Click to enlarge page
Patients with major depressive disorder (MDD) who have responded to or remitted from antidepressant treatment frequently have persistent residual symptoms (eg, fatigue, sleep problems, cognitive symptoms) that interfere with their functioning and quality of life and significantly increase the risk of relapse. Clinicians need to systematically evaluate for all depressive symptoms at baseline, so that some are not missed and the individual patient’s symptoms can be tracked across all phases of treatment. Developing a list of target problems that the patient would like to see improve is a helpful tool in this process.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of a series of teleconferences on “Residual Symptoms in Major Depressive Disorder: Prevalence, Effects, and Management,” which were held in November 2012. The teleconference series was chaired by John Zajecka, MD, Director, Depression Treatment Research Center, and Associate Professor of Psychiatry, Rush University Medical Center, Chicago, Illinois. The faculty were Susan G. Kornstein, MD, Professor of Psychiatry and Obstetrics/Gynecology and Director of Clinical Research, Department of Psychiatry, and Executive Director, Institute for Women’s Health, Virginia Commonwealth University, Richmond, Virginia; and Pierre Blier, MD, PhD, Professor, Department of Psychiatry and Cellular and Molecular Medicine, at the University of Ottawa and Director of the Mood Disorders Research Unit at the University of Ottawa Institute of Mental Health Research, Royal Ottawa Mental Health Centre in Ottawa, Ontario, Canada.
Financial disclosures: Dr Zajecka has received grants/research support from AstraZeneca, Cyberonics, Euthymics, Hoffmann-LaRoche, Otsuka, Pfizer, Shire, and Takeda and has served as a consultant for Abbott, Eli Lilly, Lundbeck, Otsuka, PamLab, Takeda, and Shire. Dr Kornstein has received grants/research support from Bristol-Myers Squibb, Euthymics, Forest, Otsuka, and Pfizer and has served as a consultant for Allergan, Eli Lilly, Forest, Lundbeck, Pfizer, Takeda, and Trovis. Dr Blier has received grants/research support from AstraZeneca, Bristol-Myers Squibb, Janssen, Labopharm, Lundbeck, Merck, Pfizer, and Servier and has served as a consultant or lecturer for AstraZeneca, Bristol-Myers, Eli Lilly, Janssen, Lundbeck, Merck, Pfizer, Servier, and Takeda.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this Academic Highlights was provided by Takeda Pharmaceuticals U.S.A., Inc. and Lundbeck. The faculty acknowledges Ruth Ross, Project Manager, Healthcare Global Village, for editorial assistance in developing the manuscript. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc, the publisher, or the commercial supporter.
This article is distributed by Takeda and Lundbeck for educational purposes only.
J Clin Psychiatry 2013;74(4):407-414 (doi:10.488/JCP.12059ah1)
© Copyright 2013 Physicians Postgraduate Press, Inc.
INTRODUCTION
Patients with major depressive disorder (MDD) who have responded to or remitted from antidepressant treatment frequently have persistent residual symptoms (eg, fatigue, sleep problems, cognitive symptoms) that may interfere with their functioning and quality of life.1-3 Residual MDD symptoms also significantly increase the risk of depressive relapse.4 To effectively target residual symptoms, clinicians need to assess for depressive symptoms throughout all phases of treatment. The goal of this article is to help psychiatrists, primary care physicians, and other mental health professionals identify and manage residual symptoms of MDD and understand their impact on long-term functioning and risk of relapse or recurrence of a major depressive episode (MDE).
Acute Phase of MDD
Acute Symptom Presentations of MDD
Depressive symptoms should be systematically assessed in the acute phase of MDD to obtain a reliable baseline against which symptoms can be tracked over time. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)5 lists 9 symptom criteria for an MDE, 5 or more of which must have been present during the same 2-week period. MDD symptoms can be conceptualized in 3 categories: emotional, physical, and cognitive. At least 1 symptom must be depressed mood (criterion A1) or loss of interest or pleasure (criterion A2), and the individual must have other symptoms from a list of 7 additional criteria: significant changes in weight or appetite; changes in sleep pattern; psychomotor agitation or slowing; physical or mental fatigue or lack of energy; guilt/feelings of worthlessness; diminished ability to think, concentrate, and make decisions; and recurrent thoughts of death or suicidal ideation, plans, or attempts (A3-A9). To be diagnosed with MDD, the patient must not have a history of manic, mixed, or hypomanic episodes, and the symptoms must not be due to substance use (eg, cocaine withdrawal) or the direct effects of a general medical condition (eg, multiple sclerosis, hypothyroidism).5 The Baltimore Epidemiologic Catchment Area 13-year follow-up study6 found that the most common MDD symptoms were depressed mood, sleep problems, and diminished ability to think and concentrate, followed by thoughts of death, appetite problems, tiredness/fatigue, loss of interest, and, finally, worthlessness and slowness/restlessness. Presentations of MDD vary significantly from one patient to another, reflecting the fact that MDD appears to be a multifactorial disease that requires clinicians to assess and manage multiple symptoms.
Establishing a Symptomatic Baseline: The Target Problem List
It is important to differentiate symptoms that emerge during treatment from those that persist from baseline, because this has important treatment implications. A systematic evaluation of symptoms at baseline helps ensure some symptoms are not missed and can help the clinician track the patient’s symptoms over the course of illness. Because each patient experiences MDD in an individual way (the “fingerprint” of that person’s illness), it is useful to ask the patient, when first assessed, to provide a list of target problems that the patient would like to see improve. This approach allows patients to express in their own words how they are experiencing depression. The clinician can then ask follow-up questions to better understand each symptom and what may be causing it. This list serves as a tracking tool that the clinician can ask about at each visit to learn which symptoms have improved or worsened and if any new problems have emerged.
