Objective: No highly effective pharmacologic interventions to prevent delirium have been identified. We examined whether suvorexant, a potent and selective orexin receptor antagonist, is effective for the prevention of delirium.
Methods: We conducted a multicenter, rater-blinded, randomized, placebo-controlled clinical trial in intensive care units and regular acute wards between April 2015 and March 2016. Eligible patients were 65 to 89 years old, newly admitted due to emergency, and able to take medicine orally and had an expected stay or life expectancy of 48 hours or more. Seventy-two patients were randomly assigned using the sealed envelope method to receive suvorexant (15 mg/d; 36 patients) or placebo (36 patients) every night for 3 days. The primary outcome measure was incidence of delirium as determined by the DSM-5. Trained psychiatrists assessed for delirium.
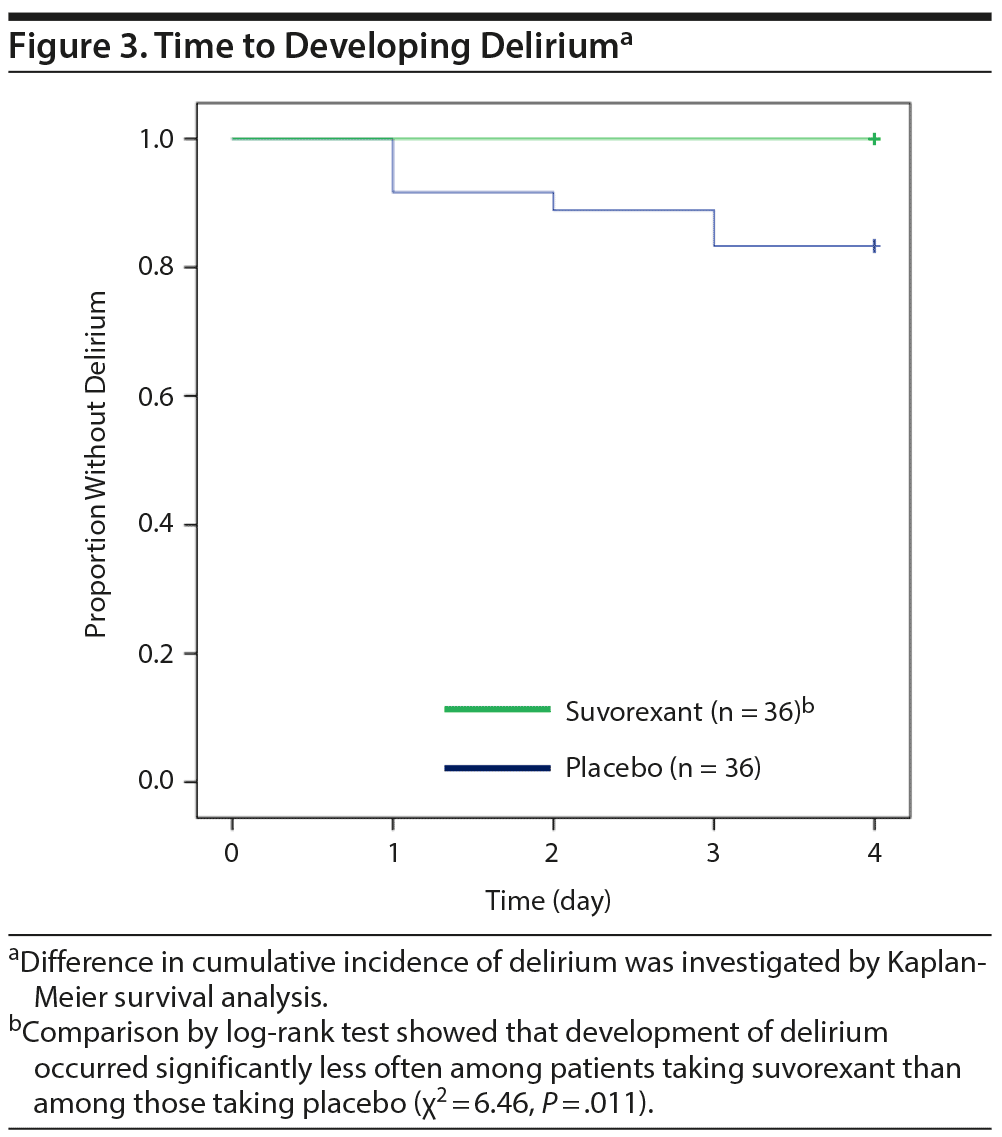
Results: We found that delirium developed significantly less often among patients taking suvorexant than among those taking placebo (0% [n/N = 0/36] vs 17% [6/36], respectively, P = .025). Comparison by log-rank test also showed that delirium developed significantly less often among patients taking suvorexant than among those taking placebo (χ2 = 6.46, P = .011). Analysis of variance revealed a tendency for main effect of treatment (F = 3.79, P = .053) on the sleep-wake cycle disturbance score (item 1) of the Japanese version of the Delirium Rating Scale-Revised-98 (DRS-R-98-J). There were no significant differences in adverse events.
Conclusions: Suvorexant administered nightly to elderly patients admitted for acute care may provide protection against delirium. Larger studies are needed to show the potential of suvorexant to improve the circadian core domain of delirium.
