Guidelines for Preventing Common Medical Complications of Catatonia: Case Report and Literature Review
ABSTRACT
Objective: Comprehensive hospital-based care for individuals with catatonia relies on preventive approaches to reduce medical morbidity and mortality. Without syndrome-specific guidelines, psychiatrists must draw from measures used for general medical and surgical inpatients. We employ a prototypical case to highlight medical complications of catatonia and review preventive guidelines for implementation in the inpatient setting.
Data Sources: Searches of the PubMed and Ovid databases were conducted from September–November 2013 using keywords relevant to 4 medical complications of catatonia: deep vein thrombosis/pulmonary embolism, pressure ulcers, muscle contractures, and nutritional deficiencies. A complementary general web-browser search was performed to help ensure that unpublished guidelines were considered.
Study Selection: A search for deep vein thrombosis/pulmonary embolism guidelines yielded 478 articles that were appraised for relevance, and 6 were chosen for review; the pressure ulcer guideline search yielded 5,665 articles, and 5 were chosen; the muscle contractures guideline search yielded 1,481 articles, and 3 were chosen; and the nutritional deficiencies guideline search yielded 16,937 articles, and 4 were chosen.
Data Extraction: Guidelines were reviewed for content and summarized in a manner relevant to the audience. No quantitative analyses were conducted.
Results: Guidelines for deep vein thrombosis/pulmonary embolism prophylaxis support use of anticoagulant therapies for patients with catatonia who are at lower risk for acute bleeding. Pressure ulcer prevention hinges on frequent skin evaluation, use of support surfaces, and repositioning. Muscle contracture data are less clear and must be extrapolated from studies of patients with neurologic injuries. Early initiation of enteral nutrition should be considered in patients with prolonged immobility.
Conclusions: As medical complications are common with catatonia, implementation of preventive measures is imperative.
J Clin Psychiatry 2014;75(6):644–651
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: November 6, 2013; accepted March 31, 2014
(doi:10.4088/JCP.13r08870).
Corresponding author: Kimberly Clinebell, MD, 3811 O’Hara St, Pittsburgh, PA 15213 ([email protected]).
Catatonia is a diverse syndrome of psychomotor disturbances that is associated with various psychiatric disorders or, in up to 25% of cases, with general medical or neurologic conditions.1 Characteristic features, including immobility, posturing, and intermittent hyperactivity, may result in an array of medical complications, some of which are life threatening. Successful detection of concurrent medical conditions is often made difficult by the behavioral and motor disturbances of catatonia: mutism, limited interpersonal engagement, and resistance to instruction (negativism). Psychiatrists who treat patients with catatonia must maintain vigilance for indicators of physical pathology, starting with awareness of common offenders. Medical complications of catatonia are cited widely and include aspiration pneumonia, muscle contractures, skin breakdown that may lead to pressure ulcers, nutritional deficiencies that may lead to feeding tube placement, electrolyte disturbances, urinary tract infections, and venous thromboembolism (VTE).2–8 Malignant catatonia is another possible medical complication with progression to autonomic instability and, in some cases, death.8–10
Clinical protocols are far from uniform in their approach to the identification and management of patients who are at high risk for these complications. Even when guidelines provide standardized recommendations for the prevention of a specific condition, for instance VTE, preventive adherence rates are as low as 40%.11 We present a medically complex case of catatonia (Figure 1) and review preventive guidelines and pertinent research for 4 medical complications of the syndrome.
