See Commentary by Lauriello et al
Objective: Schizoaffective disorder is a complex illness for which optimal treatment is not well established. Results of the first controlled, relapse-prevention study of paliperidone palmitate once-monthly injectable (paliperidone monthly) in schizoaffective disorder are presented.
Method: The study was conducted between September 20, 2010, and October 22, 2013. Patients with schizoaffective disorder (confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders) experiencing acute exacerbation of psychotic and depressive/manic symptoms were stabilized with paliperidone monthly as monotherapy or as adjunctive therapy to mood stabilizers or antidepressants and randomly assigned (1:1) to paliperidone monthly or placebo in a 15-month, double-blind, relapse-prevention phase. Randomization was stratified by administration as monotherapy or adjunctive therapy and by study center. The primary endpoint was time to relapse.
Results: 334 patients were evaluated. Paliperidone monthly significantly delayed time to relapse for psychotic, depressive, and manic symptoms compared with placebo (P < .001, log-rank test). Relapse risk was 2.49 times greater for placebo (hazard ratio = 2.49; 95% CI, 1.55 to 3.99; P < .001, Cox proportional hazards model). Overall relapse rates were 33.5% for placebo and 15.2% for paliperidone monthly. For monotherapy, relapse risk was 3.38 times greater with placebo (P = .002), and for adjunctive treatment it was 2.03 times greater with placebo (P = .021). Paliperidone monthly was superior to placebo in maintaining functioning as measured by the Personal and Social Performance scale (P = .014, mixed-model repeated-measures analysis). The most common adverse events (placebo, paliperidone monthly) were increased weight (4.7%, 8.5%), insomnia (7.1%, 4.9%), schizoaffective disorder (5.9%, 3.0%), headache (3.5%, 5.5%), and nasopharyngitis (3.5%, 5.5%). Incidence of any extrapyramidal-related adverse event was 7.1% for placebo and 8.5% for paliperidone monthly.
Conclusions: Paliperidone monthly as monotherapy or adjunctive therapy significantly delayed psychotic, depressive, and/or manic relapses; reduced their risk; and better maintained functioning in patients with schizoaffective disorder. Results support the value of maintenance treatment with paliperidone monthly in schizoaffective disorder.
Trial Registration: ClinicalTrials.gov identifier: NCT01193153
ABSTRACT
Objective: Schizoaffective disorder is a complex illness for which optimal treatment is not well established. Results of the first controlled, relapse-prevention study of paliperidone palmitate once-monthly injectable (paliperidone monthly) in schizoaffective disorder are presented.
Method: The study was conducted between September 20, 2010, and October 22, 2013. Patients with schizoaffective disorder (confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders) experiencing acute exacerbation of psychotic and depressive/manic symptoms were stabilized with paliperidone monthly as monotherapy or as adjunctive therapy to mood stabilizers or antidepressants and randomly assigned (1:1) to paliperidone monthly or placebo in a 15-month, double-blind, relapse-prevention phase. Randomization was stratified by administration as monotherapy or adjunctive therapy and by study center. The primary endpoint was time to relapse.
Results: 334 patients were evaluated. Paliperidone monthly significantly delayed time to relapse for psychotic, depressive, and manic symptoms compared with placebo (P < .001, log-rank test). Relapse risk was 2.49 times greater for placebo (hazard ratio = 2.49; 95% CI, 1.55 to 3.99; P < .001, Cox proportional hazards model). Overall relapse rates were 33.5% for placebo and 15.2% for paliperidone monthly. For monotherapy, relapse risk was 3.38 times greater with placebo (P = .002), and for adjunctive treatment it was 2.03 times greater with placebo (P = .021). Paliperidone monthly was superior to placebo in maintaining functioning as measured by the Personal and Social Performance scale (P = .014, mixed-model repeated-measures analysis). The most common adverse events (placebo, paliperidone monthly) were increased weight (4.7%, 8.5%), insomnia (7.1%, 4.9%), schizoaffective disorder (5.9%, 3.0%), headache (3.5%, 5.5%), and nasopharyngitis (3.5%, 5.5%). Incidence of any extrapyramidal-related adverse event was 7.1% for placebo and 8.5% for paliperidone monthly.
Conclusions: Paliperidone monthly as monotherapy or adjunctive therapy significantly delayed psychotic, depressive, and/or manic relapses; reduced their risk; and better maintained functioning in patients with schizoaffective disorder. Results support the value of maintenance treatment with paliperidone monthly in schizoaffective disorder.
Trial Registration: ClinicalTrials.gov identifier: NCT01193153
J Clin Psychiatry 2015;76(3):253-262
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: July 28, 2014; accepted November 11, 2014.
Online ahead of print: December 5, 2014 (doi:10.4088/JCP.14m09416).
Corresponding author: Dong-Jing Fu, MD, PhD, Clinical Development, Janssen Scientific Affairs, 1125 Trenton-Harbourton Rd, Titusville, NJ 08560 ([email protected]).
As defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),1 schizoaffective disorder presents with mixed symptoms of both schizophrenia and affective disorders.2 It is about one-third as common as schizophrenia, with an estimated lifetime prevalence of 0.3%.3 Like schizophrenia, onset of schizoaffective disorder is typically in early adulthood; however, consistent diagnosis usually occurs several years following initial recognition of psychiatric illness.4-7 Persons with schizoaffective disorder experience significant functional impairment4,8 and higher rates of hospitalization, suicidality, and substance abuse than persons with schizophrenia.9 Optimal management of schizoaffective disorder requires long-term treatment, and its prognosis is intermediate to that of schizophrenia and affective disorders.4,8,10,11
To manage psychotic, depressive, and manic symptoms, schizoaffective disorder is treated with complex pharmacologic regimens that include antipsychotics, mood stabilizers, and antidepressants.12-14 Only oral paliperidone, administered as monotherapy or adjunctively with mood stabilizers and/or antidepressants, has received regulatory approval for acute treatment of schizoaffective disorder5-7,15; however, no widely accepted clinical guidelines for acute or maintenance treatment exist.
Poor treatment adherence is prevalent with schizoaffective disorder,16-19 contributing to suboptimal treatment response and poorer long-term outcomes.20,21 Although some individuals with schizoaffective disorder achieve and maintain symptom stability with available oral antipsychotics and mood stabilizers or antidepressants, many have difficulty adhering to a daily oral regimen.22 Long-acting injectable therapies, such as once-monthly paliperidone palmitate (paliperidone monthly), provide consistent therapeutic plasma concentrations over several weeks, eliminating the need for daily oral medication and facilitating monitoring of treatment adherence.23,24 Building on efficacy data of oral paliperidone in acute management of schizoaffective disorder,5,6 this relapse-prevention study was designed to compare paliperidone monthly given as monotherapy or with adjunctive antidepressants or mood stabilizers to placebo in patients with schizoaffective disorder.
METHOD
Study Design
This randomized, double-blind, placebo-controlled, international study of relapse prevention in schizoaffective disorder with paliperidone monthly treatment (ClinicalTrials.gov identifier NCT01193153; clinical registry number CR016618) was conducted between September 20, 2010, and October 22, 2013. It was designed as a pivotal study for the schizoaffective indication. The study was approved by participating ethics committees/institutional review boards and conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects. The highest proportion of subjects were enrolled from the United States.
