Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: The only first-line pharmacologic treatments recommended for obsessive-compulsive disorder (OCD) are serotonin reuptake inhibitors (SRIs). We found that a single intravenous (IV) dose of ketamine, an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, rapidly decreases symptoms in unmedicated patients with OCD, demonstrating that a drug affecting glutamate transmission can reduce OCD symptoms without an SRI. After study participation, patients often requested a drug with similar effects to ketamine that could be taken as an outpatient.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Open-Label Trial on the Effects of Memantine in Adults With Obsessive-Compulsive Disorder After a Single Ketamine Infusion
To the Editor: The only first-line pharmacologic treatments recommended for obsessive-compulsive disorder (OCD) are serotonin reuptake inhibitors (SRIs).1 We found that a single intravenous (IV) dose of ketamine, an N-methyl-d-aspartate (NMDA) glutamate receptor antagonist, rapidly decreases symptoms in unmedicated patients with OCD,2 demonstrating that a drug affecting glutamate transmission can reduce OCD symptoms without an SRI. After study participation, patients often requested a drug with similar effects to ketamine that could be taken as an outpatient. Memantine shares a similar mechanism of action as ketamine, ie, NMDA glutamate receptor antagonism, albeit with less affinity3 to the receptor than ketamine. It has been shown to decrease OCD symptoms in some patients when given orally over 12 weeks.4-11 Given that memantine is available in oral dosing and may be easier to access than ketamine in outpatient treatment, we explored in an open-label pilot trial whether response to one NMDA receptor antagonist (IV ketamine) might predict response to a second NMDA receptor antagonist (oral memantine) in a sample of convenience—unmedicated adults with OCD who had recently finished participation in a trial of IV ketamine. We also sought to explore memantine’s clinical effects and tolerability when given after ketamine.
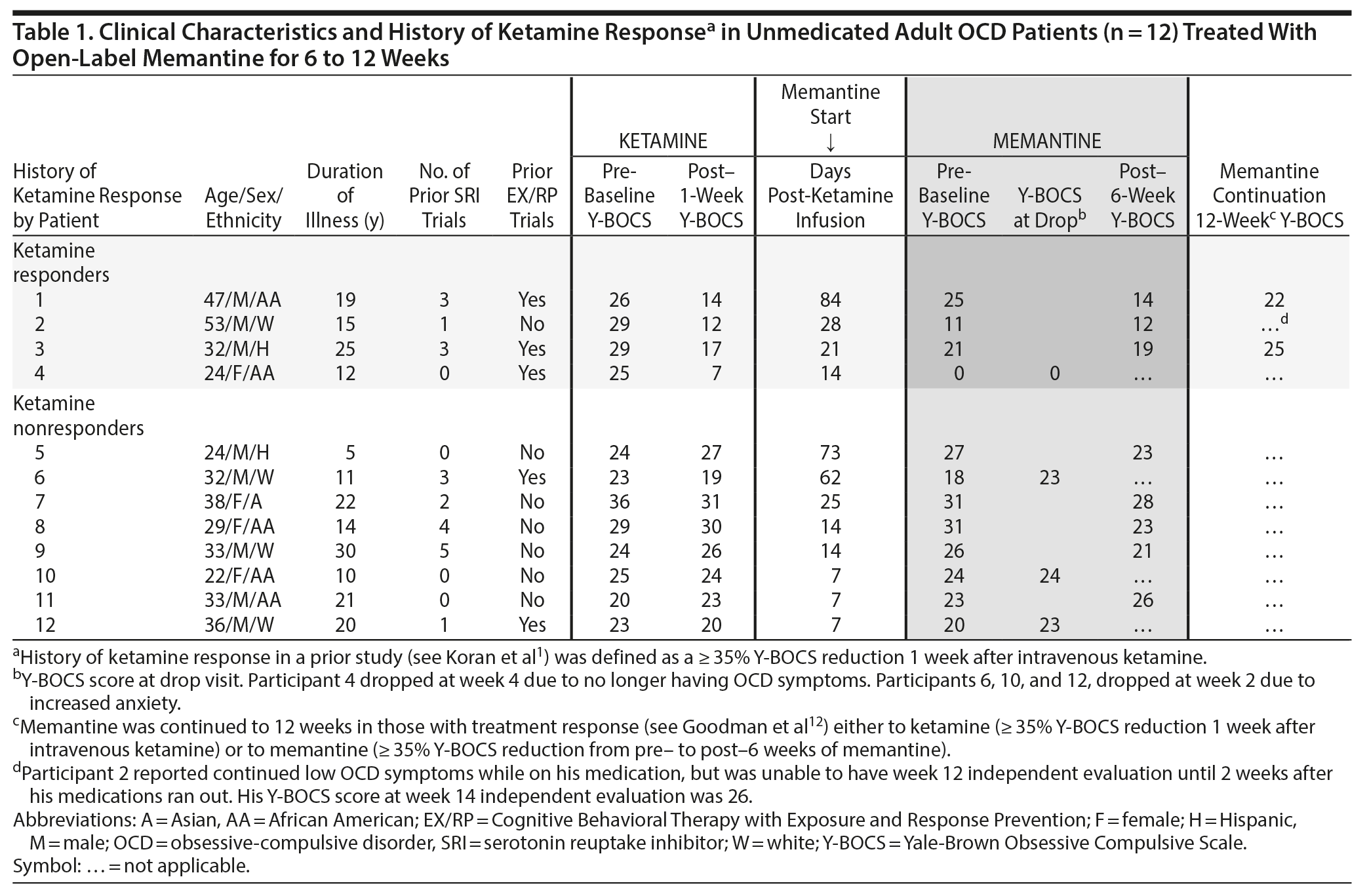
Methods. To accurately document history of ketamine effects, we recontacted (with institutional review board approval) 15 adults with OCD who participated in a prior ketamine study in which half met response criteria (≥ 35% reduction on Yale-Brown Obsessive Compulsive Scale [Y-BOCS]12) 7 days after a single 0.5 mg/kg IV dose. Twelve agreed to participate and provided informed consent. At the time of the ketamine