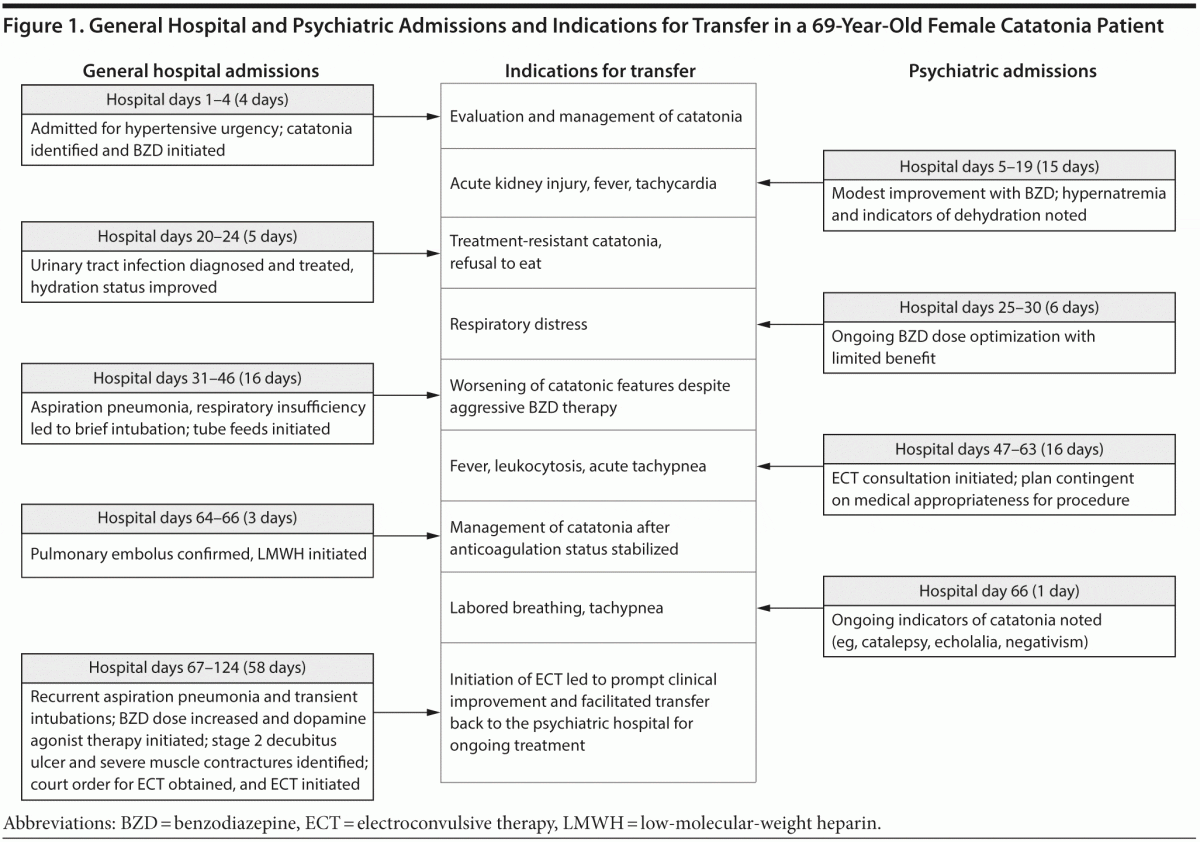
CASE REPORT
Ms A, a 69-year-old woman with a history of hypertension, hypothyroidism, and bipolar II disorder, was admitted to a community hospital for evaluation of hypertensive urgency.
Medical Hospitalization 1 (4 hospital days)
Upon the patient’s hospitalization, a psychiatric consultation was requested for psychotropic medication management. Ms A had been maintained for several years on lithium, venlafaxine, and risperidone and 1 month prior to admission was instructed to discontinue lithium due to declining renal function. At the time of consultation, the psychiatrist noted features of catatonia, including mutism, staring, and immobility. After a benzodiazepine challenge resulted in increased spontaneous activity, the psychiatrist started a scheduled regimen of lorazepam 1 mg 4 times daily. Four days later, Ms A was transferred to an inpatient psychiatric unit for further treatment of catatonia.
Psychiatric Hospitalization 1 (15 hospital days)
On presentation to the psychiatric inpatient unit, Ms A appeared disoriented, anxious, and agitated. After continued lorazepam titration, she displayed more purposeful movements and speech. Despite intravenous (IV) hydration and modest improvements to oral intake, Ms A developed refractory hypernatremia and pre-renal acute kidney injury, requiring medical consultation. Fifteen days into psychiatric admission, an emergency medical response was called for altered mental status, fever, shortness of breath, and tachycardia, and Ms A was transferred to a medical inpatient unit.
Medical Hospitalization 2 (5 hospital days)
Upon admission, Ms A was found to have a urinary tract infection and started on IV antibiotics. Five days later, she was transferred back to the psychiatric unit for further treatment of catatonia.
Psychiatric Hospitalization 2 (6 hospital days)
Upon Ms A’s arrival, the psychiatric inpatient team noted persistent catatonia marked by profound psychomotor retardation and negativism, including refusal to eat. Six days later, Ms A was transferred back to the general medical unit after developing respiratory distress, with pulse oximetry readings as low as 81%.
Medical Hospitalization 3 (16 hospital days)
Once transferred, Ms A required intubation and was found to have aspiration pneumonia. While in the intensive care unit, Ms A was seen by the nephrology service for persistent hypernatremia and was diagnosed with nephrogenic diabetes insipidus, presumed to be due to chronic lithium use. After evaluation by the speech pathology service, tube feeds were initiated, due to continued concerns for aspiration risk. During this time, the patient’s features of catatonia worsened; she became mute and developed frequent staring episodes. Fifteen days after readmission to the general medical unit, Ms A was deemed to be medically stable for transfer back to the psychiatric unit.