Trial Registration: UMIN Clinical Trials Registry identifier: UMIN000015681‘ ‹
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Preventive Effects of Suvorexant on Delirium:
A Randomized Placebo-Controlled Trial

ABSTRACT
Objective: No highly effective pharmacologic interventions to prevent delirium have been identified. We examined whether suvorexant, a potent and selective orexin receptor antagonist, is effective for the prevention of delirium.
Methods: We conducted a multicenter, rater-blinded, randomized, placebo-controlled clinical trial in intensive care units and regular acute wards between April 2015 and March 2016. Eligible patients were 65 to 89 years old, newly admitted due to emergency, and able to take medicine orally and had an expected stay or life expectancy of 48 hours or more. Seventy-two patients were randomly assigned using the sealed envelope method to receive suvorexant (15 mg/d; 36 patients) or placebo (36 patients) every night for 3 days. The primary outcome measure was incidence of delirium as determined by the DSM-5. Trained psychiatrists assessed for delirium.
Results: We found that delirium developed significantly less often among patients taking suvorexant than among those taking placebo (0% [n/N = 0/36] vs 17% [6/36], respectively, P = .025). Comparison by log-rank test also showed that delirium developed significantly less often among patients taking suvorexant than among those taking placebo (χ2 = 6.46, P = .011). Analysis of variance revealed a tendency for main effect of treatment (F = 3.79, P = .053) on the sleep-wake cycle disturbance score (item 1) of the Japanese version of the Delirium Rating Scale-Revised-98 (DRS-R-98-J). There were no significant differences in adverse events.
Conclusions: Suvorexant administered nightly to elderly patients admitted for acute care may provide protection against delirium. Larger studies are needed to show the potential of suvorexant to improve the circadian core domain of delirium.
Trial Registration: UMIN Clinical Trials Registry identifier: UMIN000015681
J Clin Psychiatry 2017;78(8):e970-e979
https://doi.org/10.4088/JCP.16m11194
© Copyright 2017 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Juntendo University Nerima Hospital, Tokyo, Japan
bDepartment of Psychiatry, Nippon Medical School Musashikosugi Hospital, Kawasaki, Japan
cDepartment of Psychiatry, Hiroshima City Hospital, Hiroshima, Japan
dDepartment of Psychiatry, Tokyo Medical and Dental University, Tokyo, Japan
eDepartment of Emergency and Critical Care Medicine, Juntendo University Nerima Hospital, Tokyo, Japan
fDepartment of Environmental and Preventive Medicine, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
*Corresponding author: Kotaro Hatta, MD, PhD, Department of Psychiatry, Juntendo University Nerima Hospital, Takanodai 3-1-10, Nerima-ku, Tokyo 177-8521, Japan ([email protected]).
Delirium represents a disturbance in attention, awareness, and cognition that develops over a short period of time and tends to fluctuate.1 The prevalence of delirium is 18% to 50% on admission, and the incidence during hospitalization is 10% to 82% in general medical and geriatric wards, the intensive care unit (ICU), and postoperative and palliative care settings.2 Further increase seems likely with the increase in the aged population. Despite some success of multicomponent nonpharmacologic interventions,3,4 no highly effective pharmacologic interventions to prevent delirium have been identified. Almost all trials of cholinesterase inhibitors failed,2 and effects of antipsychotics are controversial.5-11
There is some emerging literature suggesting that melatonin prophylaxis may reduce delirium incidence or a long-lasting episode of delirium12-16 and that PER Clock gene function may be disturbed in delirium.17,18 Further, sleep-wake cycle disturbances including insomnia, excessive daytime napping, and disintegration of the expected circadian patterns have been described as a characteristic component of delirium for decades and demonstrated to be a core symptom domain of delirium.19,20 Therefore, it is of research interest to evaluate a medication related to circadian patterns in delirium.
Suvorexant, a potent and selective orexin receptor antagonist, recently approved by the US Food and Drug Administration for the treatment of insomnia, is noted for subjective measures of sleep onset and maintenance,21 without major changes in the patient’s neurophysiology as assessed by electroencephalographic power spectral density.22 In practice, we experienced that a lot of acutely admitted patients with risk factors of delirium such as advanced age, dementia, and history of delirium did not develop delirium after suvorexant was given for insomnia, without obvious side effects. The scientific rationale for suvorexant, an orexin antagonist, is related to the characteristic sleep-wake cycle disturbance in delirium. Orexin is an alerting neurochemical. Although orexin neurons send excitatory projections to cholinergic nuclei as well as monoaminergic nuclei with particular denseness,23 it has been reported that suvorexant is highly selective for orexin-1 receptor and orexin-2 receptor antagonism, having 6,000-fold intrinsic selectivity over 170 known receptors and enzymes, as demonstrated by in vitro assay panels.24 Accordingly, the Belsomra package insert says that suvorexant has not shown affinities for acetylcholine receptors (Ki > 10µM).25 The failures in trials of cholinesterase inhibitors in delirium prevention2 and our experiences in nondevelopment of delirium after suvorexant for insomnia in patients with risk factors of delirium suggest that the restoration of sleep-wake cycle can have priority over acetylcholine neurotransmission in delirium prevention. Given that the sleep-wake cycle disturbance can be sleeplessness or excessive sleepiness or a disintegration of the diurnal cycle of sleep and wakefulness, antagonizing the action of orexin can contribute to improvement of the sleep-wake cycle disturbance. Therefore, we hypothesized that suvorexant would prevent delirium.
