hyperammonemia was defined as a plasma ammonia level ≥ 35 µmol/L.5
Patients were grouped by valproate status at admission. Continuous variables were analyzed by Mann-Whitney U test for grouped analyses and Spearman Ï for correlations. Categorical variables were analyzed by χ2 tests with continuity correction for 2 group analyses. Analysis by race was limited to the 2 most populated categories. All tests were 2-tailed with statistical significance set as P ≤ .05 and performed using SPSS 21.0 (SPSS Inc, Chicago, Illinois).
Results. Median ammonia level and rate of hyperammonemia in patients taking valproate at admission were significantly greater than in patients not taking valproate at admission (Table 1). Otherwise, there were no significant differences between groups on variables assessed. In patients taking valproate, ammonia level correlated significantly with valproate dose (Ï = 0.337, n = 37, P = .042), but not with serum levels of valproate (Ï = −0.015, n = 26, P = .940), AST (Ï = 0.059, P = .718), or ALT (Ï = 0.181, P = .264). Diagnoses (DSM-IV) of the 40 patients taking valproate were mood disorders (n = 18), schizoaffective disorder (n = 10), schizophrenia (n = 6), and dementia with behavioral disturbance (n = 6). The 29 patients with valproate-related hyperammonemia were compared to the 11 patients taking valproate without hyperammonemia; the only variable demonstrating significant difference was valproate dose (U = 81, P = .033). Patients with hyperammonemia had a higher median valproate daily dose of 1,000 mg compared to 750 mg in patients without hyperammonemia. Three of 29 patients with valproate-related hyperammonemia (mean ammonia level of 74 µmol/L) had clinically significant adverse effects—cognitive impairment in all 3 and tremors in one.
To our knowledge, this is the first study to examine the frequency of hyperammonemia in a sample of older adults taking valproate compared to those not taking valproate. Valproate use increased risk for hyperammonemia 2-fold (relative risk = 2.18, confidence interval = 1.65-2.86). Clinically significant adverse effects occurred in approximately 10% of patients with valproate-related hyperammonemia at ammonia levels around twice the upper normal limit. Clinicians should obtain ammonia levels when mental status changes arise in older adults taking valproate.
References
1. Raja M, Azzoni A. Valproate-induced hyperammonaemia. J Clin Psychopharmacol. 2002;22(6):631-633. PubMed doi:10.1097/00004714-200212000-00019
2. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates and management. Gen Hosp Psychiatry. 2012;34(3):290-298. PubMed doi:10.1016/j.genhosppsych.2011.12.009
3. Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695-714. PubMed doi:10.2165/00023210-200216100-00004
4. Holroyd S, Overdyke JT. Hyperammonemia associated with valproic acid use in elderly psychiatric patients. J Neuropsychiatry Clin Neurosci. 2012;24(3):372-374. PubMed doi:10.1176/appi.neuropsych.10100151
5. Summar M, Tuchman M. Proceedings of a consensus conference for the management of patients with urea cycle disorders. J Pediatr. 2001;138(suppl 1):S6-S10. PubMed doi:10.1067/mpd.2001.111831
Author affiliation: Department of Psychiatry, Division of Geriatric Psychiatry, University of Maryland Medical Center, Baltimore, Maryland. Potential conflicts of interest: None reported.
Funding/support: None reported.
Previous presentation: Presented at the Annual Meeting of the American Association for Geriatric Psychiatry, Late-Breaking Poster Session; March 15, 2014; New Orleans, Louisiana.
Published online: April 2, 2015.
Prim Care Companion CNS Disord 2015;17(2):doi:10.4088/PCC.14l01737
© Copyright 2015 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!