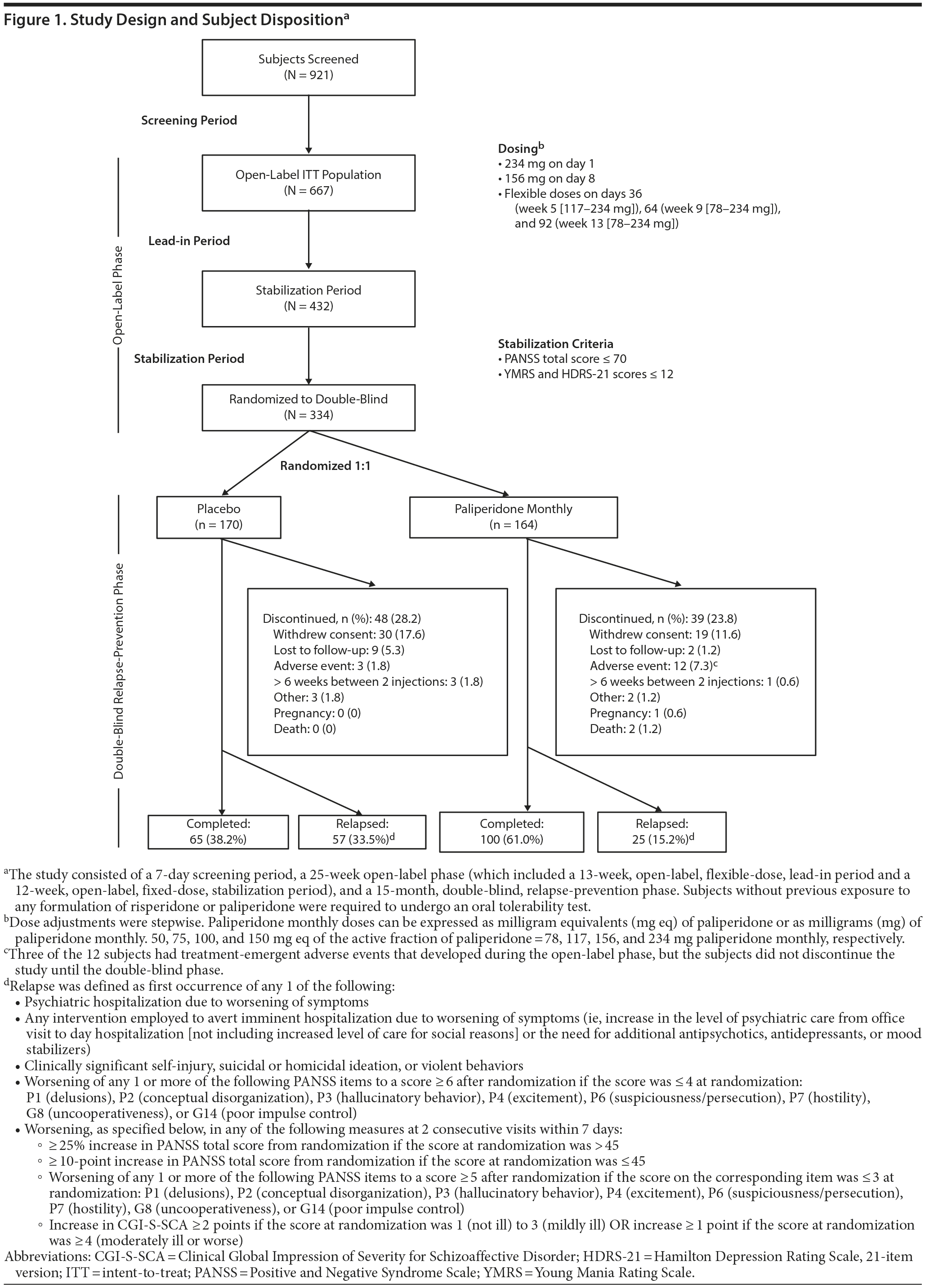
The study included multiple phases (Figure 1). After initial screening, an open-label phase consisted of a 13-week, flexible-dose, lead-in period and a 12-week, fixed-dose, stabilization period. This phase was followed by a 15-month, double-blind, relapse-prevention phase.
Stabilization criteria included Positive and Negative Syndrome Scale (PANSS)25 total scores ≤ 70, Young Mania Rating Scale (YMRS)26 scores ≤ 12, and Hamilton Depression Rating Scale, 21-item version (HDRS-21)27 scores ≤ 12 by the end of the lead-in period. Symptom stabilization had to be maintained throughout the stabilization period without the need for dose adjustments.
Stable subjects entered the 15-month, double-blind, relapse-prevention phase and were randomly assigned (1:1) to receive either paliperidone monthly or placebo injections. Randomization was stratified by absence or presence of mood stabilizers or antidepressants (ie, by monotherapy or adjunctive therapy) and study center. Subjects continued to receive double-blind treatment until predefined relapse, discontinuation, or completion of 15 months. Results of this double-blind phase are the focus of this report.
Study Population
Subjects were required to have a lifetime and current diagnosis of schizoaffective disorder according to the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Clinician Version)28 conducted during screening. Men and women aged ≥ 18 years with an acute exacerbation of psychotic symptoms ≥ 4 days and ≤ 4 weeks in duration before screening and willing to accept long-acting injectable treatment were eligible. To ensure recruitment of patients who were experiencing exacerbation of psychotic symptoms, subjects had to have a score of ≥ 4 (moderate) on ≥ 3 of the following PANSS items: P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), P4 (excitement), P6 (suspiciousness/persecution), P7 (hostility), G4 (tension), G8 (uncooperativeness), or G14 (poor impulse control). Subjects were also required to have prominent mood symptoms (YMRS and/or HDRS-21 scores ≥ 16) at screening. (See eAppendix 1 at PSYCHIATRIST.COM for an outline of key exclusion criteria.)
Treatments
All subjects received intramuscular injections of paliperidone monthly during the 25-week open-label treatment phase. Prestudy antipsychotic therapy was discontinued before the first injection. No oral supplementation was allowed during the study. Subjects entering the double-blind phase received either a fixed dose of paliperidone monthly or matching placebo once every 4 weeks. Paliperidone monthly initiation and dosing are described in Figure 1. Concomitant antidepressants, mood stabilizers, and benzodiazepines were allowed at prestudy stable doses. Limited use of newly started benzodiazepine and nonbenzodiazepine hypnotics was permitted. Subjects were required to be adherent to the study medication, and they were withdrawn from the study if more than 6 weeks had elapsed since the time of their last injection. Sites were permitted to contact patients to remind them of their scheduled visits.
Assessments
The primary objectives were to evaluate the efficacy of paliperidone monthly compared with placebo in delaying relapse of psychotic, depressive, and/or manic symptoms and to assess safety and tolerability. The definition for relapse used in the study is provided in Figure 1 and included criteria that would identify an impending relapse. The treatment effect of paliperidone monthly versus placebo was evaluated as monotherapy and adjunctive therapy.
The predefined key secondary objective was to evaluate the effect of paliperidone monthly on subject functioning as measured by the Personal and Social Performance scale (PSP).29,30 Functioning during the month prior to assessment was determined for each of the 4 PSP domains: socially useful activities, personal/social relationships, self-care, and disturbing/aggressive behavior.
Measures of symptomatic change included the PANSS, YMRS, and HDRS-21 total scores. Illness severity was assessed on the Clinical Global Impression of Severity for Schizoaffective Disorder (CGI-S-SCA).31 The Medication Satisfaction Questionnaire (MSQ)32 was assessed as a patient-reported outcome. Only qualified raters who are trained professionals were allowed to administer the SCID, PANSS, YMRS, HDRS-21, CGI-S-SCA, and PSP.