Psychiatric Hospitalization 3 (15 hospital days)
During this admission, Ms A was referred for an electroconvulsive therapy (ECT) consultation, and although it was recommended, Ms A was deemed not to be medically stable for ECT. Six days later, Ms A experienced intermittent fevers (of 38.4°C) with an elevated white blood cell count (15.8 × 109/L); the infectious disease team was consulted and initiated an extensive evaluation for infectious etiology. Fourteen days after transfer, Ms A acutely developed tachypnea with respiratory rate in the 30s despite oxygen saturation of 96% on 2 L of oxygen via nasal cannula, and she was transferred emergently to the medical unit.
Medical Hospitalization 4 (3 hospital days)
Upon admission, computed tomography angiography of the chest confirmed a pulmonary embolus, and anticoagulation efforts were undertaken using subcutaneous enoxaparin. Two days later, Ms A was transferred back to the psychiatric unit.
Psychiatric Hospitalization 4 (1 hospital day)
On the day of transfer, tachypnea and labored breathing prompted a subsequent emergency transfer back to the medical unit.
Medical Hospitalization 5 (58 hospital days)
Following transfer and review by the psychiatry consultation service, the patient was noted to be staring and was rigid in all extremities, with catalepsy and mild echolalia. The next day, Ms A experienced respiratory failure requiring intubation and transfer to the intensive care unit.
With the approval of her pulmonologists, lorazepam was titrated slowly to 3.75 mg 4 times daily, and amantadine was started adjunctively at 100 mg twice daily. Nevertheless, Ms A remained catatonic with waxy flexibility, rigidity, staring, mutism, and catalepsy. ECT was again considered, but the patient was deemed to be too medically unstable to proceed. She continued to experience recurrent aspirations with resultant pneumonia and intermittent fevers, requiring frequent extubation and reintubation, until a tracheostomy was performed at 1 month into admission. Despite daily wound care, physical therapy, and occupational therapy, Ms A developed a stage 2 decubitus ulcer and severe muscle contractures.
As Ms A’s respiratory status stabilized, consultation by the anesthesiology and pulmonology services supported the initiation of court-ordered ECT while she was an inpatient on the medical unit. Ms A received her first 3 ECT treatments while on the medical unit, almost 4 months into her hospital course. After the first ECT session, Ms A abruptly began to communicate in full sentences for the first time in more than 2 months; her rigidity decreased, and she no longer exhibited staring, waxy flexibility, or catalepsy. Ms A was transferred back to the psychiatric unit to complete her course of ECT, with continued improvement in her catatonic presentation.
LITERATURE REVIEW
Data Sources
A search of the PubMed and Ovid databases was conducted across a 3-month span (September–November 2013), using the following keywords: DVT prophylaxis guidelines, immobility, PE prophylaxis guidelines, pressure ulcer prevention, prolonged immobility, muscle contracture prevention, nutritional deficiencies, enteral nutrition guidelines, prolonged NPO, and prolonged NPO status. The following limits were placed on all searches: human studies, full-text articles, and English language. A general web-browser search of the same keywords, using Google, helped to ensure that unpublished guidelines from major national organizations were not omitted. References from downloaded articles were checked for completeness. National and international health care organization Web sites were searched individually for guidelines, including the US Preventive Services Task Force, Centers for Disease Control and Prevention, World Health Organization, Department of Health and Human Services, Joint Commission, and the Center for Medicare and Medicaid Services.
Study Selection
Deep vein thrombosis (DVT)/pulmonary embolism (PE). DVT prophylaxis guidelines was used as a search term in PubMed, yielding 281 articles; when limits were applied, 207 articles were identified. When immobility was used as an additional search term, 3 articles were found, none of which was relevant to our patient population. A search of DVT prophylaxis guidelines in Ovid yielded 6 results; when limits were applied, 2 articles were identified, neither of which applied to our target patient population. When PE prophylaxis guidelines was used as a search term in PubMed, 197 articles were found, and 146 with limits. When PE prophylaxis guidelines was used as a search term in Ovid, no articles were found. Articles were reviewed, and 6 were selected on the basis of relevance to our patient population.
Pressure ulcers. Pressure ulcer prevention was used as a search term in PubMed, yielding 5,084 articles; when limits were applied, 2,543 articles were identified. When results were further limited by prolonged immobility, 3 articles were found, none of which bore relevance to our patient population. When pressure ulcer prevention was used as a search term in Ovid, 581 articles were found; when limits were applied, 122 articles were found. When prolonged immobility was added as an additional term, no articles were identified. Five articles were selected on the basis of relevance to our patient population.