The second point of this study was the prediction of delirium from the viewpoints of inflammation, the permeability of blood-brain barrier, and neuroprotection. As systemic inflammation is considered to be a risk factor of delirium, we have reported the predictive value of a change in natural killer cell activity for delirium.26 Increase in the permeability of blood-brain barrier and decline in neuroprotection are of further interest to investigate the influence of systemic inflammation on the central nervous system. Increase in the accuracy of delirium prediction may be an effective strategy in delirium prevention.
METHODS
Design
We conducted this multicenter, rater-blinded, parallel group, randomized, placebo-controlled trial between April 2015 and March 2016 in 3 university hospitals and 1 general hospital. The study was approved by the institutional review board of Juntendo University Nerima Hospital, Tokyo, Japan, with local approval from the other participating centers. Patients or their proxy decision-makers provided written informed consent after receiving a complete description of the study. This activity was conducted by the DELIRIA-J study group. This trial was registered with the UMIN Clinical Trials Registry (identifier: UMIN000015681).

- Despite some success of multicomponent nonpharmacologic interventions, no highly effective pharmacologic interventions to prevent delirium have been identified.
- In a randomized controlled trial of 72 patients who were 65 to 89 years old, delirium developed significantly less often among patients taking suvorexant than among patients taking placebo.
- For insomnia patients with delirium risk factors, suvorexant is a viable consideration.
Participants and Setting
Eligible patients were 65 to 89 years old, newly admitted due to emergency, and able to take medicine orally and had an expected stay or life expectancy of 48 hours or more. Patients were admitted via emergency rooms to ICUs or acute wards. Exclusion criteria included patients taking strong CYP3A inhibitors such as itraconazole, clarithromycin, ritonavir, saquinavir, nelfinavir, indinavir, telaprevir, and voriconazole, which are contraindications to the use of suvorexant because of a major interaction with suvorexant; patients with narcolepsy, cataplexy, severe liver dysfunction, and severe respiratory dysfunction to whom the package insert requires careful administration of suvorexant; patients who were already delirious at admission; and patients taking antipsychotics. Also, patients with alcohol dependence and drug abuse were excluded. We used Triage-DOA (Alere Inc, Tokyo, Japan) for urine-based abuse screening of patients. Patients were assessed and excluded by trained psychiatrists.
Randomization and Masking
After completion of the baseline assessment, patients were randomly assigned using the sealed envelope method in a rater-blind manner to receive either suvorexant or placebo (1:1 ratio). For randomization, we referred to a random number table, with sequentially numbered, opaque, sealed envelopes used to conceal the allocation sequence. The randomization mechanism was in permuted blocks with a block size of 4. Study medication was set by pharmacists and administered daily at 21:00 hours. The dose of suvorexant was 15 mg/night as a single tablet, representing the standard dose for the approved indication of sleep disturbance for elderly people. As the placebo tablet matched the suvorexant active agent in appearance, patients were blinded with the study drug. A study physician in each emergency department of 4 attending hospitals kept the randomization code, and no rater became aware of treatment allocations until requesting unmasking on March 4, 2016. There was blinding of nurses and other staff such as physiotherapists. Nurses provided all patients equally with preventive care. The study physician in each emergency department never unblinded the study drug identity to others such as each patient’s attending physicians, clinical staff, or patients’ families. However, physicians in each emergency department treated patients as a team including the study physician. Therefore, we did not describe the study design as a double-blind trial.
Procedure
Before starting the trial, site coordinators, who were experienced psychiatrists, were trained to assess outcomes as raters. They approached patients at their bedside within 24 hours after admission, screened for eligibility, and asked them to participate. After randomization, patients received the study medication on 3 consecutive evenings, starting the day of admission at 21:00 hours.
At baseline, we recorded patients’ demographic and clinical characteristics. Patients’ demographics were ascertained from the patients themselves, family members, and the medical record. According to the Beers Criteria,27 the prescription of potentially "deliriogenic" medication during hospitalization was recorded and analyzed. Attending physicians continued to prescribe psychotropic medication taken before admission without any change and were not allowed to prescribe additional psychotropic medication after admission. The Acute Physiology Score component of the Acute Physiology and Chronic Health Evaluation II (APACHE II)28 and Charlson Comorbidity Index29 were assessed to evaluate physical condition. Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) performance status. It is a 1-item scale, and it ranges from 0 to 4, with higher scores indicating more difficulty with activities of daily living.30 Clinical Dementia Rating (CDR) was assessed to evaluate the existence and severity of dementia.31
In a phase 3 randomized, placebo-controlled trial aiming to assess the clinical profile of suvorexant, the efficacy of suvorexant was reportedly characterized by subjective measures of sleep onset and maintenance due to its superiority to placebo in improving subjective time to sleep onset and subjective total sleep time.21 Therefore, as sleep metrics, subjective time to sleep onset, subjective number of awakenings, waking too early, subjective quality of sleep, and subjective total sleep time were recorded. Raters interviewed patients for the subjective items on every morning round and tabulated and scored the items. Subjective quality of sleep was rated using a 1 to 4 scale: very good (1), fairly good (2), fairly bad (3), very bad (4). In addition, difficulty falling asleep, difficulty staying asleep, waking too early, total sleep time, and disturbance of natural sleep-wake rhythm during study drug administration were tabulated and scored based on the nursing record that was checked with comments on every 15-minute round.32 Disturbance of natural sleep-wake rhythm was determined based on the nursing record and rater observations. Internal consistency was not high, with a Cronbach α coefficient for the sleep parameters of 0.68, which referred to the ratings of 50 elderly hospitalized patients who tried sleep metrics preliminarily.