Statistics
The intent-to-treat (ITT) population was defined as all randomized subjects who received at least 1 injection of double-blind study drug. This population was used for efficacy and safety analyses. Time to first relapse was assumed to follow an exponential distribution with a hazard ratio (HR) of 1.96. With this assumption, ≥ 286 subjects were to be randomly assigned in a 1:1 ratio to paliperidone monthly or placebo to obtain ≥ 95 relapses in order to show whether treatment was significantly different from placebo at a 2-sided significance level of .05, with 90% power to detect an HR of 1.96.
The primary efficacy endpoint was time between day 1 of the double-blind phase and first documentation of relapse. Treatment differences for relapse were evaluated using a log-rank test stratified by concomitant medication stratum (mood stabilizers/antidepressants or no concomitant treatment). A cumulative distribution function of time to relapse was estimated by the Kaplan-Meier method. Risk of relapse for the subgroup of subjects on monotherapy and adjunctive therapy was also examined using Cox proportional hazards models. In addition, a Cox proportional hazards model was extended to include 3 types of mood events: manic, depressive, and mixed. Test of hypotheses for any difference in risk of relapse in mood event types was examined by the Global Competing Risk test.33 The Cox proportional hazards model was also used to examine differences between treatment groups for psychotic relapses. Details of analytic methods are described in eAppendix 1.
Mean change from double-blind baseline in PSP score at endpoint (month 15) was the predefined key secondary efficacy variable. Change in PSP score was analyzed using a mixed-model repeated-measures (MMRM) analysis of covariance model. Additional sensitivity analyses were performed to assess the robustness and consistency of findings at double-blind endpoint. Biases associated with missing data were carefully considered. Control of overall Type I error for testing treatment versus placebo for the primary efficacy endpoint and key secondary endpoint and statistical analysis of other secondary efficacy endpoints are described in eAppendix 1.
The number of subjects with ≥ 1 adverse event for each preferred term was summarized regardless of severity and relationship to study medication. Relative risk and corresponding 95% confidence intervals (CIs) associated with these treatment-emergent adverse events (TEAEs) are provided.
RESULTS
Subjects and Disposition
Subject disposition is summarized in Figure 1. A total of 667 subjects were enrolled in the 25-week open-label phase, and 334 were stabilized and subsequently randomized to placebo (n = 170) or paliperidone monthly (n = 164) in the double-blind relapse-prevention phase.
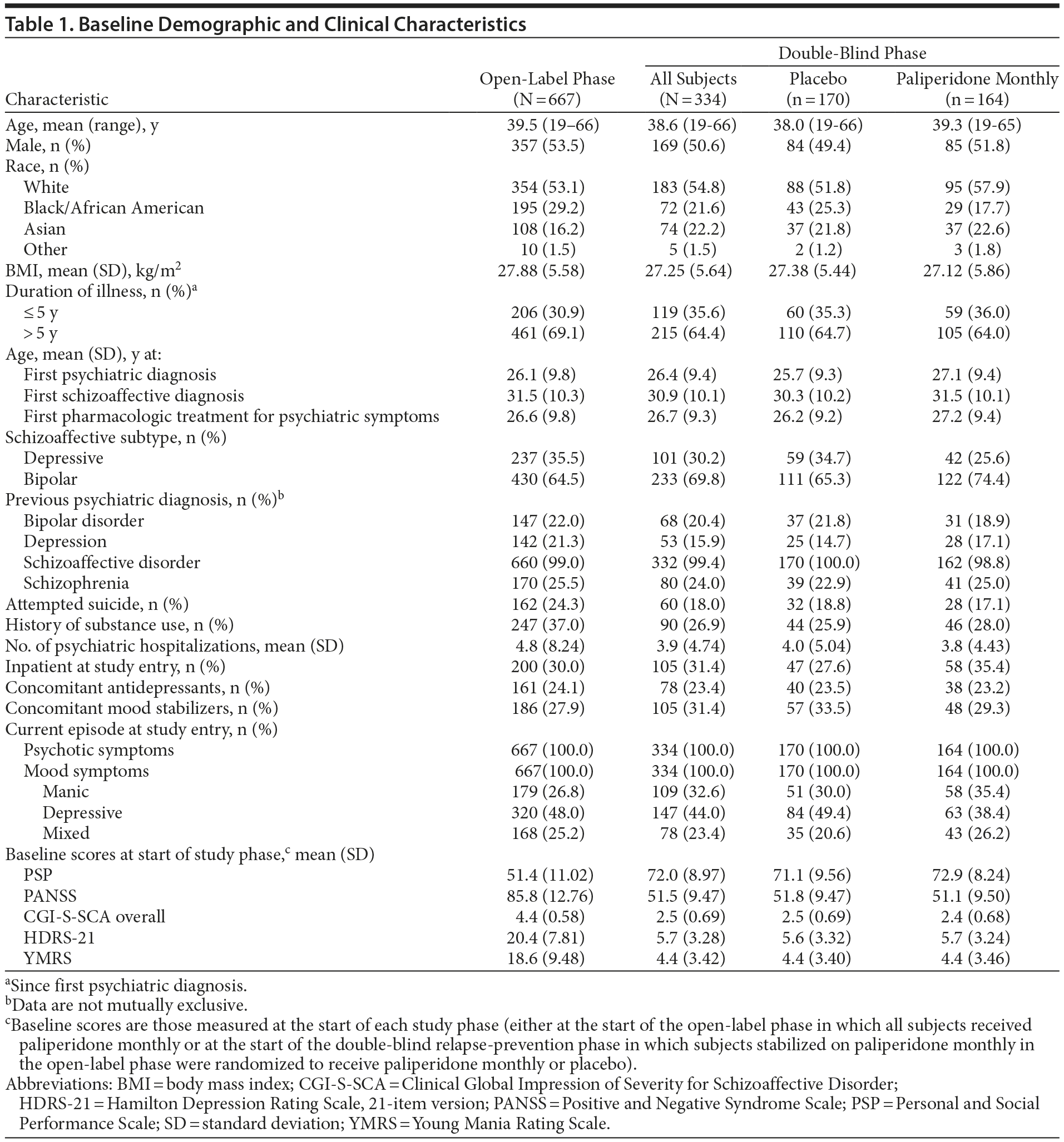
Overall baseline demographics and clinical characteristics were consistent across treatment groups for both enrolled and randomized subjects. Clinical symptom scores improved considerably during 25-week open-label treatment. The mean (SD) open-label baseline scores in this acutely ill population were PANSS, 85.8 (12.76); PSP, 51.4 (11.02); CGI-S-SCA overall, 4.4 (0.58); HDRS-21, 20.4 (7.81); and YMRS, 18.6 (9.48). At study entry, 30.0% of subjects were inpatients, and 24.3% had a history of suicide attempt (Table 1). Percentages of subjects using concomitant antidepressants, mood stabilizers, benzodiazepine and nonbenzodiazepine hypnotics, and anxiolytics were 23.5%, 33.5%, 11.2%, and 17.6%, respectively, in the placebo group and 23.2%, 29.3%, 9.1%, and 18.9%, respectively, in the paliperidone monthly group.