Muscle contractures. Muscle contracture prevention was used as a search term in PubMed, yielding 1,481 articles; when limits were applied, 626 articles were identified. When prolonged immobility or immobility was added as an additional search term, no articles were found. Search of muscle contracture prevention in Ovid yielded no results. Four articles were selected on the basis of relevance to our patient population.
Nutritional deficiencies. When nutritional deficiencies was used as a search term in both PubMed and Ovid, no results were found. Instead, enteral nutrition guidelines was entered as a search term in PubMed, which yielded 937 results. Prolonged NPO was entered as the search term in an additional PubMed search, which yielded 37 results; when this was extended to prolonged NPO status, 3 articles were identified. Enteral nutritional deficiencies was also used as a search term in Ovid, which yielded 15,960 results; when these results were limited to English language and human subjects, 11,383 articles were identified. When prolonged NPO was used in an additional search, 2 articles were found, neither of which was relevant. After careful review of abstracts and full text, 4 articles were selected on the basis of relevance to our patient population.
The reference lists of all articles recovered for our topics were cross-checked, and a general Google web search was completed to ensure that all potential studies were considered.
Data Extraction
As no quantitative analyses were conducted, guidelines were reviewed for content and summarized for each of the 4 complications of prolonged immobility chosen for this review.
Results and Discussion
In the care of catatonic patients who require ECT, caregivers are placed in the challenging position of preventing and treating medical complications that interfere with administration of ECT, even while ECT may be the most effective means of treating the psychomotor disturbances driving those medical comorbidities. In the case of Ms A, administration of ECT had rapid effects on mobility, speech, and rigidity that facilitated improvements to breathing status; however, this did not occur until hospital day 54. After spending over 2 months in the general medical unit for recurrent aspiration pneumonia and respiratory failure with renal disease, sacral wounds, and strictures, Ms A achieved sufficient respiratory stability for transfer to the psychiatric unit after receiving only 3 ECT sessions.
Given the potential severity of medical complications of catatonia, and the risk for associated mortality, preventive measures are vital to achieving successful outcomes. We review, below, the recommendations for prophylaxis of 4 common complications of catatonia: DVT and PE, pressure ulcers, muscular contractures, and nutritional deficits. Where specific guidelines do not exist, we have reviewed pertinent studies and draw inferences for individuals who are hospitalized with catatonia (Table 1).