Outcomes
The primary outcome was incidence of delirium during the first 3 days after initiation of the study medication. We used criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),1 to diagnose delirium. Simultaneously, we censored patients developing delirium using the Delirium Rating Scale-Revised-98 (DRS-R-98)33 total score according to a cutoff score of 14.5, established for the Japanese population through investigation of the reliability and validity of the Japanese version of the DRS-R-98 (DRS-R-98-J).34 The method for interviewing patients about 16 items of the scale was the same, as we had arranged it in advance. Interrater reliability measured by interclass correlation coefficient for pairs of independent raters was very high for the DRS-R-98-J total score: 0.997 for Juntendo University Nerima Hospital, 0.994 for Nippon Medical School Musashikosugi Hospital, 0.970 for Tokyo Medical and Dental University, and 0.918 for Hiroshima City Hospital. DRS-R-98-J was measured daily between 10:00 hours and 11:00 hours up to day 4. Raters reviewed all nursing records before morning rounds and collected information about each patient directly from bedside nurses. Raters then assessed each patient to determine if delirium had occurred. Raters also made their rounds every afternoon. Once delirium occurred, the etiology was recorded according to the Delirium Etiology Rating Checklist.33 Adverse events such as somnolence, headache, fatigue, dizziness, bad dreams, sleep paralysis, hypnagogic/hypnopompic hallucinations, and complex sleep-related behaviors, which are listed in the insert form of suvorexant as potential side effects, were formally assessed.
Secondary objectives were cumulative incidence of DSM-5 delirium over a 3-day period of administration of the study drug as analyzed using Kaplan-Meier survival analysis; incidence of delirium including follow-up data up to day 7, although the study drug was not given on and after the night of day 4; and the exploration of change in serum biomarkers associated with delirium prediction such as C-reactive protein (CRP) and high-sensitivity CRP (hs-CRP) for inflammation, S100 protein β for increase in the permeability of blood-brain barrier, and insulinlike growth factor 1 (IGF-1) for neuroprotection.
The medication period of 3 days was determined referring to the shortest period to find the significant difference in the incidence of delirium in our previous trial about preventive effects of ramelteon conducted in almost the same settings.15 The reason for setting the shortest period of medication was the intention of the lowest burden to patients and real clinical practice for the experimental procedure. We would consider that suvorexant is inferior to ramelteon in the effects on delirium prevention if there were no significant difference in the incidence of delirium between the groups in the present study. Thereafter, patients were followed-up until discharge.
Measurement of Biomarkers
Serum levels of CRP, hs-CRP, IGF-1, and S100β were measured on the first and second mornings after admission. We have previously reported changes in blood natural killer cell activity prior to the onset of delirium.26 Therefore, we focused on other markers in the context of delirium prediction and presumed that values of the markers on the second morning would increase or decrease compared to those on the first morning after admission. Blood samples were obtained by cubital puncture at 6:00 am before breakfast. The CRP levels were measured according to the latex agglutination method using Nanopia CRP reagent (Sekisui Medical Co, Ltd, Tokyo, Japan) and JCA-BM8060 (JEOL, Ltd, Tokyo, Japan). The hs-CRP levels were determined by latex-enhanced immunonephelometric assay using CardioPhase hsCRP reagent (Siemens Healthcare KK, Tokyo, Japan). Serum IGF-1 concentrations were determined by immunoradiometric assay kit (IGF-1 assay "Daiichi"; Fujirebio Inc, Tokyo, Japan). Serum S100β concentrations were determined by a sandwich enzyme immunoassay for the quantitative measurement of human S100β (the RD192090100R Human S100β ELISA; BioVendor, Brno, Czech Republic). All measurement was performed by a clinical laboratory testing company (SRL, Inc, Tokyo, Japan).
Sample Size
As the degree of the effects of suvorexant on delirium prevention is unknown, we quoted our previous randomized placebo-controlled trial of ramelteon.15 In the study,15 the incidence of delirium in patients treated with placebo was 32%, whereas that in patients treated with ramelteon was 3%. As there was little difference in setting and participants between studies, we assumed that the incidence of delirium in patients receiving placebo would be 32%, while the incidence in patients receiving suvorexant would be 3% as well. The statistical power was set as power = 1 – β = 80% and sensitivity as α = 5% to enable detection of differences. Power analysis consequently set the required number of patients at 26 patients per group. However, the period of administration of the study drug quoted was 6 days, whereas that of the present study was 3 days. In the study quoted,15 the incidence of delirium in patients treated with placebo until day 4 was 26%, whereas that in patients treated with ramelteon was 0%. Power analysis consequently set the required number of patients also at 26 patients per group.