Of 334 randomized subjects, 82 (24.6%) experienced relapse, 165 (49.4%) completed the entire 15-month double-blind phase without relapse, and 87 (26.0%) discontinued the study early for reasons other than relapse (Figure 1). The most common reason for discontinuation was withdrawal of consent. All-cause discontinuations for the placebo and paliperidone monthly groups were 61.8% and 39.0% (P < .001, Cochran-Mantel-Haenszel [CMH] test), respectively. Time to all-cause discontinuation in the double-blind phase was significantly shorter for placebo than paliperidone monthly (P < .001). Monthly dosing distribution during the double-blind phase was 4.9% of subjects at 78 mg, 9.8% at 117 mg, 47.0% at 156 mg, and 38.4% at 234 mg. Median duration of exposure was 268.5 and 446.0 days for the placebo and paliperidone monthly groups, respectively.
Efficacy
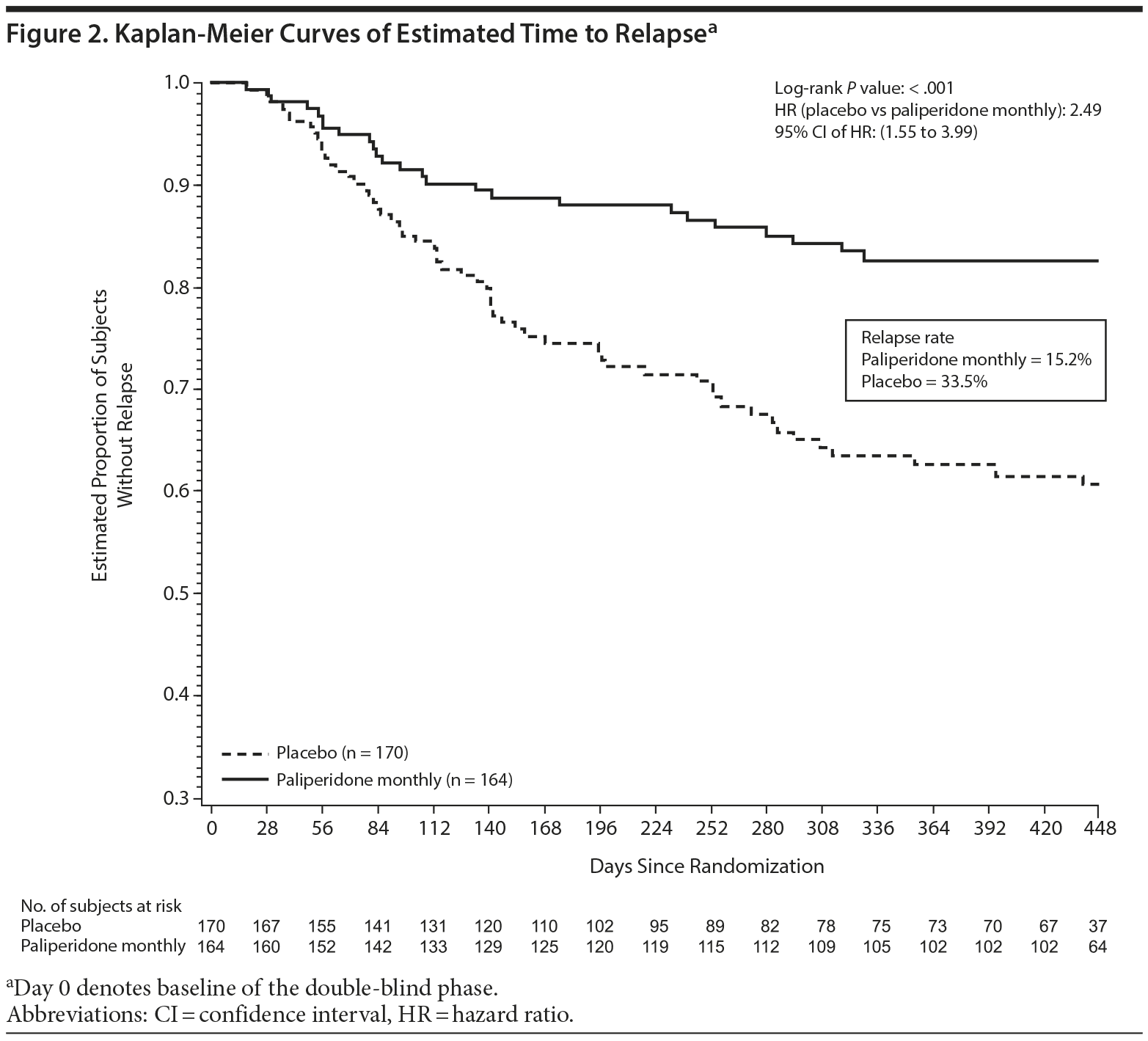
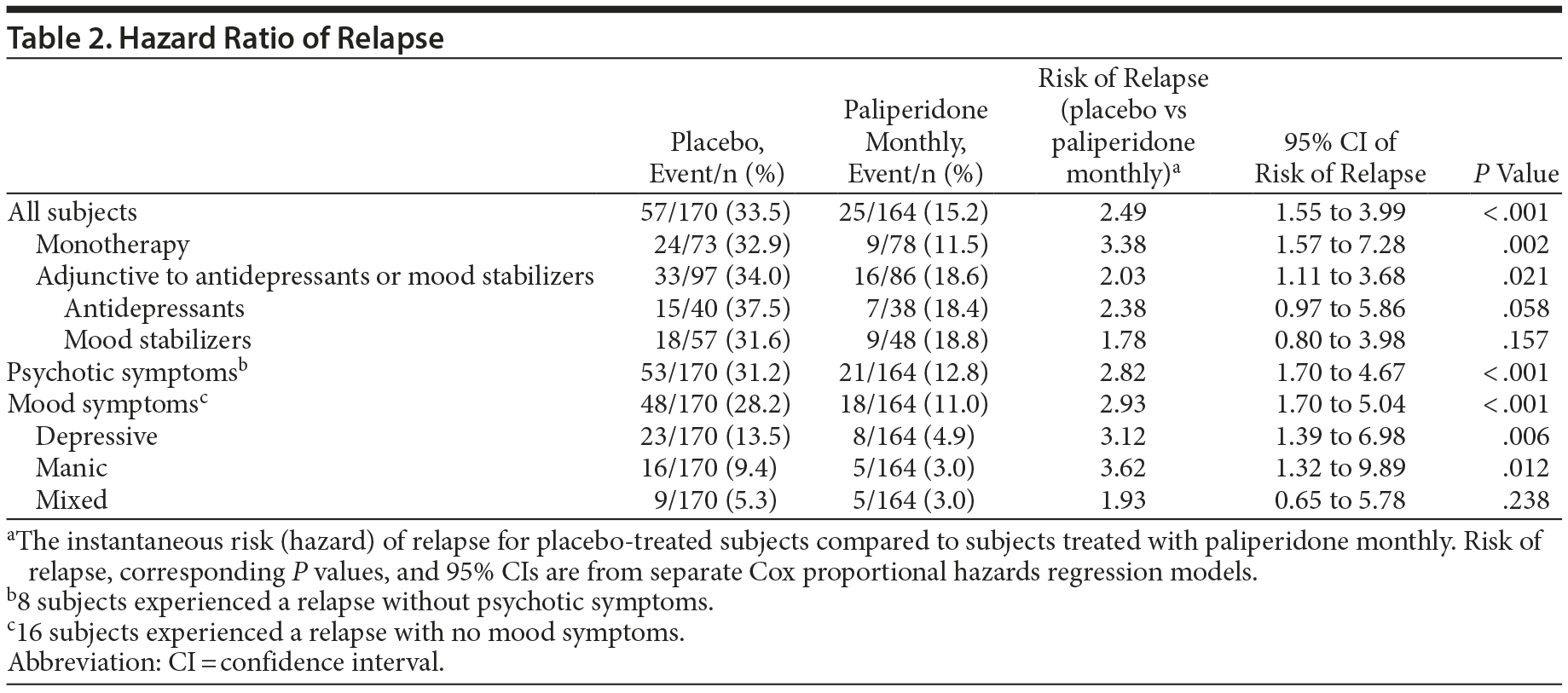
Paliperidone monthly treatment was associated with significant delay in time to relapse compared with placebo (P < .001) using the log-rank test controlling for concomitant medication strata (with or without adjunctive mood stabilizers/antidepressants) (Figure 2). Correspondingly, a significantly lower percentage of subjects treated with paliperidone monthly experienced a relapse event (P < .001, CMH test), with relapse rates of 33.5% (n = 57) and 15.2% (n = 25) in the placebo and paliperidone monthly groups, respectively. Relapse risk in the double-blind phase was 2.49-fold higher for placebo compared with paliperidone monthly (HR = 2.49; 95% CI, 1.55 to 3.99; P < .001), corresponding to a 60% decrease in relapse risk with maintenance treatment (Table 2).