study, all met DSM-IV and DSM-5 criteria for OCD with at least moderate symptoms (Y-BOCS score ≥ 16). At the time of the memantine trial, all were unmedicated and 2 had symptoms of mild to moderate depression. As shown in Table 1, the weeks since their ketamine infusion varied as did their OCD severity as measured by the Y-BOCS prior to starting memantine. Ketamine responders averaged 36.8 days (SD = 32.0) before memantine administration, and ketamine nonresponders averaged 26.1 days (SD = 26.4) (t10 = 0.615, P = .552).
Open-label memantine was started at 5 mg daily and titrated by 5 mg weekly to 10 mg twice daily for up to 6 weeks. Memantine was continued to 12 weeks in those with treatment response,13 either previous response to ketamine (≥ 35% Y-BOCS reduction 1 week after IV ketamine) or current response to memantine (≥ 35% Y-BOCS reduction from pre- to post-6 weeks of memantine). At baseline and 6 and 12 weeks, an independent evaluator blinded to study design evaluated OCD symptoms (Y-BOCS), depression (17-item Hamilton Depression Rating Scale [HDRS-17]14), and anxiety (Hamilton Anxiety Rating Scale [HARS]15). Paired t tests assessed change from pre-memantine (baseline) to post-memantine (6 weeks) using the last available observation.
Results. Of the 12 who started memantine, 8 completed 6 weeks and 3 completed 12 weeks. Overall, the 12 patients showed no significant changes 6 weeks after memantine initiation on Y-BOCS (t11 = 1.28, P = .23), HDRS-17 (t11 = 1.85, P = .09), and HARS (t11 = -0.09, P = .93). In those who did not respond to IV ketamine (n = 8), they did not respond to oral memantine either (Table 1). However, they did report side effects of dizziness (n = 2) and anxiety (n = 3), which led 3 to drop out early. In those who did respond to IV ketamine (n = 4), the story was more complex. Subject 1 lost his ketamine response at the time of starting memantine and regained his treatment response after 6 weeks of memantine but not after 12 weeks of memantine. Subject 2 seemed to maintain his response to ketamine (although it is not clear how long the beneficial effects of ketamine would have lasted without the memantine) and, by his verbal report, continued to maintain it after 12 weeks of mematine; however, when he discontinued his mematine at week 12, he relapsed 2 weeks later. Subject 3 also seemed to maintain his ketamine response after 6 weeks of memantine (34% decrease from initial score of 29 on the Y-BOCS). Subject 4 responded so robustly to ketamine that it was impossible to assess further benefit from memantine.