Statistical Analysis
Outcomes were assessed by intention-to-treat. Data were collected on standardized forms, and statistical analyses were performed using SPSS version 23-J software (IBM Japan, Tokyo, Japan). Differences between categorical variables in patient demographics and clinical characteristics were calculated using Fisher exact test. Differences between sequential variables were calculated using the unpaired t test (with Welch correction if applicable). If data were not sampled from Gaussian distributions, a nonparametric test (Mann-Whitney test) was used. Difference in cumulative incidence of delirium was investigated by Kaplan-Meier survival analysis. We performed post hoc analyses to compare DRS-R-98-J total score and severity scale score between groups over the study period, using 2- (suvorexant and placebo) by-4 (day 1, day 2, day 3, and day 4 time points) repeated-measures analysis of variance (ANOVA). Also, comparing sleep-wake cycle disturbance score (item 1) of DRS-R-98-J between groups over the study period, ANOVA was used because the item was only a 4-grade scale ranging from 0 to 3. All statistical tests were 2-tailed. Values of P < .05 were regarded as statistically significant.
RESULTS
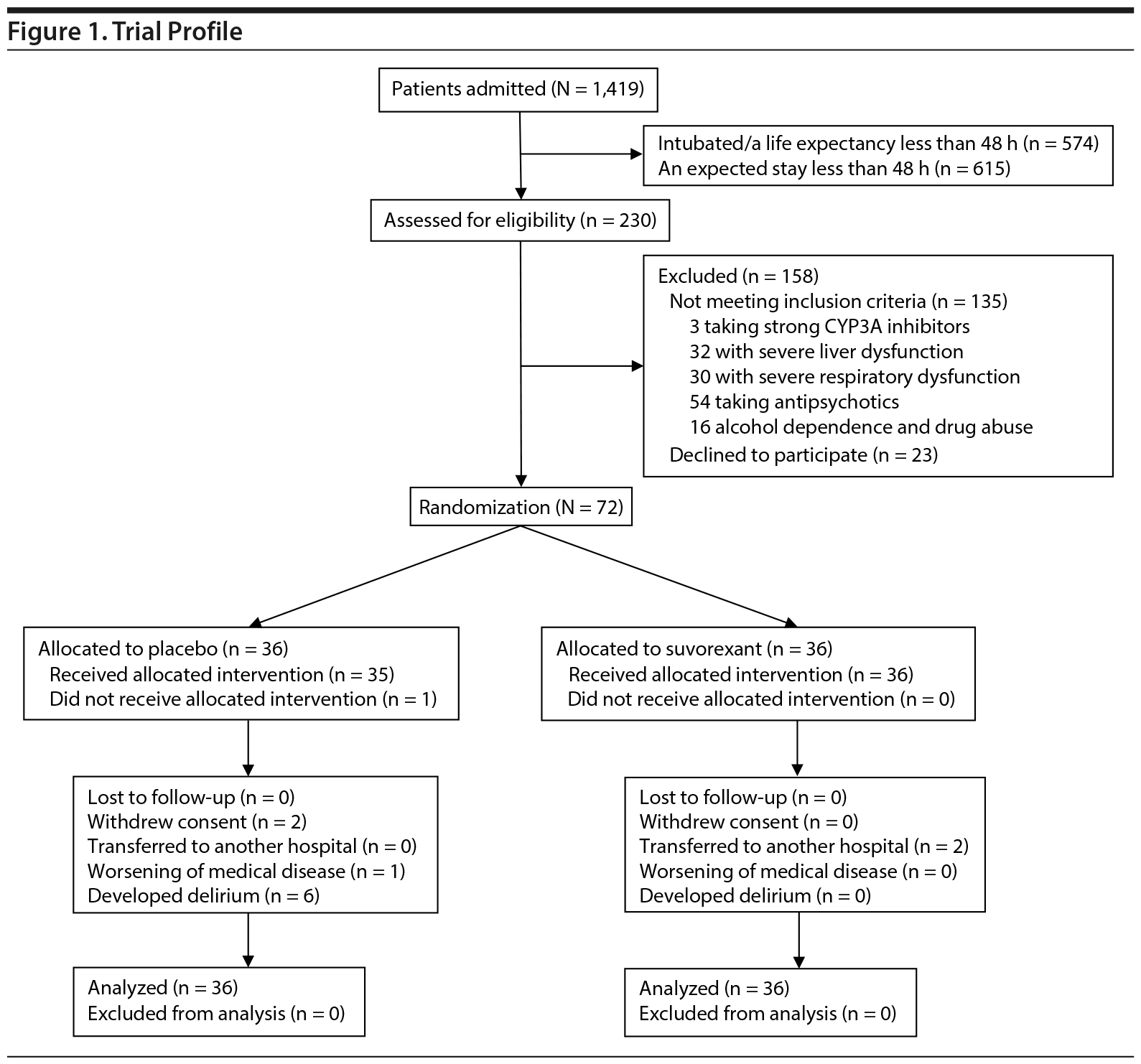
Trial recruitment started on April 1, 2015, and ended on February 29, 2016 as planned. During the study period, 1,419 patients were admitted via emergency rooms to ICUs (656 patients) or regular acute wards (763 patients). Among them, 574 patients were intubated or had a life expectancy of less than 48 hours, and 615 patients were expected to stay less than 48 hours. The rest, 230 patients, were assessed for eligibility (Figure 1). The contraindication of suvorexant in Japan was only taking strong CYP3A inhibitors; therefore, 3 patients were excluded. Careful administration was required for patients with severe liver dysfunction and severe respiratory dysfunction despite noncontraindication. In the process of planning this study, patient’s safety preceded the high rate of enrolling, as subjects were emergency patients. Therefore, 62 patients with such conditions were excluded. Fifty-four patients taking antipsychotics were excluded because antipsychotics would prevent the development of delirium. Among 95 eligible patients, 23 patients refused to participate (participation rate, 76%). Thus, 72 patients, (ICUs, 37 patients; regular acute wards, 35 patients) (Juntendo University Nerima Hospital, 18 patients; Nippon Medical School Musashikosugi Hospital, 20 patients; Tokyo Medical and Dental University, 14 patients; Hiroshima City Hospital, 20 patients) were randomly assigned to the 2 treatment groups. These were nondelirious patients per DSM-5 criteria and the DRS-R-98-J.