Relapse risk was significantly higher for placebo versus paliperidone monthly in both monotherapy (HR = 3.38; P = .002) and adjunctive therapy (HR = 2.03; P = .021) subgroups. For the monotherapy subgroup, 32.9% (n = 24) and 11.5% (n = 9) of placebo and paliperidone monthly subjects, respectively, experienced a relapse. For the adjunctive therapy subgroup, relapse rates were 34.0% (n = 33) and 18.6% (n = 16), respectively.
When treatment effect was evaluated by relapse type, relapse risk was significantly higher for placebo versus paliperidone monthly in subjects with psychotic (P < .001), depressive (P = .006), or manic (P = .012) relapse. The risk ratio for relapse favoring treatment did not differ across different mood episode types (P = .718, Global Competing Risk test).
The most common reasons for relapse across both treatment groups were worsening of clinical symptom scores and interventions to avert hospitalizations (ie, increase in the level of psychiatric care from office visit to day hospitalization [not including increased level of care for social reasons] or the need for additional antipsychotics, antidepressants, or mood stabilizers) (Supplementary eTable 1). Hospitalization for decompensating schizoaffective disorder symptoms occurred in 7.1% of subjects in the placebo group and 3.0% in the paliperidone monthly group.
Mean change in PSP from baseline at month 15 significantly favored paliperidone monthly over placebo (P = .014) using MMRM analysis, and the least squares mean difference between groups in change scores at month 15 was 3.3 (95% CI, 0.68 to 5.95) (Supplementary eFigure 1). To evaluate the validity of the missing-at-random assumption for PSP, several sensitivity analyses based on missing-not-at-random were performed to assess robustness and consistency of findings at the month 15 endpoint. These analyses confirmed that paliperidone monthly was superior to placebo for maintaining functioning as measured by PSP at endpoint (Supplementary eTable 2).
To further evaluate the clinical relevance and consistency of PSP results, subject-level PSP data were examined as a categorical endpoint. The proportion of placebo-treated subjects with good functioning (PSP total score > 70) was 50.6% at double-blind baseline and 41.1% at endpoint, whereas it was 57.9% at double-blind baseline and 59.0% at endpoint (between-group difference, P = .002) for paliperidone monthly treated subjects with good functioning.
Additional secondary efficacy analyses are summarized in Supplementary eFigure 2. The LS-mean between-group differences for change in HDRS-21, YMRS, PANSS, and CGI-S-SCA total scores all significantly favored paliperidone monthly over placebo: HDRS-21 total scores: -2.5; 95% CI, −3.93 to -1.12; P < .001; YMRS total scores: -3.2; 95% CI, -4.53 to -1.83; P < .001; PANSS total scores: -6.9; 95% CI, -10.41 to -3.37; P < .001; CGI-S-SCA scores: -0.5; 95% CI, -0.69 to -0.24; P < .001.
The proportions of subjects with CGI-S-SCA scores of “not ill” to “mildly ill” at double-blind baseline were 95.9% (88/170) and 97.6% (74/164) for the placebo and paliperidone monthly groups, respectively. These percentages decreased at double-blind endpoint to 64.9% (45/168) and 83.9% (46/161), respectively (between-group difference, P < .001) (Supplementary eFigure 3A).
The proportion of subjects who were satisfied with their antipsychotic medication per the MSQ scale favored paliperidone monthly treatment: for placebo (93.5% of subjects at double-blind baseline and 69.6% at endpoint) compared with paliperidone monthly (94.5% at double-blind baseline and 85.7% at endpoint) (between-group difference, P < .001) (Supplementary eFigure 3B).
Safety
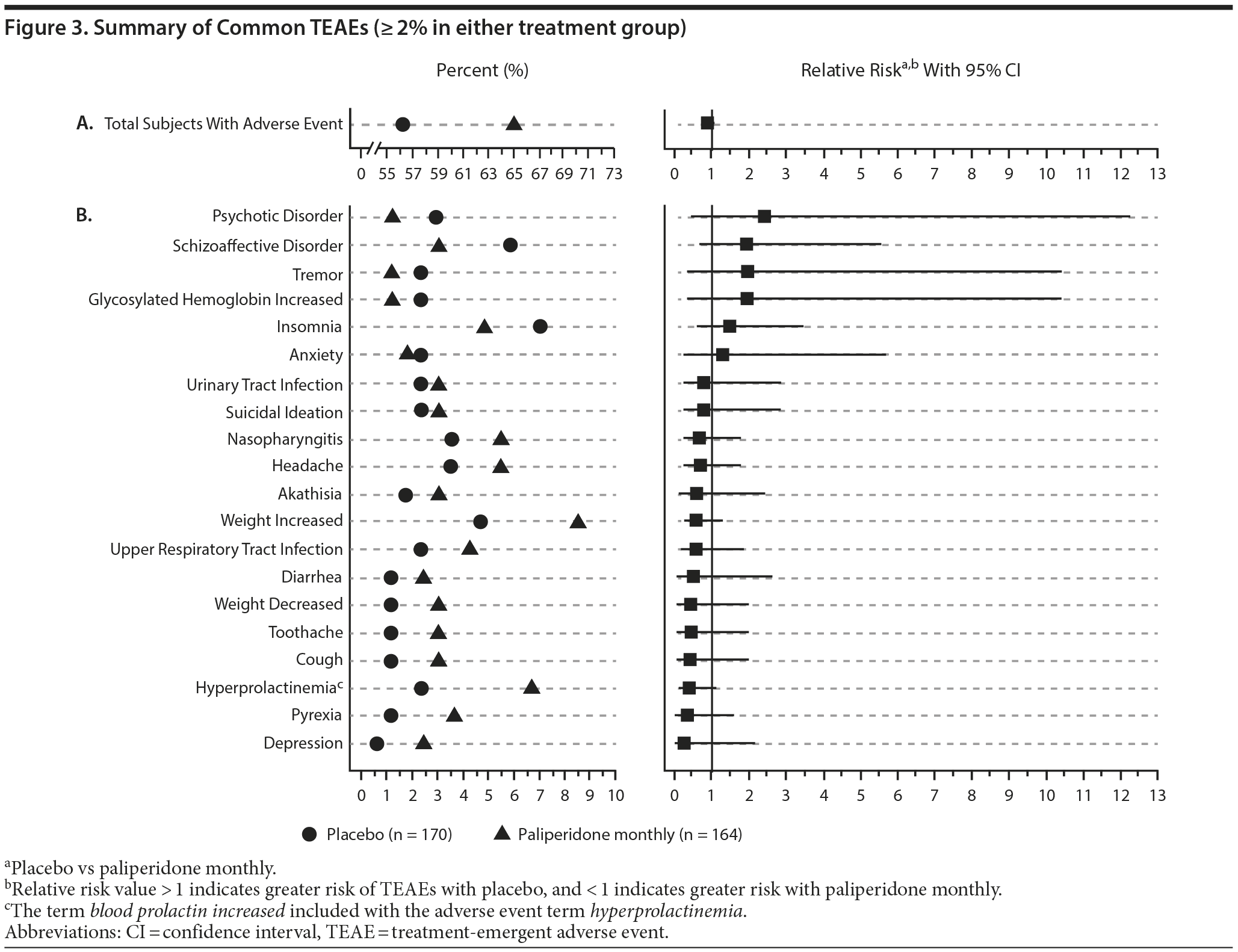
Common TEAEs reported during the double-blind phase are shown in Figure 3. Most TEAEs were mild to moderate. The most common (≥ 5% incidence in either treatment group) included schizoaffective disorder (placebo vs paliperidone monthly: 5.9%, 3.0%), weight increased (4.7%, 8.5%), nasopharyngitis (3.5%, 5.5%), headache (3.5%, 5.5%), and insomnia (7.1%, 4.9%). Serious TEAEs were reported in 9.4% and 5.5% of subjects in the placebo and paliperidone monthly groups, respectively, with events related to psychiatric disorders the most frequent. Two deaths occurred in the paliperidone monthly group. These included 1 death due to overdose of sleeping pills and another due to coronary artery disease. Both deaths were assessed by the investigator as not related to study drug. Three subjects (1.8%) in the placebo group discontinued therapy because of TEAEs (Méniרre’s disease, hepatitis C, and insomnia), while 12 subjects (7.3%) in the paliperidone monthly group discontinued due to TEAEs (cardiac failure congestive, fatigue, blood glucose increased, weight increased, musculoskeletal stiffness, akathisia, schizoaffective disorder, galactorrhea [2 patients], dermatitis, cognitive disorder, and abdominal discomfort). No significant differences between treatment groups in TEAE incidence were identified (ie, the CI of the relative risk values consistently crossed the boundary of 1).
Rates of extrapyramidal symptom (EPS)-related TEAEs were 7.1% in the placebo group and 8.5% in the paliperidone monthly group. Specific events included hyperkinesia (2.9%, 3.7%), parkinsonism (1.8%, 3.0%), tremor (2.4%, 1.2%), dyskinesia (1.8%, 0.6%), and dystonia (1.2%, 0). The percentage of subjects using anti-EPS medications at any time during double-blind treatment was 18.8% in the placebo group and 18.9% in the paliperidone monthly group.