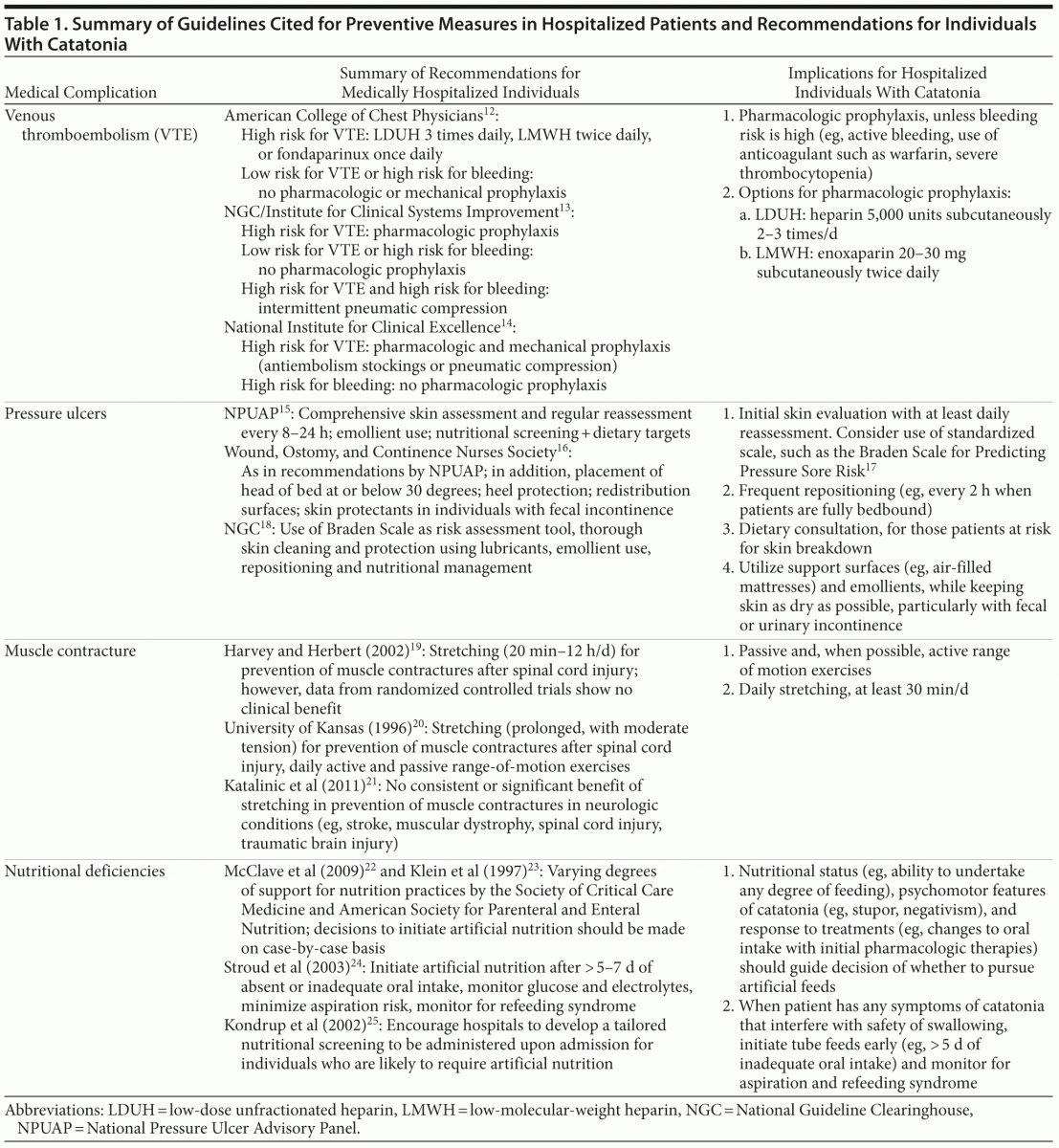
Deep vein thrombosis and pulmonary embolism. For hospitalized nonsurgical patients, the American College of Physicians recommends DVT prophylaxis with subcutaneous heparin or a low-molecular-weight heparin (LMWH) such as enoxaparin, unless the risk of bleeding outweighs the likely benefits of prophylaxis. The American College of Physicians makes no specific recommendations for duration of therapy and advises generally against mechanical prophylaxis with compression stockings.26
The American College of Chest Physicians (ACCP),12 National Guideline Clearinghouse (NGC), and Institute for Clinical Systems Improvement (ICSI)13 stratify recommendations for nonsurgical hospitalized patients on the basis of risk for DVT. For patients who are at increased risk for thrombosis, the ACCP recommends prophylaxis with 1 of the following 3 options: low-dose unfractionated heparin 3 times per day; LMWH twice daily; or once-daily fondaparinux, a factor Xa inhibitor.12 For patients who are at low risk for thrombosis or high risk for bleeding, the ACCP, NGC, and ICSI uniformly advise against the use of pharmacologic prophylaxis.12,13 The NGC does, however, recommend intermittent pneumatic compression for patients with elevated risk for both bleeding and VTE. Established and therapeutic use of warfarin (ie, for reasons other than VTE prophylaxis) will likely provide sufficient protection against DVT/PE, although use of aspirin is not sufficient as monotherapy for VTE prophylaxis.13
The United Kingdom’s National Institute for Clinical Excellence (NICE) provides a pathway for reducing risk of VTE in hospitals. The recommended algorithm includes an initial assessment of risks for VTE (eg, reduced mobility for 3 days or more, dehydration, obesity) against risks for bleeding (eg, acute hepatic failure, concurrent use of anticoagulants), with use of pharmacologic prophylaxis when the former outweigh the latter. The NICE pathway differs from other guidelines in the recommendation of routine mechanical prophylaxis for hospitalized patients with high risk of VTE. The NICE pathway also outlines contraindications to mechanical prophylaxis, including peripheral neuropathy, peripheral arterial disease, and cardiac failure.14
Overall consensus on guidelines for DVT/PE prophylaxis supports the role of anticoagulant therapies, such as heparin or enoxaparin, for hospitalized patients with catatonia who are not bleeding or at high risk for acute bleeding. These measures are underscored by calls for standardized DVT/PE prophylaxis from the Joint Commission, the National Quality Forum, and the Centers for Medicare and Medicaid Services.27 Pharmacologic prophylaxis is most prudent for those patients with stuporous catatonia who are bedbound and largely immobile. In our patient, the likely benefits of DVT prophylaxis outweighed the risks, yet she was not started on DVT prophylaxis and subsequently developed a PE.