Screening Tools
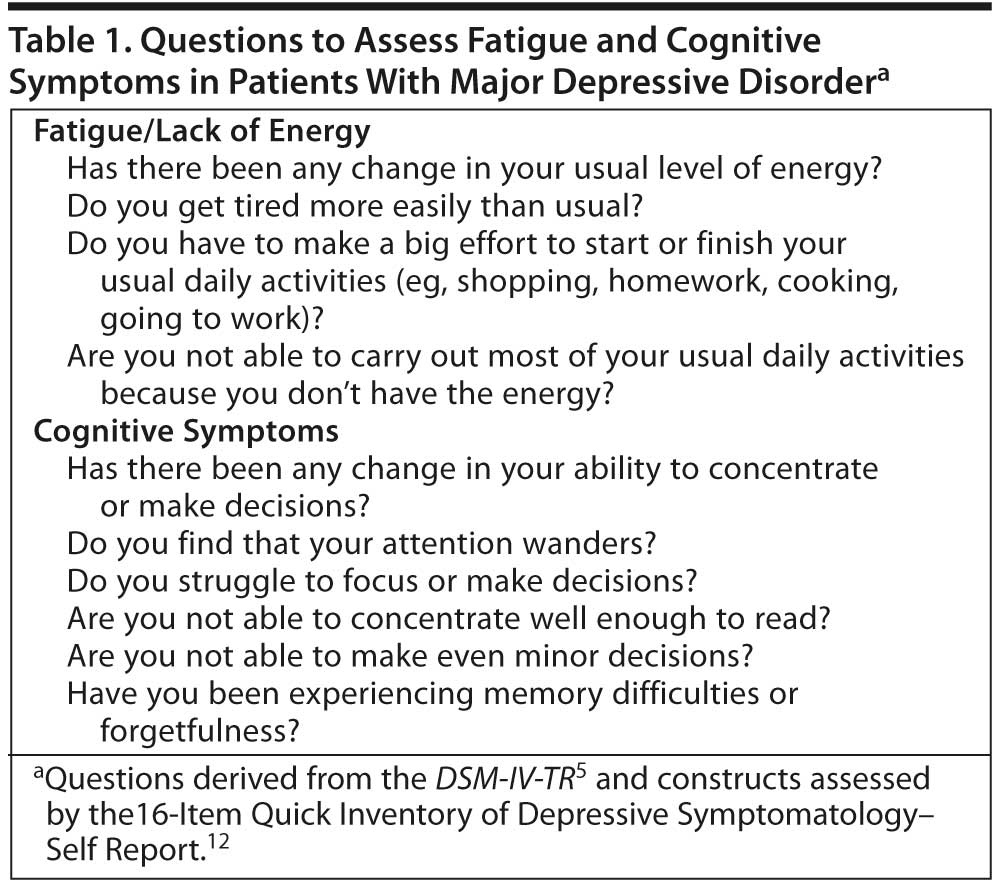
A number of screening instruments have been developed for use in clinical practice, such as the 9-item Patient Health Questionnaire (PHQ-9),7 which screens for major depression; however, a limitation of the PHQ-9 is that it does not assess impairment and differential diagnostic criteria.8 A number of scales are widely used in research, including the Hamilton Depression Rating Scale (HDRS),9 the Montgomery-Asberg Depression Rating Scale,10 and the Inventory of Depressive Symptomatology (IDS).11 The 16-item self-report version of the IDS, the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR16),12 is more suitable for use in clinical practice. Specific aspects of depression are assessed by scales such as the Sheehan Disability Scale,13 which assesses functional impairment in work/school, social life, and home/family life, and the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ),14 which measures cognitive and executive dysfunction and fatigue in mood and anxiety disorders. Although some of these scales are not practical for use in day-to-day clinical settings, they can help clinicians learn what types of questions to ask to elicit information about depressive symptoms at baseline and as treatment progresses (Table 1). This type of careful clinical evaluation helps ensure that the clinician is adequately assessing for all MDD symptoms, including fatigue and cognitive symptoms. To track progress from one visit to the next, it is also helpful to ask patients what percentage change would be needed for them to get back to what they consider 100% well.
Symptoms of MDD That May Not Be Recognized
Clinicians may not recognize fatigue/lack of energy (criterion A6) and diminished ability to think/concentrate or indecisiveness (criterion A8) as symptoms of MDD, perhaps because these symptoms are also associated with many other medical and psychiatric disorders. Patients may not report these symptoms because they do not recognize them as part of the depression.
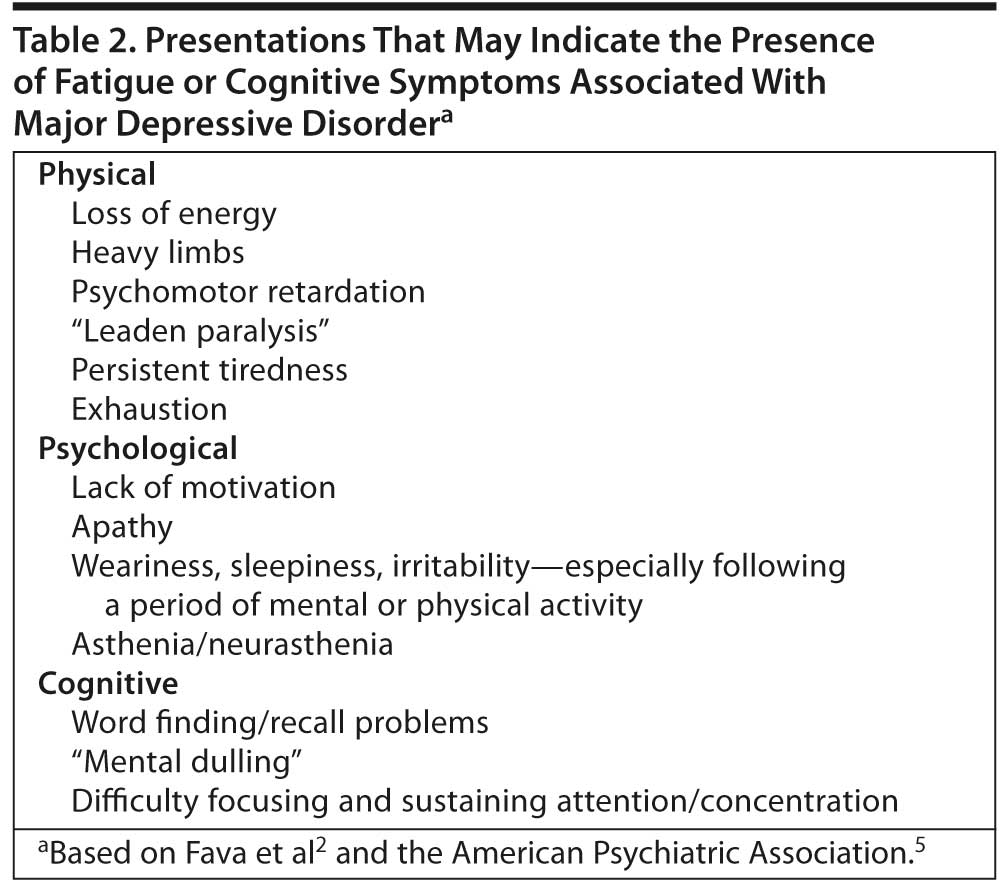
Fatigue/lack of energy. Fatigue is among the most common residual symptoms of MDD.1,3 Patients may complain of sustained fatigue with no physical exertion and that even minor tasks require more energy than they feel they have. They may be less efficient and complain that it takes them much longer than usual to complete activities of daily living.5 The differential diagnosis of fatigue in MDD is complicated by the many other conditions associated with fatigue (eg, stress, primary sleep disorders, general medical conditions such as fibromyalgia, anemia, heart failure, and hypothyroidism), many of which, when identified, can be effectively treated. Table 2 lists presentations that may suggest fatigue associated with MDD.