Prolactin-related TEAEs were reported in 5.8% of women who received placebo and in 13.9% receiving paliperidone monthly; the most common were hyperprolactinemia/blood prolactin increase (3.5%, 8.9%), amenorrhea (2.3%, 3.8%), and galactorrhea (1.2%, 3.8%). Prolactin-related TEAEs were reported in 1.2% of men receiving placebo and 7.1% receiving paliperidone monthly; the most common of these was hyperprolactinemia/blood prolactin increase (0%, 4.7%).
Glucose-related TEAEs occurred in 2.4% of subjects receiving placebo and in 1.8% receiving paliperidone monthly. The proportion of subjects with a ≥ 7% weight increase was 6.0% for placebo and 13.0% for paliperidone monthly. The mean (SD) weight change was -0.8 (4.5) kg and -0.2 (6.1) kg for placebo and paliperidone monthly subjects, respectively.
DISCUSSION
This double-blind study supports the efficacy and safety of paliperidone monthly when used as monotherapy or adjunctive therapy for maintenance treatment of schizoaffective disorder. Withdrawal of treatment for patients in the placebo group previously stabilized on paliperidone monthly treatment resulted in a 2.49-fold increased relapse risk compared to continued treatment with paliperidone monthly, supporting a protective effect in the schizoaffective disorder population at high risk for poor adherence and relapse into psychosis, depression, and/or mania. Consistent with this potential benefit of paliperidone monthly therapy, the median duration of exposure during the 15 months of observation was 160 days longer with continued treatment compared with withdrawal to placebo. These findings extend observations from schizophrenia trials34 demonstrating the ability of paliperidone monthly to maintain efficacy for psychosis and support maintenance effects for preventing emergence of depression and mania symptoms in schizoaffective disorder. These results support prior evidence that the paliperidone molecule is effective and safe for acute management of these schizoaffective disorder symptoms following relapse.5,6
The efficacy of paliperidone monthly monotherapy suggests that it may serve as a foundation for treatment of patients with schizoaffective disorder. As monotherapy, paliperidone monthly eliminates requirements for simultaneous management of multiple psychotropic medications for physicians and daily oral medication adherence by patients. In this study, the effect of treatment appeared smaller with adjunctive therapy, which may reflect partial efficacy of adjunctive treatment and/or more refractory illness in patients requiring adjunctive therapies.
Relapse criteria in this study were designed to identify early indicators of relapse, such as symptom worsening or need for increase in psychotropic medications. These criteria minimized the potential for patients to experience a fully symptomatic relapse. Patients with an impending relapse were immediately discontinued from the study for rapid institution of standard treatment. This study design aspect may have contributed to the relatively small observed treatment effect for some secondary efficacy variables during double-blind treatment phase and differences in changes in overall functioning.
Improving and maintaining patient functioning beyond symptom control is a key long-term treatment goal. The PSP score reflects the composite of 4 functional domains (socially useful activities, personal/social relationships, self-care, and disturbing/aggressive behavior). Results from this study demonstrate that paliperidone monthly better maintains long-term functioning as measured by the PSP, providing potential value beyond maintenance of symptom control. Biases associated with missing PSP data were carefully considered; however, the inherent issue of informative censoring in a relapse-prevention study design may require additional interpretation of the PSP data. Patient experience with medication contributes to adherence. In this study, patient satisfaction was better maintained with paliperidone monthly treatment compared to placebo.
Enrolled subjects had a current and lifetime diagnosis of schizoaffective disorder according to DSM-IV. On the basis of investigator assessments following the release of DSM-5, 95% of randomized subjects also met DSM-5 diagnostic criteria for schizoaffective disorder, confirming that the study population represents patients with schizoaffective disorder according to current diagnostic criteria.
The pattern of TEAEs was similar to those reported in the previously conducted maintenance study of paliperidone monthly in schizophrenia,34 and no new safety concerns were detected. Only patients who could be stabilized on and tolerated treatment were included in the double-blind, placebo-controlled study phase. The 25-week prior open-label treatment with paliperidone monthly may have minimized observations of TEAEs associated with treatment. Tolerability and efficacy findings for the open-label phase of this study will be reported elsewhere.
Consistent with most controlled efficacy studies, a limitation of this study is that the subject population was chosen to minimize confounding factors such as substance dependence. It was also limited to patients who consented to receive long-acting injectable therapy. Therefore, results may not be generalizable to all patients with schizoaffective disorder.
In conclusion, this study provides evidence that paliperidone monthly significantly delayed and reduced risk of relapse of psychotic, depressive, and manic symptoms and better maintains functioning when used as monotherapy or adjunctive therapy in patients with schizoaffective disorder.