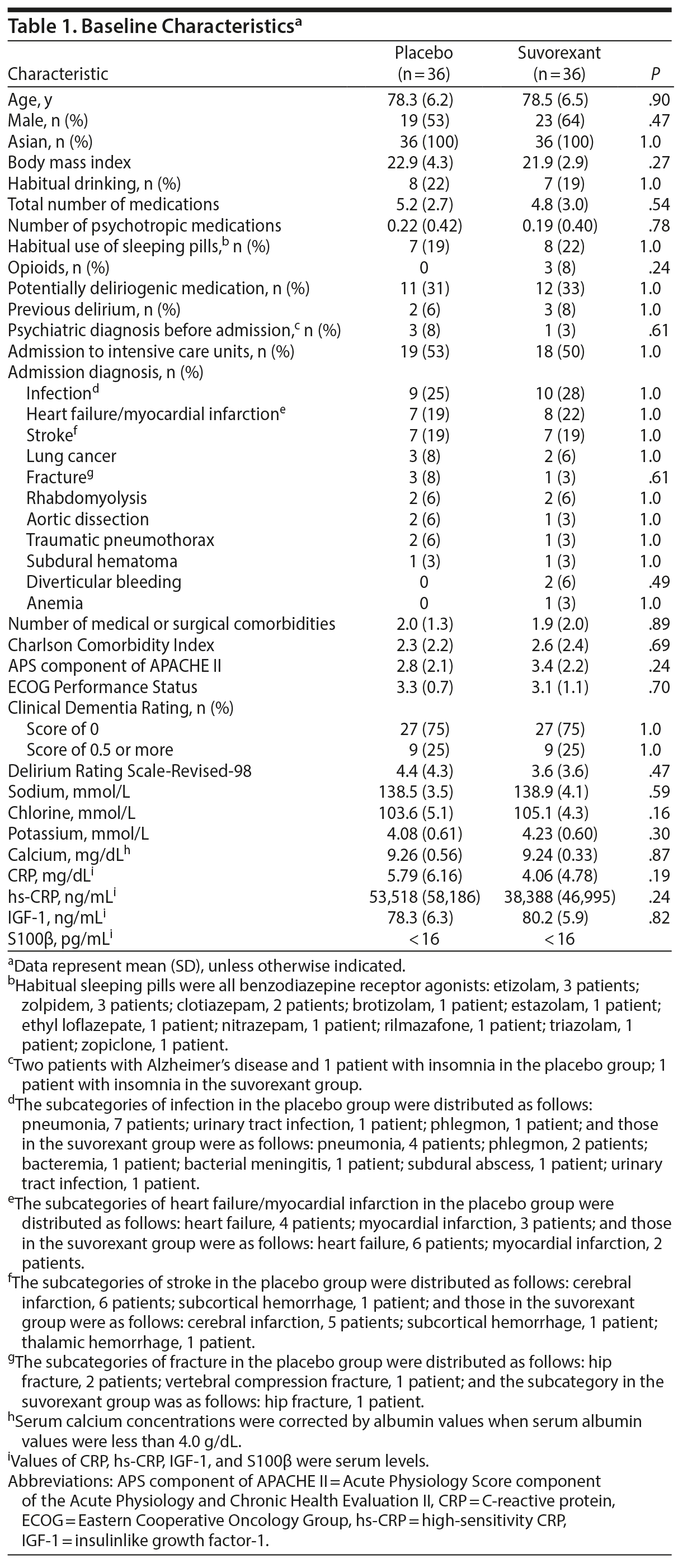
Baseline characteristics of randomized patients were much the same between groups (Table 1). There was no significant difference in the rate of the Clinical Dementia Rating score of 0.5 or more that indicates dementia or mild cognitive impairment between groups. With respect to other known risk factors for delirium such as advanced age and previous incidence of delirium, there were no significant differences between groups. There were no significant differences in baseline levels of CRP, hs-CRP, and IGF-1 between the groups.