To conceptualize fatigue in MDD, it is helpful to consider research concerning rheumatoid arthritis (RA). Fatigue is a common symptom of RA, and its absence characterizes remission. Pollard et al15 demonstrated that, while fatigue improves with effective RA treatment, fatigue associated with RA is linked to pain and depression, and improvement of fatigue is linked to improvement of pain. These findings suggest that fatigue may be centrally mediated and secondary to the RA pathophysiology. Applying this model to MDD may help clinicians understand that fatigue may not be part of the same entity as the other core symptoms of MDD, but may be mediated by other pathophysiologic processes that current treatments do not adequately address.
Cognitive symptoms. Many patients with MDD have cognitive symptoms (eg, difficulty thinking and concentrating, memory difficulties and forgetfulness, and trouble making decisions) that significantly interfere with their ability to function and may make it difficult for them to participate in and benefit from psychotherapeutic interventions (eg, cognitive-behavioral therapy). Cognitive symptoms can be particularly problematic for active patients and those with academically or occupationally challenging roles.5 Because patients may not recognize that these problems are related to depression, reports from significant others may be helpful. The DSM-IV-TR indicates that this MDD criterion (A8) can be diagnosed on the basis of the patient’s subjective account or the observation of others.5 Cognitive symptoms can present in a variety of ways, making them difficult to identify (Table 2). However, they are an important treatment target, since they are among the most common residual symptoms of MDD3,16 and, as such, may be less likely to respond to available treatments.
Cognitive symptoms must be distinguished from antidepressant side effects.17 They may also be mistaken for early dementia in the elderly. However, in depression-related “pseudodementia,” the memory problems largely resolve when the depression is successfully treated. If they do not, this suggests possible dementia.5 Cognitive symptoms associated with MDD also tend to develop over a brief time, unlike the more gradual decline seen in dementia.5
Defining Response and Remission
To track patients’ progress and evaluate treatment efficacy, consensus is needed on how to define nonresponse, response without remission, remission, and relapse.18 Many patients respond to treatment for MDD without achieving remission, and even patients who meet criteria for remission often have some residual symptoms. Ideally, remission should involve an absence of all depressive symptoms accompanied by functional improvement. Response is generally defined as ≥ 50% reduction from baseline in score on a depression rating scale, most commonly the HDRS, in research studies.18 Remission is usually defined as an HDRS score ≤ 7, as in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study.19 However, a recent analysis16 of residual symptoms in the STAR*D study used the secondary outcome, the QIDS-SR16, with response defined as ≥ 50% improvement and remission as a score ≤ 5, because the QIDS-SR16 provided more complete data on depressive symptoms. Ongoing monitoring of response and remission is best accomplished using a rating scale. The QIDS-SR16 can be used to monitor patients in clinical practice by having the patient fill it out in the waiting room before being seen.
Continuation/Maintenance Phase of MDD Treatment
The goal of treating MDD, as with other medical illnesses, is to sustain remission during long-term treatment. Although relapse is common, there are strategies clinicians can use to avoid it.
Predictors of Relapse and Recurrence in MDD
Failure to achieve and maintain remission. Failing to achieve and maintain remission after acute treatment is among the strongest predictors of relapse and poor functional and psychosocial outcomes. In a classic 1995 study of 57 patients who no longer met full criteria for MDD, Paykel et al20 found that early relapse occurred in 76% of those who had not achieved full remission but in only 25% of those who had achieved full remission (no residual symptoms, defined as HDRS score < 8). These findings, replicated in subsequent studies,4,21 highlight a major problem with the current treatment of MDD, since failure to achieve full remission is common. In the STAR*D study,22 only approximately one-third of the patients achieved full remission after an adequate trial of a selective serotonin reuptake inhibitor (SSRI), and a substantial percentage remained symptomatic after 3 or more adequate antidepressant trials.
Presence of residual symptoms. A major predictor of relapse in MDD is the presence of residual symptoms, not only in those who have responded without full remission but also in those who have achieved a full remission during acute treatment. Residual symptoms include both symptoms that have persisted from baseline as well as new onset symptoms. On the basis of a 10-year study, Judd et al4 reported that patients with even mild residual MDD symptoms relapsed more than 3 times faster to their next MDE (median 68 vs 231 weeks) and more than 5 times faster to any depressive episode (median 33 vs 184 weeks) than recovered asymptomatic patients. These findings highlight the importance of treating all symptoms, even in patients who achieve remission. The STAR*D study23 also found that the chance of responding decreased with each subsequent treatment. It is important to try to achieve full sustained remission as early in the course of treatment as possible, as is done for other illnesses for which acute remission is possible with adequate treatment (eg, bacterial infections, certain cancers). Sustained full remission from depression is associated with improved long-term outcomes and a reduced risk of adverse outcomes such as treatment resistance.24
Number of previous depressive episodes. Number of prior MDEs is another predictor of relapse and recurrence,25 with at least 60% of those who have had a single episode expected to have a second, while those who have had 2 episodes have a 70% chance of having a third, and those who have had 3 episodes have a 90% chance of having a fourth.5 Solomon et al25 followed 318 patients with MDD for 5 to 10 years, during which nearly two-thirds suffered at least 1 recurrence; the risk of recurrence increased by 16% with each successive recurrence, while the risk of recurrence progressively decreased as the duration of recovery increased.