Drug names: paliperidone palmitate (Invega Sustenna), risperidone (Risperdal and others).
Author affiliations: Scientific Affairs, Janssen Scientific Affairs, LLC (Drs Fu and Alphs); Biostatistics, Janssen Research & Development, LLC (Dr Turkoz); Global Clinical Operations, Janssen Research & Development, LLC (Mr Simonson); and Central Nervous System, Janssen Research & Development, LLC (Dr Canuso), Titusville, New Jersey; Collaborative Neuroscience Network, Inc, Garden Grove, California (Dr Walling); Department of Psychiatry, SUNY Downstate Medical Center, Brooklyn, New York (Dr Schooler); and Department of Psychiatry, New York University Langone Medical Center, New York (Dr Lindenmayer).
Author contributions: Drs Fu, Walling, Schooler, Lindenmayer, Canuso, and Alphs and Mr Simonson were responsible for study concept and design, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. Dr Turkoz was responsible for study concept and design, acquisition of data, analysis and interpretation of the data, statistical analysis of the data, drafting of the manuscript, and critical revision of manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
Study investigators: The authors thank the following investigators who contributed data to the study: Volodymyr Abramov, MD, PhD, ScD (Donetsk, Ukraine); Neal Adams, MD, MPH, and Ira Glick, MD (Oakland, CA); Maria Alexandrova, MD, PhD (Pleven, Bulgaria); George M. Badescu, MD (Craiova, Romania); Michael D. Banov, MD (Marietta, GA); Vinay L. Barhale, MD (Aurangabad, India); James T. Barker II, MD (Charlotte, NC); Norhayati Binti Nordin, MD (Kota Kinabalu, Malaysia); Yuliya Blazhevych, MD (Kiev, Ukraine); Ronald L. Brenner, MD (Cedarhurst, NY); Joel S. Breving, MD (Staten Island, NY); David W. Brown, MD (Austin, TX); Rowena G. Cosca, MD (Iloilo City, Philippines); Doina C. Cozman, MD (Cluj-Napoca, Romania); Monina G. Cruz, MD (Quezon City, Philippines); Irina A. Dan, MD (Bucharest, Romania); Bethany B. Davis, MD, and Brian S. Bortnick, MD (Atlanta, GA); Temenuzhka Dechkova-Novakova, MD (Ruse, Bulgaria); Vladyslav Demchenko, MD (Kiev, Ukraine); Michael Downing, MD (Dallas, TX); Esther G. Ebenezer, Assoc Prof, MBBS (Ipoh, Malaysia); Tsveteslava Galabova, MD (Novi Iskar, Bulgaria); Hitendra A. Gandhi, MD (Ahmedabad, India); Donald Garcia, Jr, MD (Austin, TX); Eduard Gfeller, MD (Maitland, FL); Elena Gherman, MD (Bucharest, Romania); Liana Giurgiuca, MD (Bucharest Romania); Armen K. Goenjian, MD (Torrance, CA); Gerhard P. Grobler, MD (Pretoria, Republic of South Africa); Daniel Gruener, MD (Philadelphia, PA); Thomas A. Grugle, MD (Dallas, TX); Mariano S. Hembra, MD (Iloilo City, Philippines); Luchezar Hranov, PhD, MD (Sofia, Bulgaria); Vishal India, MD (Andhra, India); Karl Jacobs, MD (San Diego, CA); Suarn Singh Jasmit Singh, MBBS (Tanjung Rambutan, Malaysia); Venu G. Jhanwar, MD (Varanasi, India); Emil Kaludiev, MD, PhD (Sofia, Bulgaria); Mary A. Knesevich, MD (Irving, TX); Joseph Kwentus, MD (Flowood, MS); Maria Ladea, MD (Bucharest, Romania); Olga M. Lapeyra, MD, and Peter P. Ventre, MD (North Miami, FL); Igor Linskiy, MD, PhD, Dr Med Sc (Kharkiv, Ukraine); Jennifer M. Lytle, MD, MPH (Denver, CO); Mirela Manea, MD (Bucharest, Romania); Raymond Manning, MD (Pico Rivera, CA); Gabriela Marian, MD (Bucharest, Romania); Victor Marinescu, MD (Bucharest, Romania); Nataliya Maruta, MD, PhD (Kharkiv, Ukraine); Svitlana Moroz, MD, PhD (Dnipropetrovsk, Ukraine); Paul W. Murphy, MD (Wichita, KS); Unmesh Nagapurkar, MD (Nashik, India); Daniel J. Niehaus, Prof (Cape Town, Republic of South Africa); Anil Nischal, MD (Lucknow, India); Gheorghe Oros, MD (Oradea, Romania); Bogdan M. Pacala, MD (Sibiu, Romania); Agnes B. Padilla, MD (Davao City, Philippines); Pavlo Palamarchuk, MD (Stepanivka, Ukraine); Sanjay Phadke, MD (Pune, India); Delia Podea, MD (Arad, Romania); Stefan Popov, MD (Plovdiv, Bulgaria); Satheesh Rao, MD (Mangalore, India); Sathyanarayana Rao, MBBS, DPM, MD (Mysore, India); Oleksandr Romaniv, MD (Uzhgorod, Ukraine); Sofiya Rymsha, MD, PhD, Dr Med Sc (Vinnytsa, Ukraine); Martha Sajatovic, MD (Cleveland, OH); Ramanathan Sathianathan, MBBS, DPM, MD (Chennai, India); Juan P. Schronen, MD (Cape Town, Republic of South Africa); Scott Segal, MD (Ft. Lauderdale, FL); Sandip H. Shah, MD (Vadodara, India); Rajinder Shiwach, MD, and Ernest Brownlee, Jr, MD (DeSoto, TX); Franco Sicuro, MD (Creve Coeur, MO); Dorina Sima, MD (Bucharest, Romania); Dinesh Singh, MD (Durban, Republic of South Africa); Andrii Skrypnikov, MD, PhD (Poltava, Ukraine); Sherry A. Soefje, MD, and Jelena Kunovac, MD (Oceanside, CA); Mary Stedman, MD (Tampa, FL); Ahamad H. Sulaiman, MBBS (Kuala Lumpur, Malaysia); Rajagopal Sunder, MD; Lily S. Chung, MD; Sadashiv Rajadhyaksha, MD; and Samuel Dey, Jr, MD (Riverside, CA); Simona C. Tamasan, MD (Timisoara, Romania); Anil Tambi, MD (Jaipur, India); Alexandru Tiugan, MD (Craiova, Romania); Mauricio Tohen, MD, DrPH, MBA (San Antonio, TX); Maria Silvi Trandafir, MD (Bucharest, Romania); Tram Tran-Johnson, PharmD, PsyD (San Diego, CA); Jitendra Trivedi, MD (Lucknow, India); Catalina Tudose, MD (Bucharest, Romania); Lidia N. Udangiu, MD (Bucharest, Romania); Daniel Vasile, MD (Bucharest, Romania); Gert J. Venter, MD (Gauteng, Republic of South Africa); Viktoriya Verbenko, MD, PhD (Symferopol, Ukraine); Mykhaylo Vynnyk, MD, PhD, Dr Med Sc (Ivano-Frankovsk. Ukraine); David P. Walling, PhD (Garden Grove, CA); Blaise Wolfrum, MD (Schaumburg, IL); Rakesh Yadav, MD (Jaipur, India); and Gennadii Zilberblat, MD (Glevakha, Ukraine).