Two patients in the placebo group withdrew consent at day 1 before the first study drug was given and at day 4, respectively. One patient in the placebo group was not assessed at day 4 due to worsening of cerebral infarction, which was the admitting diagnosis. No patients were discharged within 48 hours after admission in either group, but 2 patients in the suvorexant group transferred to another hospital at day 3 due to the improvement of their illness. No patients died prior to completion of the trial.
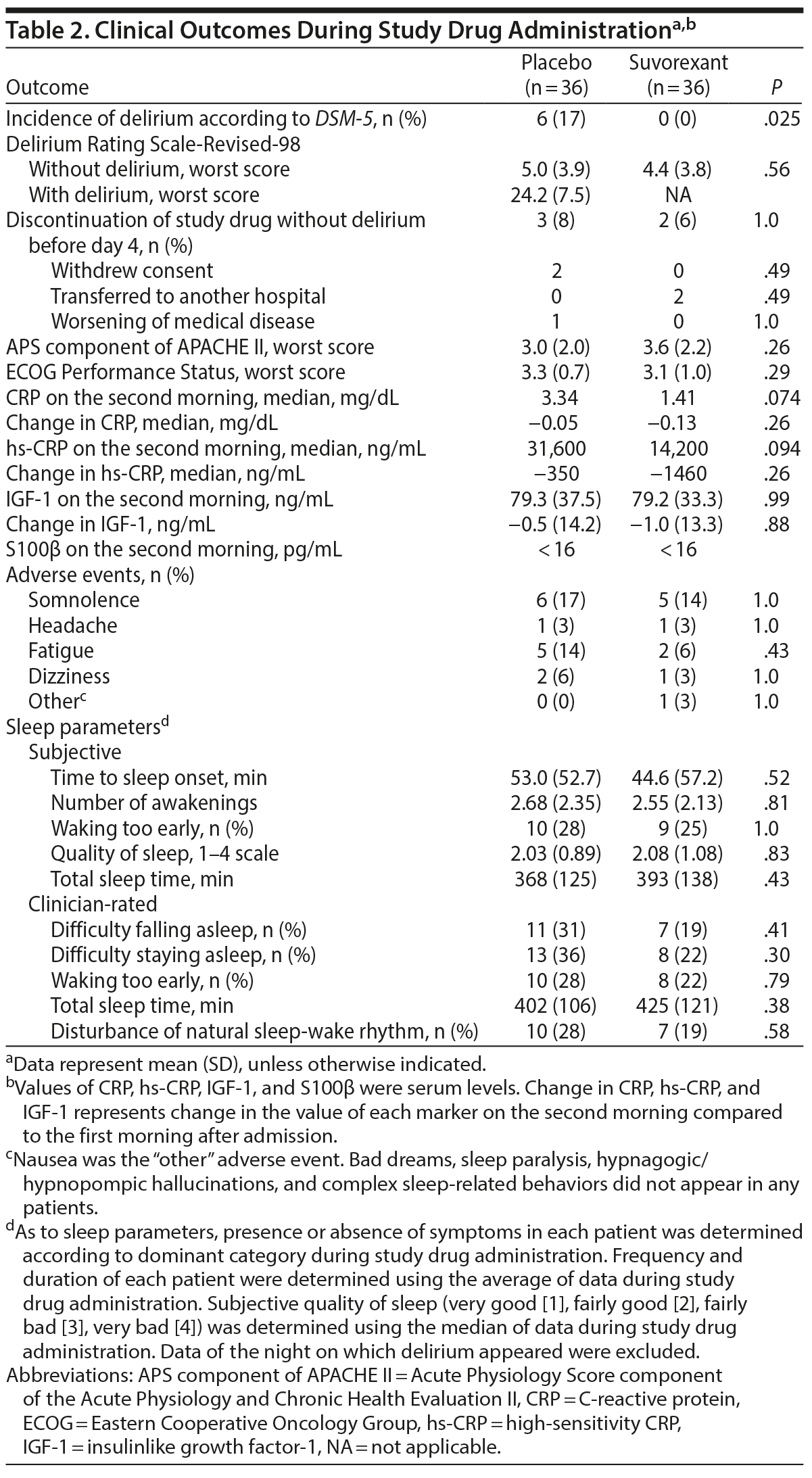
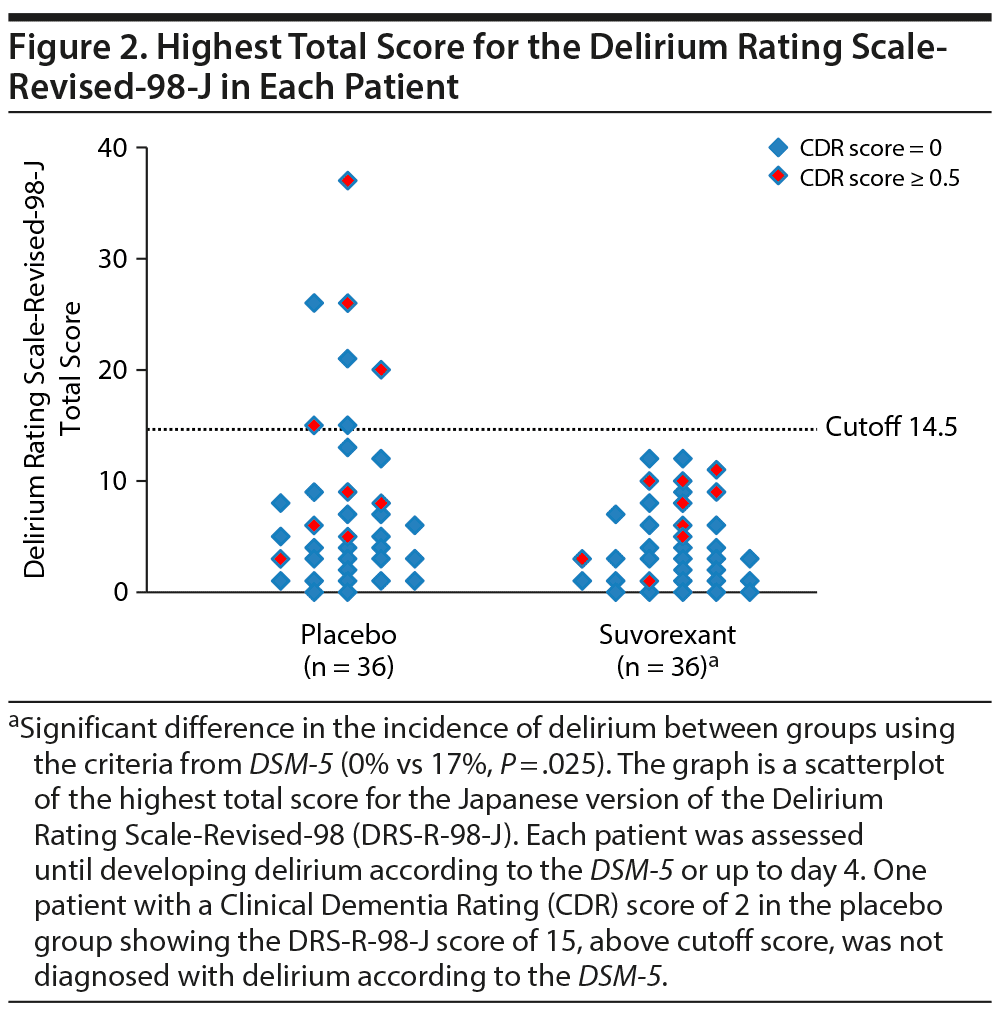
Table 2 shows outcomes. In the placebo group, a total of 6 patients including 4 patients in ICUs and 2 patients in regular acute wards developed delirium, whereas no one developed delirium in the suvorexant group. Causes of delirium in 6 patients according to the Delirium Etiology Checklist data were as follows: respiratory infection (definite cause), stroke (definite cause), respiratory infection and fracture (likely cause), cardiac and renal insufficiency (likely cause), cardiac insufficiency (likely cause), and traumatic brain injury (likely cause). None of these 6 patients had prior episodes of delirium. Figure 2 shows scattergrams of the highest total score of DRS-R-98-J in each patient. There was a significant difference in the rate of developing delirium between the suvorexant group and the placebo group (0% vs 17%, P = .025). As the incidence of delirium in the suvorexant group was 0, the risk ratio was not calculable/estimable. The Mantel-Haenszel test was used to assess the preventive effect of suvorexant on delirium, while controlling for gender, the DRS-R-98-J at baseline, and CRP value at baseline. A significant negative correlation between the administration of suvorexant and the occurrence of delirium was found, as evidenced by a correlation coefficient of −0.317 (P = .009). A significant difference in the rate of developing delirium