Prevalence and Types of Residual Symptoms
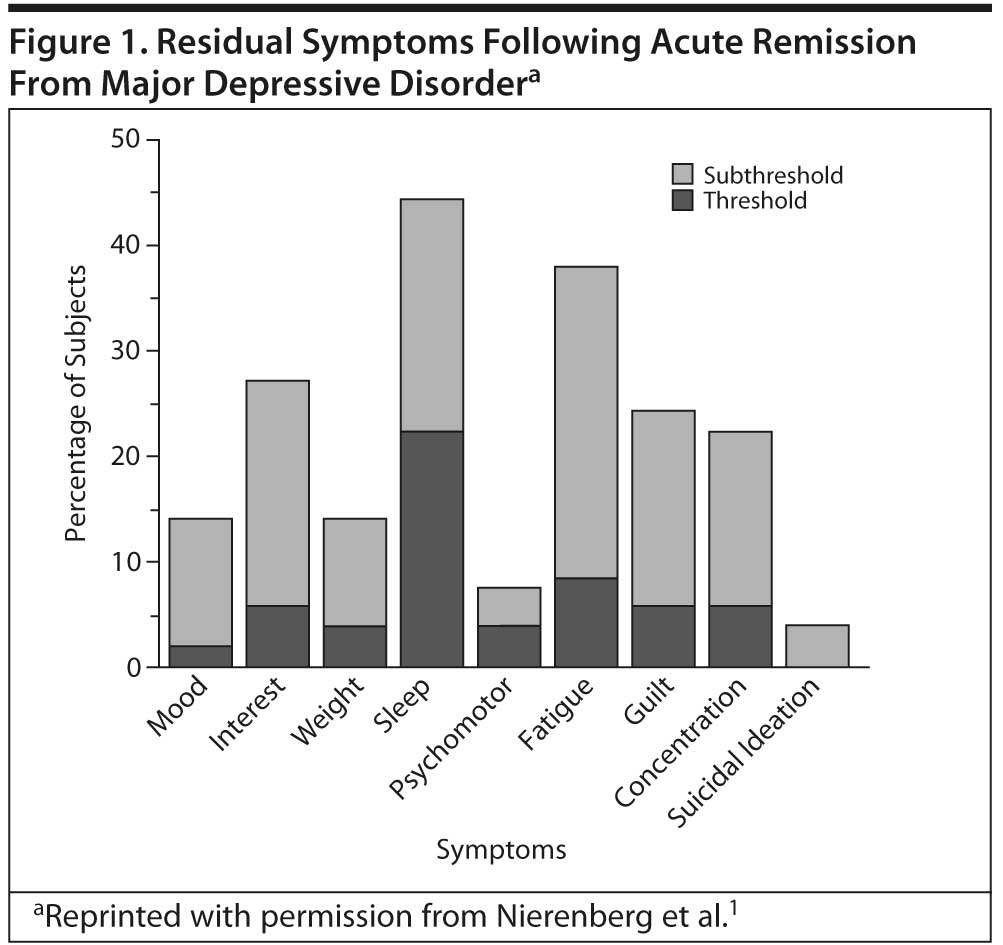
In a study of 215 outpatients with MDD, Nierenberg et al1 found that, of the 108 patients who achieved remission (HDRS score ≤ 7), 26% had 1 and 57% had 2 or more residual symptoms, while fewer than 20% were free of residual symptoms after 8 weeks of SSRI treatment. In the 82% of the sample who remitted but continued to have at least 1 residual symptom, sleep problems (44%) and fatigue (38%), followed by lack of interest, guilt, and concentration problems (Figure 1), were the most common residual symptoms.
Bolling and Kohlenberg17 found that at least 75% of 161 depressed patients who had completed a course of SSRI treatment had experienced at least 1 unwanted psychological effect, with the most frequently reported being narrowed range of affect (46%), not feeling like self (33%), loss of creativity (24%), inability to cry (21%), apathy (19%), vivid dreams (18%), loss of concentration (17%), loss of ambition (16%), loss of empathy (14%), increased anger (14%), and memory loss (13%).
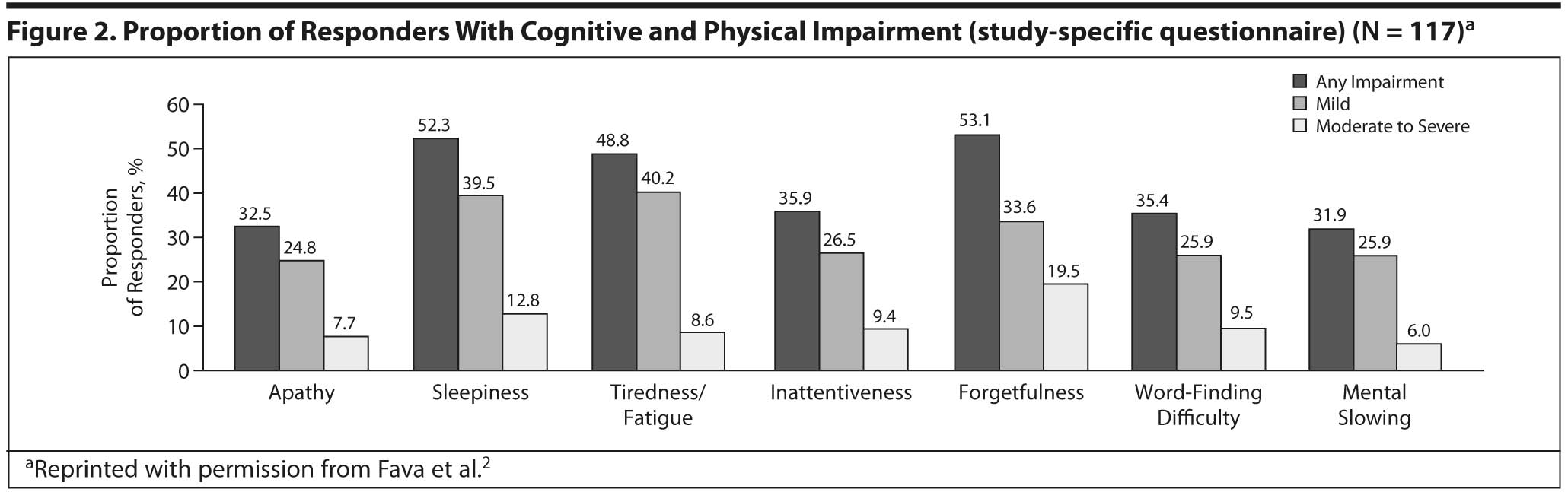
Fava et al2 assessed cognitive and physical symptoms in 117 patients who were responders (score < 9 on the Harvard Department of Psychiatry/National Depression Screening Day scale) after 3 months of treatment for MDD using a number of assessments beyond DSM-IV-TR criteria, including the CPFQ and a study-specific questionnaire. Consistent with earlier findings, more than 30% of responders reported cognitive symptoms (apathy, inattentiveness, forgetfulness, word-finding difficulty, and mental slowing), 49% reported fatigue, and 52% reported sleepiness/sedation (Figure 2).2
In a 3-year prospective study of 267 patients with MDD, Conradi et al3 found an average of 2 residual symptoms during remission. They also found that cognitive problems (diminished ability to think/concentrate or indecisiveness), lack of energy, and sleep problems were present 85%-94% of the time during depressive episodes and 39%-44% of the time during remissions.