In ketamine nonresponders, none responded subsequently to oral memantine. In ketamine responders, memantine may have helped 1 patient temporarily regain the ketamine effects and 2 maintained the ketamine effects, although it is not possible to rule out whether ketamine’s effect would have persisted without memantine. Another responded so well to ketamine it was not possible to assess memantine’s effects. In this sample of convenience with variable time between treatments, we learned that in ketamine responders, ketamine affects individuals in unique trajectories. How these trajectories intersect with memantine’s effects awaits a confirmatory large randomized trial.
This sample had a low rate of response to memantine compared to prior studies,4-11 which could have occurred for 2 reasons: (1) in contrast to prior trials, our sample was not taking an SRI, and (2) 8 of the 12 participants had no response to ketamine, which means we may have selected patients unresponsive to NMDA receptor modulation, the putative mechanism of ketamine and memantine.
Limitations of this exploratory open-label pilot study include small sample size, lack of randomization and blinding, and high drop rate due to side effects and nonresponse. It is also important to highlight that ketamine and memantine have similar (but not identical) mechanisms of action via NMDA receptor antagonism.
References
1. Koran LM, Hanna GL, Hollander E, et al; American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(suppl):5-53. PubMed
2. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483. PubMed doi:10.1038/npp.2013.150
3. Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6(1):61-67. PubMed doi:10.1016/j.coph.2005.09.007
4. Pasquini M, Biondi M. Memantine augmentation for refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1173-1175. PubMed doi:10.1016/j.pnpbp.2006.04.013
5. Poyurovsky M, Weizman R, Weizman A, et al. Memantine for treatment-resistant OCD. Am J Psychiatry. 2005;162(11):2191-2192. PubMed doi:10.1176/appi.ajp.162.11.2191-a
6. Aboujaoude E, Barry JJ, Gamel N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J Clin Psychopharmacol. 2009;29(1):51-55. PubMed doi:10.1097/JCP.0b013e318192e9a4
7. Feusner JD, Kerwin L, Saxena S, et al. Differential efficacy of memantine for obsessive-compulsive disorder vs generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81-93. PubMed
8. Stewart SE, Jenike EA, Hezel DM, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34-39. PubMed doi:10.1097/JCP.0b013e3181c856de
9. Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47(2):175-180. PubMed doi:10.1016/j.jpsychires.2012.09.015
10. Bakhla AK, Verma V, Soren S, et al. An open-label trial of memantine in treatment-resistant obsessive-compulsive disorder. Ind Psychiatry J. 2013;22(2):149-152. PubMed doi:10.4103/0972-6748.132930
11. Haghighi M, Jahangard L, Mohammad-Beigi H, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013;228(4):633-640. PubMed doi:10.1007/s00213-013-3067-z
12. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed doi:10.1001/archpsyc.1989.01810110048007
13. Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown Obsessive Compulsive Scale. J Clin Psychiatry. 2005;66(12):1549-1557. PubMed doi:10.4088/JCP.v66n1209
14. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
15. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
aDepartment of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California
bVeterans Affairs Palo Alto Health Care System, Palo Alto, California
cDepartment of Psychology, Stony Brook University, Stony Brook, New York
dNew York State Psychiatric Institute, New York
eDepartment of Psychiatry, College of Physicians and Surgeons, Columbia University, New York
Potential conflicts of interest: Dr Rodriguez and Mss Levinson, Zwerling, and Vermes report no additional financial or other relationships relevant to the subject of this letter. Dr Simpson has received royalties from Cambridge University Press and UpToDate, Inc.
Funding/support: This investigation was supported by grants from National Institute of Mental Health K23MH092434 (Dr Rodriguez) and K24MH09155 (Dr Simpson).
Role of the sponsor: The funding organization is a public institution and had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
Acknowledgment: The authors thank those individuals with OCD who generously donated their time to participate in this research study.
Trial registration: ClinicalTrials.gov identifier: NCT00956085.
J Clin Psychiatry 2016;77(5):688-689
dx.doi.org/10.4088/JCP.15l10318
© Copyright 2016 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!