Pressure ulcers. The National Pressure Ulcer Advisory Panel (NPUAP)15; Wound, Ostomy, and Continence Nurses Society (WOCN)16; and ICSI28 provide guidelines for pressure ulcer prevention, including dermatologic assessment and skin care, nutritional support, repositioning, and provision of support surfaces. The NPUAP and WOCN recommend comprehensive skin assessment in all health care settings and on a regular basis for those patients at risk for ulceration; this includes an evaluation for heat, edema, and induration along all skin surfaces every 8–24 hours, depending on degree of risk. Recommendations for dermatologic care focus on emollient use to reduce risk of skin damage and prevention of moisture accumulation on areas at risk for ulceration. Caretakers are advised against turning a patient onto an already reddened skin surface and against applying massage techniques or friction to skin at risk for breakdown. For all patients with an elevated risk for pressure ulcers, the NPUAP and WOCN recommend nutritional screening; if nutritional deficits are likely to interfere with skin care and wound healing, dietary referral is suggested, along with the following dietary targets: > 30–35 kcal/kg, ~ 1.25 g/kg protein, and 1 mL fluid intake/kcal per day.15,16
The NGC guidelines for prevention of pressure ulcers target nursing staff and include recommendations by Ayello and Sibbald18 that outline skin assessment, risk determination, and documentation. Specific instructions include adequate examination of the skin, including under devices, for changes to color and temperature, with mind to cultural differences in skin pigmentation. The NGC guidelines utilize the Braden Scale as a standardized risk assessment tool.17 Skin care recommendations include assessing skin regularly, cleaning and protecting the skin thoroughly using lubricants, and managing moisture with emollients. The NGC also recommends nutritional management and repositioning and gives detailed guidelines for preventing and managing skin tears proactively.18
Repositioning to prevent ulcers is paramount, with frequency based on tissue tolerance, activity level, skin condition, and general medical conditions. The WOCN recommends maintaining the head of bed at or below 30 degrees, providing heel protection, utilizing redistribution surfaces when possible, and using skin protectants in patients with fecal incontinence. Changes to skin condition should be reported to all nursing and primary medical providers involved in the patient’s care to coordinate efforts at managing complications and mitigating risk of further skin breakdown.16
Providers for catatonic patients should pay special attention to preventing pressure ulcers, particularly in the setting of prolonged immobility, catalepsy, and limited response to initial treatments. Patients must receive an initial thorough skin evaluation, with daily reassessment as a minimum goal. Frequent repositioning will remove pressure from at-risk surfaces, particularly if erythema, edema, induration, or warmth is noted. In the care of patients deemed to be at risk for skin breakdown, providers are encouraged to consult a dietitian for recommendations on calorie counts, protein, and fluid intake. Optimizing support surfaces is prudent, and areas at risk for ulceration should be kept dry and protected with emollients. The use of a validated scale for assessment, such as the Braden Scale,17 is encouraged for standardization.
Muscle contractures. To our knowledge, no definitive guidelines have been published for the prevention of muscle contractures in patients with catatonia or similar conditions. Recommendations can be drawn, however, from reviews of contracture prevention in patients with structural neurologic damage.
Reviews by Harvey and Herbert19 and a group at the University of Kansas20 identify regular stretching as the widely observed approach for treatment and prevention of muscle contractures after spinal cord injury. At the same time, Harvey et al29 showed no clinical benefit from an implemented stretching protocol in recently injured individuals with paraplegia and tetraplegia. In spite of potential limitations, Harvey and Herbert19 recommend that physical therapists apply muscle stretching in patients with spinal cord injuries as long as practically possible, from 20 minutes to 12 hours per day. Clinical data do not consistently guide an optimal duration of stretching, rendering such a large daily range difficult to interpret and apply. The University of Kansas guidelines20 also recommend daily range-of-motion exercises, both active and passive, as a prophylactic measure and describe prolonged stretch of moderate tension as an effective method to reverse a developing contracture.
Katalinic and colleagues’21 review published in 2011 analyzes the outcomes and recommendations from 25 articles that investigated the effects of stretch on treating or preventing contractures in patients with neurologic conditions such as stroke, cerebral palsy, traumatic brain injury, spinal cord injury, and muscular dystrophy. Conclusions suggest no consistent or significant benefit of stretching to joint mobility, pain, spasticity, or activity level, although all studies followed effects for less than 6 months at a time.
Established data that guide prevention of muscle contractures are based largely on patients with structural and persisting neurologic damage; in these circumstances, prophylactic benefit of stretching has not been demonstrated consistently. For Ms A, as in most individuals with catatonia, no structural or persistent neurologic injury was detected. In these cases, catatonia may be viewed as a transient functional neurologic syndrome for which immobility is often reversed with somatic therapies; as such, preventive measures may be expected to render some benefit, even if small. Whenever possible, we suggest instituting both range-of-motion exercises (ie, passive and, when possible, active) and stretching in an effort to prevent or mitigate severity of contractures. This may prove challenging in the face of negativism, oppositional paratonia, frank rigidity, or hyperactivity; assertive and prompt management of the catatonic syndrome is therefore vital.