Finally, McClintock et al16 found that the 15% of patients who responded but did not remit after 12 weeks of SSRI treatment in phase 1 of the STAR*D study endorsed 2 to 9 residual symptoms, with 75% reporting 5 or more residual symptoms. The most common symptoms persisting from baseline were midnocturnal insomnia (82%), sad mood (71%), and decreased concentration/decision making (71%). The most common treatment-emergent symptoms were midnocturnal insomnia (51.4%) and decreased general interest (40.0%).16
Preventing Relapse and Recurrence During Continuation/Maintenance Treatment
Many factors that predict relapse or recurrence in MDD can be prevented, modified, or potentially avoided by employing a systematic, personalized approach to treatment. This involves continued assessment for signs and symptoms during all phases of treatment and early intervention to prevent relapse. (One way to assess whether patients are really symptom-free is to ask if they can “belly laugh.”) As mentioned above, it is helpful to use the list of target symptoms created for the specific patient at the beginning of treatment to track that patient’s progress over time.
Differential Diagnosis of Residual and New Onset Symptoms
Clinicians need to distinguish persisting residual symptoms, newly emerging or re-emerging symptoms, and late-onset antidepressant side effects (Table 3). If new onset symptoms actually represent late onset antidepressant side effects, this has important treatment implications. Patients who have responded or remitted after treatment with an SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) may complain several weeks, if not months, later that they “don’ t feel the same as when they first responded.” They may develop side effects such as apathy, “feeling blah,” lack of motivation, fatigue, sleepiness, mental “dulling,” and word-finding problems. This syndrome, often referred to as antidepressant-induced tachyphylaxis, significantly increases risk of relapse.26 Both clinicians and patients can confuse these iatrogenic effects with the new onset or return of depressive symptoms. A good question to ask patients who begin to present with such symptoms is, “Do you feel blah?” Clinicians should assess for tachyphylaxis early in treatment and, if it is present, consider switching to a medication with a broader or different pharmacologic profile,26,27 since such late-onset side effects may be associated with overly potent effects on the serotonin (5-HT) transporter in the brain of some patients, resulting in reduced norepinephrine (NE) and dopamine (DA) activity (see next section).
Clinicians need to consider a number of other differential diagnostic issues in evaluating new onset symptoms27:
- accuracy of the diagnosis (eg, bipolar disorder misdiagnosed as MDD)
- effects of comorbid substance use disorders
- symptoms of new onset medical disorders (eg, endocrine, rheumatologic, or neurologic conditions; cancer; dementia)
- comorbid psychiatric conditions (eg, anxiety disorders, sleep disorders, attention-deficit/hyperactivity disorder), which may not have been evident until the depression was treated
- side effects and interactions of other medications (including over-the-counter agents and medications prescribed by other physicians)
- psychosocial factors (eg, chronic and new life stressors, such as from returning to work/school)
- lack of adherence (as they recover, patients may cut back on medications, especially if cost is an issue) leading to a discontinuation syndrome presenting with physical (eg, fatigue, flu-like symptoms), cognitive, and/or mood (dysphoria, irritability) symptoms that can be misinterpreted as new onset depressive symptoms or early relapse.
Managing Residual Symptoms
The following strategies are useful in assessing and managing residual symptoms27:
- identify baseline “target symptoms” and follow up over time
- utilize differential diagnostic skills (including laboratory workup)
- consider neuropsychological testing to aid diagnosis and monitor treatment effectiveness over time
- intervene early for new onset symptoms or side effects
- consider reducing dose of the medication that may be contributing to symptoms, but not at the cost of loss of efficacy
- consider adjunctive therapy (pharmacologic, cognitive-behavioral therapy)
- consider switching treatments (additional or different pharmacologic mechanism).
Reconceptualizing the Approach to MDD Symptoms
The acute treatment of MDD can result in early improvement of the core MDD symptoms that clinicians frequently assess, such as mood, sleep, appetite, and feelings of hopelessness, helplessness, and guilt. However, the evidence outlined above suggests that symptoms such as fatigue and cognitive problems, which are common after treatment despite response and remission of the other core depressive symptoms, can hinder functional recovery and contribute to relapse. Thus, it is important when evaluating criteria for remission from MDD to include remission of these specific symptoms.
Neurochemistry of MDD: Targeted Treatment
An understanding of the neurochemical mechanisms involved in MDD can help clinicians better target interventions to the specific patient’s presentation to try to achieve full remission.