Potential conflicts of interest: Drs Fu and Alphs are employees of Janssen Scientific Affairs, LLC, and Johnson & Johnson stockholders. Drs Turkoz and Canuso and Mr Simonson are employees of Janssen Research & Development, LLC, and Johnson & Johnson stockholders. Dr Walling has received grant/research support from Janssen Research Foundation. Dr Schooler has received grant/research support from Otsuka, Neurocrine, and Genentech and has served as advisory board member/speaker for Roche, Forum, Sunovion, and Lundbeck. Dr Lindenmayer has received grant/research support from Janssen, Eli Lilly, Pfizer, Otsuka, Dainippon Sumitomo, Sunovion, Neurocrine, EnVivo, and Roche and is a consultant for Janssen.
Funding/support: This study was funded by Janssen Scientific Affairs, LLC (Primary Study Protocol Number R092670-SCA-3004), which was responsible for the design and conduct of the study and for the collection, management, analysis, and interpretation of the data. Writing and editorial assistance were also funded by Janssen Scientific Affairs, LLC.
Previous presentations: This work has been presented in part at the American Psychiatric Association 167th Annual Meeting; May 3-7, 2014; New York, New York ▪ Society of Biological Psychiatry 69th Annual Scientific Meeting; May 8-10, 2014; New York, New York ▪ ASCP Annual Meeting; June 16-19, 2014; Hollywood, Florida ▪ XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain ▪ American Psychiatric Nurses Association 28th Annual Conference, October 22-25, 2014; Indianapolis, Indiana ▪ 27th ECNP Congress of Applied and Translational Neuroscience; October 18-21, 2014; Berlin, Germany ▪ Institute on Psychiatric Services Annual Meeting; October 30-November 2, 2014; San Francisco, California.
Acknowledgments: The authors thank Tricia Newell, PhD; Matthew Grzywacz, PhD; and ApotheCom, LLC, Yardley, Pennsylvania, for providing writing and editorial assistance for this manuscript (funded by Janssen Scientific Affairs, LLC). The authors also thank Cynthia A. Bossie, PhD (Janssen Scientific Affairs, LLC), for her scientific input and discussions and Erin Lee, RN (Janssen Scientific Affairs, LLC), for her assistance in data review.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing; 2013.
2. Malaspina D, Owen MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25. PubMed doi:10.1016/j.schres.2013.04.026
3. Perפlפ J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19-28. PubMed doi:10.1001/archpsyc.64.1.19
4. Nasrallah HA, Goldberg JF, Correll CU; SAD Working Group. Differential diagnosis and therapeutic management of schizoaffective disorder. Ann Clin Psychiatry. 2010;22(suppl 1):S1-S12.. PubMed
5. Canuso CM, Schooler N, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized, controlled study comparing a flexible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30(5):487-495. PubMed doi:10.1097/JCP.0b013e3181eeb600
6. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo-controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71(5):587-598. PubMed doi:10.4088/JCP.09m05564yel
7. Canuso CM, Turkoz I, Fu DJ, et al. Role of paliperidone extended-release in treatment of schizoaffective disorder. Neuropsychiatr Dis Treat. 2010;6:667-679. PubMed doi:10.2147/NDT.S12612
8. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long-term course. Acta Psychiatr Scand. 1990;82(5):352-358. PubMed doi:10.1111/j.1600-0447.1990.tb01400.x
9. Cheniaux E, Landeira-Fernandez J, Lessa Telles L, et al. Does schizoaffective disorder really exist? a systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106(3):209-217. PubMed doi:10.1016/j.jad.2007.07.009
10. Murru A, Pacchiarotti I, Nivoli AM, et al. What we know and what we don’ t know about the treatment of schizoaffective disorder. Eur Neuropsychopharmacol. 2011;21(9):680-690. PubMed doi:10.1016/j.euroneuro.2011.03.001
11. Keck PE Jr, McElroy SL, Strakowski SM. Schizoaffective disorder: role of atypical antipsychotics. Schizophr Res. 1999;35(suppl):S5-S12. PubMed doi:10.1016/S0920-9964(98)00165-0
12. McElroy SL, Keck PE Jr, Strakowski SM. An overview of the treatment of schizoaffective disorder. J Clin Psychiatry. 1999;60(suppl 5):16-21, discussion 22.. PubMed
13. Flynn J, Grieger TA, Benedek DM. Pharmacologic treatment of hospitalized patients with schizoaffective disorder. Psychiatr Serv. 2002;53(1):94-96. PubMed doi:10.1176/appi.ps.53.1.94
14. Lerner V, Libov I, Kotler M, et al. Combination of “atypical” antipsychotic medication in the management of treatment-resistant schizophrenia and schizoaffective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):89-98. PubMed doi:10.1016/j.pnpbp.2003.09.024
15. Invega (paliperidone) extended-release tablets [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2007, 2011.
16. Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159(1):103-108. PubMed doi:10.1176/appi.ajp.159.1.103
17. Murru A, Pacchiarotti I, Nivoli AM, et al. Rates and clinical correlates of treatment non-adherence in schizoaffective bipolar patients. Acta Psychiatr Scand. 2012;125(5):412-418. PubMed doi:10.1111/j.1600-0447.2012.01837.x
18. Murru A, Pacchiarotti I, Amann BL, et al. Treatment adherence in bipolar I and schizoaffective disorder, bipolar type. J Affect Disord. 2013;151(3):1003-1008. PubMed doi:10.1016/j.jad.2013.08.026
19. Perkins DO, Johnson JL, Hamer RM, et al; HGDH Research Group. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63. PubMed doi:10.1016/j.schres.2005.10.016
20. Lindenmayer JP, Liu-Seifert H, Kulkarni PM, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70(7):990-996. PubMed doi:10.4088/JCP.08m04221
21. Bodén R, Brandt L, Kieler H, et al. Early non-adherence to medication and other risk factors for rehospitalization in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;133(1-3):36-41. PubMed doi:10.1016/j.schres.2011.08.024
22. Karve S, Markowitz M, Fu DJ, et al. Assessing medication adherence and healthcare utilization and cost patterns among hospital-discharged patients with schizoaffective disorder. Appl Health Econ Health Policy. 2014;12(3):335-346. PubMed doi:10.1007/s40258-014-0095-8
23. Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235-244. PubMed doi:10.1097/JCP.0b013e3181dd3103
24. Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218-226. PubMed doi:10.1016/j.pnpbp.2010.11.008
25. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
26. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429-435. PubMed doi:10.1192/bjp.133.5.429
27. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. PubMed doi:10.1111/j.2044-8260.1967.tb00530.x
28. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press Inc; 1996.
29. Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323-329.. PubMed
30. Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213-224. PubMed doi:10.1016/j.psychres.2007.11.012
31. Allen MH, Daniel DG, Revicki DA, et al. Development and psychometric evaluation of a clinical global impression for schizoaffective disorder scale. Innov Clin Neurosci. 2012;9(1):15-24.. PubMed
32. Canuso CM, Grinspan A, Kalali A, et al. Medication satisfaction in schizophrenia: a blinded-initiation study of paliperidone extended release in patients suboptimally responsive to risperidone. Int Clin Psychopharmacol. 2010;25(3):155-164. PubMed doi:10.1097/YIC.0b013e3283372977
33. Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute; 1995.
34. Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107-117. PubMed doi:10.1016/j.schres.2009.10.026
This PDF is free for all visitors!