Nutritional deficiencies. Despite the importance of proper nutrition, few standardized guidelines are available to direct providers in optimizing nutritional status for patients with critical illness or prolonged lack of enteral intake. The Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition describe varying degrees of evidence-based support for critical care practices in nutrition, making overarching and generalized recommendations difficult to establish.22 Without consensus for the most effective markers of malnutrition,23 the decision to initiate nutritional support must be made on a case-by-case basis.
When patients are unable to spontaneously eat or drink for a prolonged period, providing enteral nutrition is preferred to parenteral support. Stroud and colleagues24 recommend initiating artificial nutrition after more than 5–7 days of absent or inadequate oral intake, while monitoring glucose and electrolytes, minimizing aspiration risk, and knowing the signs of refeeding syndrome in malnourished patients. As no consistent guidelines are available to inform hospital-based screening for nutritional deficiencies,25 the authors also encourage hospitals to develop a tailored nutrition screening, to be completed on admission, for patients who are likely to require tube feeds.24
For patients with catatonia, nutritional status and psychomotor features should guide the threshold and timeline for artificial feedings. When faced with negativism and refusal to eat, or with immobility and rigidity that interfere with swallowing safety, early initiation of tube feeds should be considered, along with strict aspiration precautions and monitoring for signs of refeeding syndrome (eg, hypokalemia, hypophosphatemia, hypomagnesemia, indicators of colicky pain). Ethical and legal considerations should also be at the forefront when considering artificial feeding in patients who are unable to communicate for themselves.24
Limitations
The most significant limitation of this review is a lack of systematic data analysis; this was not undertaken, given the paucity of quantifiable indicators that were reviewed in the preventive guidelines. As many guidelines are posed by individual organizations, PubMed and Ovid searches may have missed unpublished recommendations. We attempted to mitigate this risk by conducting a complementary general web search and search of prominent health care organization Web sites; nevertheless, some preventive guidelines may still have been missed.
CONCLUSION
The syndrome of catatonia places individuals at risk for a wide range of medical complications, ranging from mildly painful (eg, stage 1 pressure ulcer) to potentially fatal (eg, pulmonary embolism). Timely recognition, immediate intervention, and prevention of these conditions can facilitate provision of effective therapies for catatonia, including ECT, thereby improving outcomes, decreasing hospital length of stay, and preventing further medical morbidity. In reviewing guidelines and literature that inform preventive measures for common complications of catatonia, we aim to support psychiatric and general medical providers in the care of individuals with this complex syndrome.
Diagnosis of catatonia should be met with swift management, including the recommendation for ECT consultation immediately following benzodiazepine nonresponse.30,31 In the case described, various systemic challenges delayed the gold standard intervention of ECT; these included obtaining a court order for ECT when the patient could not consent, facilitating ECT treatments in a medical setting, and managing multiple medical complications that prevented clearance for ECT. Complications of prolonged immobility often delay authorization for ECT, whereas treatment with ECT may itself prevent further medical complications. Psychiatrists must remain mindful of these challenges while providing care for patients in the general medical or psychiatric hospital setting.
Drug names: amantadine (Symmetrel and others), enoxaparin (Lovenox and others), fondaparinux (Arixtra and others), lithium (Lithobid and others), lorazepam (Ativan and others), risperidone (Risperdal and others), venlafaxine (Effexor and others), warfarin (Coumadin, Jantoven, and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, amantadine is not approved by the US Food and Drug Administration as augmentation treatment for catatonia.
Author affiliations: Western Psychiatric Institute and Clinic, University of Pittsburgh Department of Psychiatry, Pittsburgh, Pennsylvania (all authors); and Veterans Affairs Boston Healthcare System, Harvard Medical School, Boston, Massachusetts (Dr Azzam).
Financial disclosure: Drs Clinebell, Azzam, Gopalan, and Haskett have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: None reported.
REFERENCES
1. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371–380. doi:10.1176/appi.neuropsych.21.4.371 PubMed
2. Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry. 2008;10(2):153–155. doi:10.4088/PCC.v10n0210 PubMed
3. Levenson JL. Medical aspects of catatonia. Prim Psychiatry. 2009;16(3):23–36.
4. Larsen HH, Ritchie JC, McNutt MD, et al. Pulmonary embolism in a patient with catatonia: an old disease, changing times. Psychosomatics. 2011;52(4):387–391. doi:10.1016/j.psym.2011.01.028 PubMed
5. Devi S, Behere RV, Varambally S, et al. Physical deformity as sequela of chronic catatonia and response to electroconvulsive therapy: a case report. J ECT. 2011;27(3):e49–e50. doi:10.1097/YCT.0b013e3182107172 PubMed
6. Nomoto H, Hatta K, Usui C, et al. Vitamin K deficiency due to prolongation of antibiotic treatment and decrease in food intake in a catatonia patient. Psychosomatics. 2011;52(5):486–487. doi:10.1016/j.psym.2011.01.026 PubMed
7. Caroff S, Mann S, Campbell E, et al. Catatonia: From Psychopathology to Neurobiology. Washington, DC: APPI Press; 2004.