Association Between Neurochemical Mechanisms and Low Remission Rates
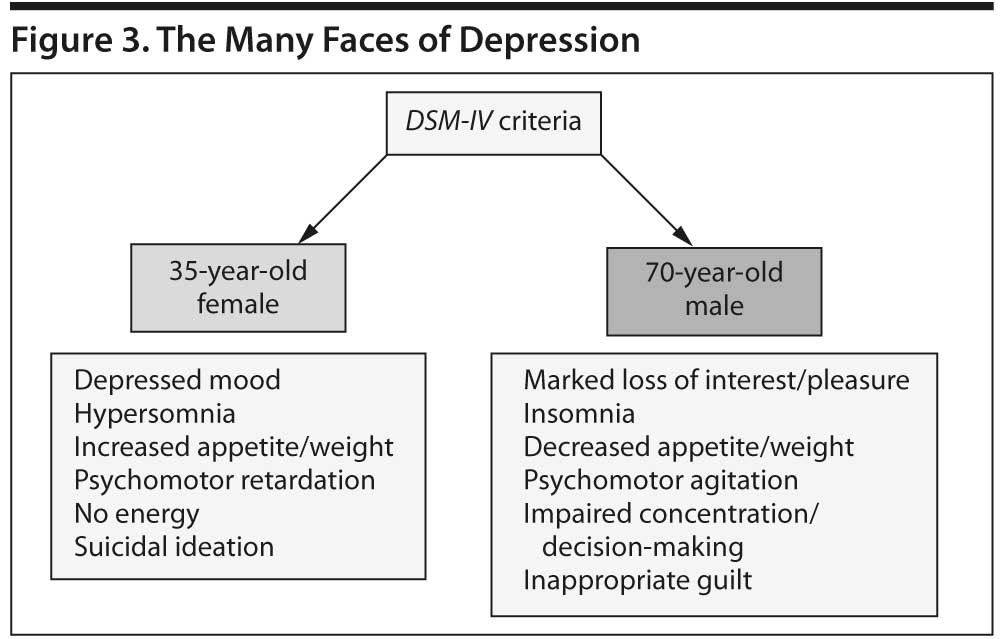
Because MDD is a multifactorial disorder, 2 patients can meet DSM-IV-TR criteria for MDD without sharing a single sign or symptom (Figure 3). It is unlikely that exactly the same neurologic factors account for the depression in these 2 patients or that a selective medication (eg, an SSRI) could be effective in all patients with depression, as demonstrated in the STAR*D study.22 Other differences in MDD presentations (eg, melancholic or atypical features, seasonal effects, psychotic symptoms) further complicate our understanding of the neurobiology and most appropriate treatment of MDD. Although a discussion of genetic factors is beyond the scope of this article, polymorphisms in various receptor regions may also influence whether patients respond to a particular medication.28-30
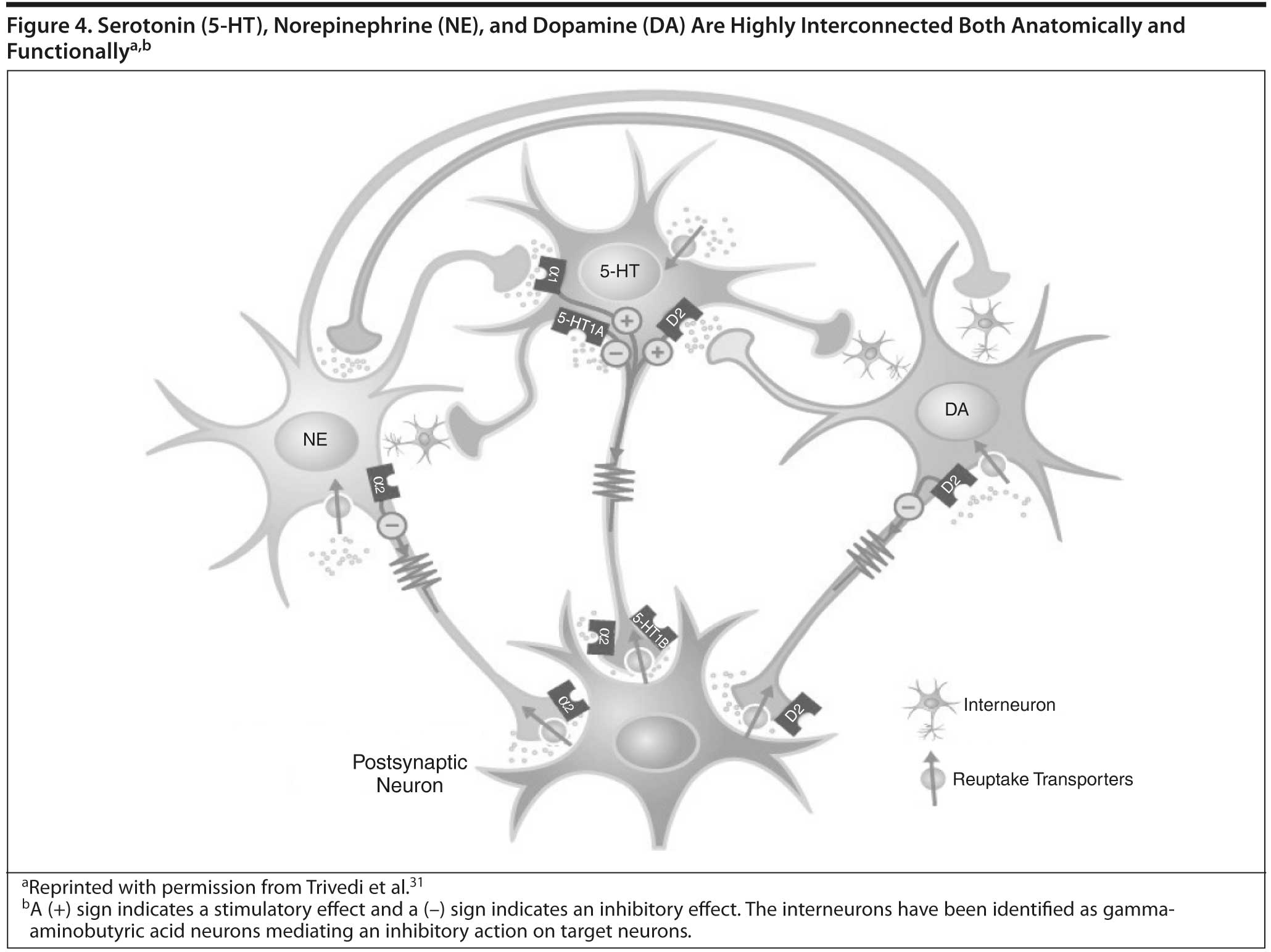
How can lack of complete response to SSRIs and other antidepressants be understood on a neurochemical basis? As shown in Figure 4, the 5-HT, NE, and DA systems are highly interconnected.31 Thus, while SSRIs are thought to increase 5-HT transmission, they also appear to dampen NE and DA neuron activity, reducing release of NE and DA,32-34 which may explain some of the residual symptoms and side effects seen with SSRIs.