8. Mann S, Caroff SN, Campbell E. et al. Identification and treatment of malignant catatonia. Child Adolesc Psychopharmacol News. 2008;8(6):7–12. doi:10.1521/capn.8.6.7.23079
9. Mann SC, Caroff SN, Bleier HR, et al. Electroconvulsive therapy of the lethal catatonia syndrome. Convuls Ther. 1990;6(3):239–247. PubMed
10. Fricchione G, Bush G, Fozdar M, et al. Recognition and treatment of the catatonic syndrome. J Intensive Care Med. 1997;12(3):135–147.
11. Cohen AT, Tapson VF, Bergmann JF, et al; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–394. doi:10.1016/S0140-6736(08)60202-0 PubMed
12. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):7S-47S. PubMed
13. Jobin S, Kalliainen L, Adebayo L, et al. Venous Thromboembolism Prophylaxis. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI); 2012.
14. National Institute for Health and Care Excellence. Reducing the risk of venous thromboembolism in hospital patients. http://pathways.nice.org.uk/pathways/venous-thromboembolism#path=view%3A/pathways/venous-thromboembolism/reducing-the-risk-of-venous-thromboembolism-in-hospital-patients.xml&content=view-node%3Anodes-mechanical-prophylaxis. Updated September 2013. Accessed April 16, 2014.
15. European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
16. Wound, Ostomy, and Continence Nurses Society (WOCN). Guideline for Prevention and Management of Pressure Ulcers. Mount Laurel, NJ: Wound, Ostomy, and Continence Nurses Society (WOCN); 2010.
17. Bergstrom N, Braden BJ, Laguzza A, et al. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987;36(4):205–210. doi:10.1097/00006199-198707000-00002 PubMed
18. Ayello EA, Sibbald RG. National Guidelines Clearinghouse. Pressure ulcer prevention: evidence-based geriatric nursing protocols for best practice. 2012. Agency for Healthcare Research and Quality. http://www.guideline.gov/content.aspx?id=43935. Updated June 19, 2008. Accessed: November 2, 2013.
19. Harvey LA, Herbert RD. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord. 2002;40(1):1–9. doi:10.1038/sj.sc.3101241 PubMed
20. Galas J, ed. Contractures. Lawrence, KS: Research and Training Center on Independent Living, University of Kansas; 1996.
21. Katalinic OM, Harvey LA, Herbert RD. Effectiveness of stretch for the treatment and prevention of contractures in people with neurological conditions: a systematic review. Phys Ther. 2011;91(1):11–24. doi:10.2522/ptj.20100265 PubMed
22. McClave SA, Martindale RG, Vanek VW, et al; Society of Critical Care Medicine. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr. 2009;33(3):277–316. doi:10.1177/0148607109335234 PubMed
23. Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr. 1997;66(3):683–706. PubMed
24. Stroud M, Duncan H, Nightingale J; British Society of Gastroenterology. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(suppl 7):vii1–vii12. doi:10.1136/gut.52.suppl_7.vii1 PubMed
25. Kondrup J, Allison SP, Elia M, et al; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421. doi:10.1016/S0261-5614(03)00098-0 PubMed
26. Randel A. Practice guidelines: ACP recommendations for VTE prophylaxis in hospitalized patients. Am Fam Physician. 2012;85(12):1204.
27. Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22(12):1762–1770. doi:10.1007/s11606-007-0369-z PubMed
28. Institute for Clinical Systems Improvement (ICSI). Pressure Ulcer Prevention and Treatment Protocol: Health Care Protocol. Bloomington, MN: Institute for Clinical Systems Improvement (ICSI); 2012.
29. Harvey LA, Herbert R, Crosbie J. Does stretching induce lasting increases in joint ROM? A systematic review. Physiother Res Int. 2002;7(1):1–13. doi:10.1002/pri.236 PubMed
30. Fricchione GL, Cassem NH, Hooberman D, et al. Intravenous lorazepam in neuroleptic-induced catatonia. J Clin Psychopharmacol. 1983;3(6):338–342. doi:10.1097/00004714-198312000-00002 PubMed
31. Kellner CH, Popeo DM, Aloysi AS. Electroconvulsive therapy for catatonia. Am J Psychiatry. 2010;167(9):1127–1128, author reply 1128. doi:10.1176/appi.ajp.2010.10020261 PubMed