Neurochemical Basis for MDD Symptoms
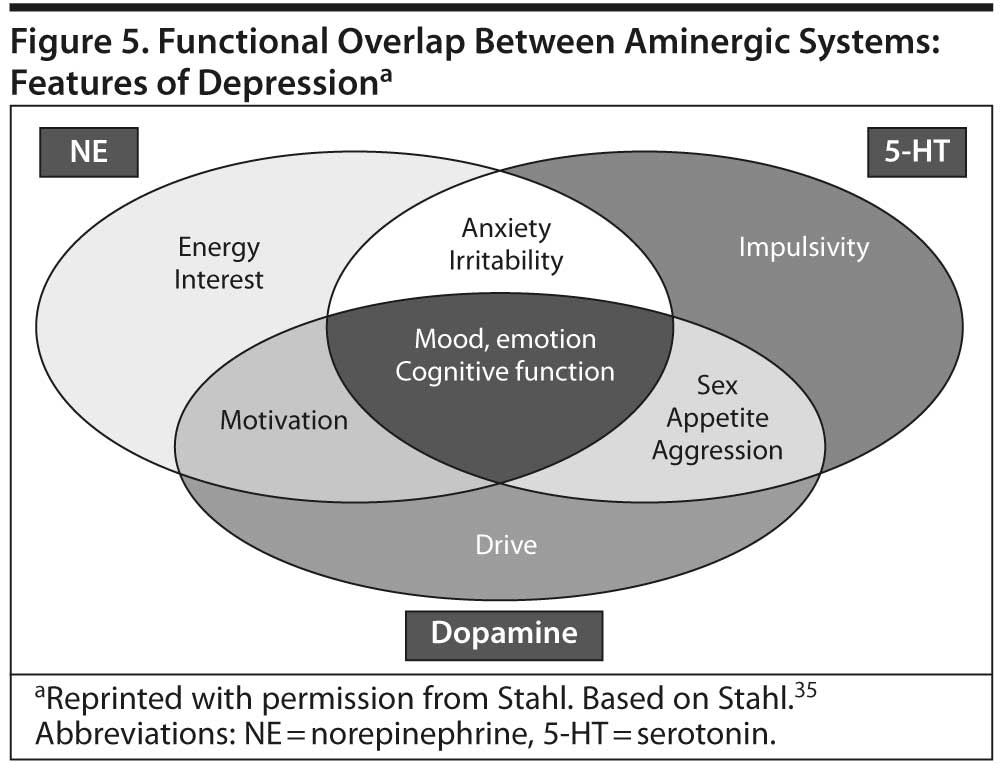
The classic monoamine hypothesis posited that depression was related to a deficit in monoamines, especially 5-HT and NE, in critical brain synapses. However, subsequent research suggests that the different symptoms of MDD are associated with dysfunctions in multiple circuits and regions in the brain and that multiple neurotransmitters, especially 5-HT, NE, and DA, are involved.35,36 While some MDD symptoms appear to be associated primarily with 1 neurotransmitter system, others appear to be associated with 2 or more systems (Figure 5). For example, depressed mood and sadness appear to be associated with 5-HT, NE, and DA.35 In contrast, apathy/loss of interest appears to be associated with circuits that regulate interest, drive, and pleasure in the hypothalamus and the “pleasure center” in the nucleus accumbens as well as the prefrontal cortex and may reflect deficiencies in DA and NE.35,36 Such deficiencies can also underlie changes in sexual function.36 Thus, overly potent serotonergic antidepressants may have the potential to worsen such symptoms, because a boost in 5-HT may reduce levels of NE and DA.35 Fatigue and loss of energy appear to be associated with deficits in NE and DA.35 Cognitive symptoms appear to be associated with numerous systems, including 5-HT, NE, DA, histamine, and acetylcholine.36
Neurotransmitters and Antidepressant Side Effects
An understanding of the neurochemistry underlying antidepressant side effects can help clinicians distinguish side effects from residual symptoms and treat them more effectively. A number of late side effects of SSRIs may be due to postulated adaptive/compensatory changes in response to overly selective 5-HT activity in the brain. The resulting reduction in NE and DA activity may produce the “blah,” apathetic, amotivational side effects and sexual side effects that often appear later in treatment with SSRIs.26,36
Optimizing the Current Treatment Regimen
To reduce risk of relapse, it is important to aim for complete remission without residual symptoms. However, patients and clinicians may be reluctant to change a treatment that produced initial response because of concern about relapse. If a patient has not achieved an adequate response, the first step is optimize the current treatment regimen, which may involve increasing the dose of the antidepressant.27 While likely to be helpful for some patients, this strategy is unlikely to help if the persisting symptoms are not already being addressed by the current antidepressant.36
Adjunctive Treatments
Deciding to use adjunctive strategies. If residual symptoms persist after the current regimen has been optimized, clinicians need to “deconstruct” the MDD to identify the specific symptoms and side effects the patient is experiencing and decide whether first to try adjunctive treatment or switch to a different agent.27,36 To reduce risk of side effects and drug interactions, clinicians should try to use as few medications as possible to achieve response and remission. It is important to use evidence-based treatments and weigh the risk/benefit ratio in introducing a new medication, including those approved as adjunctive treatments for MDD, and to consider symptoms of other comorbid illnesses if present.24 Treatment should be tailored to the needs of the individual patient (eg, choosing an adjunctive agent that may treat both residual symptoms and a side effect of the current treatment).
Neurochemical basis for adjunctive treatment strategies. The neurochemical rationale for adjunctive treatments for MDD generally involves (1) increasing the effects of 1 or more neurotransmitters (eg, 5-HT, NE, DA, γ-aminobutyric acid, glutamate) and/or (2) modulating the activity of receptors that may affect specific symptomatic and side effect domains, either directly or through their ability to increase levels of various neurotransmitters.37,38 5-HT acts via 7 families of receptors (eg, 5-HT1-5-HT7) and at least 14 receptor subtypes.38 Models suggest that stimulation of 5-HT1A and 5-HT1B receptors increases levels of NE and DA, respectively, and 5-HT1A appears to be associated with improved mood.37,38 Agonism of 5-HT1A may also help normalize libido and sexual function.38 Agonism of 5-HT1B and 5-HT2C appears to modulate appetite and reduce risk of weight gain.39,40 Antagonism of 5-HT3 receptors appears to reduce gastrointestinal side effects.37-40 5-HT7 receptors appear to be involved in cognition and regulation of circadian rhythm and sleep.38 Thus, combining an SSRI with agents that have targeted effects on specific 5-HT receptor subtypes may have the potential to improve efficacy and/or tolerability.
Atypical antipsychotics. Because of their greater affinity for 5-HT2A and 5-HT2C than D2 receptors, atypical antipsychotics can increase NE and DA activity in patients with an inadequate response to an SSRI. Dremencov et al found that a coadministered atypical antipsychotic reversed the decrease in NE neuronal firing that occurred with an SSRI, via 5-HT2A receptor antagonism,41 and that a selective 5-HT2C antagonist reversed SSRI-induced inhibition of DA activity.34 Thus, adding an atypical antipsychotics to an antidepressant may increase chances of response in patients who have not responded to several trials of antidepressant monotherapy,24,27 even when psychotic symptoms are not present, and a number of these agents have been approved for this indication. Doses used for antidepressant augmentation are lower than those used to treat psychosis.27
Other adjunctive agents. Clinicians sometimes also try adding other agents to help with residual symptoms such as fatigue, apathy, lack of energy, insomnia, and cognitive symptoms, although no agents besides the atypical antipsychotics (and the medical food l-methylfolate) have been approved as adjunctive treatments for major depressive disorder.24,27,36 Adding a cognitive enhancing agent (eg, an acetylcholinesterase inhibitor) or using antidepressants with minimal anticholinergic effects may be an appropriate option for depressed patients with comorbid early dementia.27 SSRIs and some SNRIs are associated with sexual dysfunction at minimum effective doses; this can be difficult to manage, since available adjunctive agents are rarely helpful. If a patient has responded to an SSRI or SNRI, but has sexual dysfunction, the clinician may want to switch to an antidepressant with a more favorable side effect profile in this area.27
Combinations of antidepressants with different mechanisms of action. Two double-blind studies found that beginning with a combination of 2 antidepressants with complementary mechanisms increases response and remission rates in MDD. One study42 compared monotherapy and initial combination therapy with 2 antidepressants with complementary mechanisms of action on the 5-HT and NE systems; the second study43 compared antidepressant monotherapy to 1 of 3 combinations of antidepressants with different mechanisms of action (all agents given at therapeutic doses). Both studies found that remission rates doubled with combination treatment versus monotherapy.
CONCLUSION
The goal of continuation/maintenance treatment for MDD is to sustain symptomatic and functional remission and assure safety, tolerability, and adherence to treatment. Patients who enter continuation/maintenance treatment in remission often have residual symptoms of depression that increase risk of relapse and reduce chances for recovery. Clinicians need to take a systematic personalized approach to identify and manage symptoms during long-term treatment. Growing evidence suggests that some MDD symptoms (eg, fatigue, cognitive symptoms) may not respond to conventional treatments that are effective in treating other core MDD symptoms. Early identification of these symptoms, combined with novel approaches to treatment, may improve long-term outcomes for many patients.
References
1. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225. PubMed doi:10.4088/JCP.v60n0403
2. Fava M, Graves LM, Benazzi F, et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J Clin Psychiatry. 2006;67(11):1754-1759. PubMed doi:10.4088/JCP.v67n1113
3. Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2010;41:1-10. PubMed
4. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108. PubMed doi:10.1016/S0165-0327(98)00138-4
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
6. Chen LS, Eaton WW, Gallo JJ, et al. Empirical examination of current depression categories in a population-based study: symptoms, course, and risk factors. Am J Psychiatry. 2000;157(4):573-580. PubMed doi:10.1176/appi.ajp.157.4.573
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
8. Rush AJ, First MB, Blacker D, eds. Handbook of Psychiatric Measures. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2008.
9. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
10. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
11. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. PubMed doi:10.1017/S0033291700035558
12. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
13. Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93-105. PubMed doi:10.2190/T8EM-C8YH-373N-1UWD
14. Fava M, Iosifescu DV, Pedrelli P, et al. Reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire. Psychother Psychosom. 2009;78(2):91-97. PubMed doi:10.1159/000201934
15. Pollard LC, Choy EH, Gonzalez J, et al. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford). 2006;45(7):885-889. PubMed doi:10.1093/rheumatology/kel021
16. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186. PubMed doi:10.1097/JCP.0b013e31820ebd2c
17. Bolling MY, Kohlenberg RJ. Reasons for quitting serotonin reuptake inhibitor therapy: paradoxical psychological side effects and patient satisfaction. Psychother Psychosom. 2004;73(6):380-385. PubMed doi:10.1159/000080392
18. Prien RF, Carpenter LL, Kupfer DJ. The definition and operational criteria for treatment outcome of major depressive disorder. A review of the current research literature. Arch Gen Psychiatry. 1991;48(9):796-800. PubMed doi:10.1001/archpsyc.1991.01810330020003
19. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. PubMed doi:10.1176/appi.ajp.163.1.28
20. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180. PubMed doi:10.1017/S0033291700033146
21. Pintor L, Gastó C, Navarro V, et al. Relapse of major depression after complete and partial remission during a 2-year follow-up. J Affect Disord. 2003;73(3):237-244. PubMed doi:10.1016/S0165-0327(01)00480-3
22. Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-459. PubMed doi:10.1007/s11920-007-0061-3
23. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed doi:10.1176/appi.ajp.163.11.1905
24. Zajecka J, Goldstein C, Barowski J. Combining drug treatments to achieve remission. In: Schwartz TL, Petersen T, eds. Depression: Treatment Strategies and Management. 2nd ed. New York, London: Informa Healthcare; 2009:54-100. doi:10.3109/9781420084887.003
25. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233. PubMed doi:10.1176/appi.ajp.157.2.229
26. Rothschild AJ, Dunlop BW, Dunner DL, et al. Assessing rates and predictors of tachyphylaxis during the Prevention of Recurrent Episodes of Depression With Venlafaxine ER for Two Years (PREVENT) study. Psychopharmacol Bull. 2009;42(3):5-20. PubMed
27. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
28. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239-258. PubMed doi:10.1016/j.euroneuro.2011.10.003
29. McMahon FJ, Buervenich S, Charney D, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78(5):804-814. PubMed doi:10.1086/503820
30. Lemonde S, Du L, Bakish D, et al. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7(4):501-506. PubMed doi:10.1017/S1461145704004699
31. Trivedi MH, Hollander E, Nutt D, et al. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J Clin Psychiatry. 2008;69(2):246-258. PubMed doi:10.4088/JCP.v69n0211
32. Szabo ST, de Montigny C, Blier P. Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol. 2000;3(1):1-11. PubMed doi:10.1017/S1461145700001772
33. Kawahara Y, Kawahara H, Kaneko F, et al. Long-term administration of citalopram reduces basal and stress-induced extracellular noradrenaline levels in rat brain. Psychopharmacology (Berl). 2007;194(1):73-81. PubMed doi:10.1007/s00213-007-0826-8
34. Dremencov E, El Mansari M, Blier P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J Psychiatry Neurosci. 2009;34(3):223-229. PubMed
35. Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 3rd ed. Cambridge, UK: Cambridge University Press; 2008.
36. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17. PubMed
37. Millan MJ. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 2009;6(1):53-77. PubMed doi:10.1016/j.nurt.2008.10.039
38. Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009;61(5):761-777. PubMed
39. Lam DD, Garfield AS, Marston OJ, et al. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97(1):84-91. PubMed doi:10.1016/j.pbb.2010.09.003
40. Millan MJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110(2):135-370. PubMed doi:10.1016/j.pharmthera.2005.11.006
41. Dremencov E, El Mansari M, Blier P. Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biol Psychiatry. 2007;61(5):671-678. PubMed doi:10.1016/j.biopsych.2006.05.015
42. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465. PubMed doi:10.1016/j.euroneuro.2009.01.015
43. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288. PubMed doi:10.1176/appi.ajp.2009.09020186
This PDF is free for all visitors!