among patients with CDR score ≥ 0.5 between groups was not found (the placebo group, 33% [3/9] vs the suvorexant group, 0% [0/9], P = .21). Figure 3 shows Kaplan-Meier curves. Comparison by log-rank test showed that development of delirium occurred significantly less often among patients taking suvorexant than among those taking placebo (χ2 = 6.46, P = .011).
When a cutoff point of 11 was applied as a lower cutoff value of the DRS-R-98-J at baseline to exclude subsyndromal delirium, there was still significant difference in the rate of developing delirium between the suvorexant group and the placebo group (0% [n/N = 0/34] vs 13% [4/31], P = .047). When a cutoff point of 7 was applied as a lower cutoff value of the DRS-R-98-J at baseline, there was no significant difference in the rate of developing delirium between the suvorexant group and the placebo group (0% [n/N = 0/28] vs 12% [3/26], P = .10). The reason for the diminishing difference between the groups was due to the exclusion of 2 patients who were not delirious but with dementia at baseline and developed delirium. As trained psychiatrists screened for eligibility using DSM-5 and DRS-R-98-J, a possibility of including subsyndromal delirium at baseline was low. Taking the obvious inexpediency of excluding patients who were not delirious but with dementia at baseline into account, applying a lower cutoff value of the DRS-R-98-J at baseline may be inexpedient in this study.
Repeated-measures ANOVA revealed a significant main effect of treatment (F = 4.59, P = .036) and interaction (F = 2.94, P = .045), but no significant main effect of time course (F = 1.21, P = .30) on DRS-R-98-J total score (Supplementary eFigure 1). Also, repeated-measures ANOVA revealed a significant main effect of treatment (F = 5.03, P = .028) and interaction (F = 3.53, P = .026), but no significant main effect of time course (F = 1.17, P = .32) on DRS-R-98-J severity scale score (Supplementary eFigure 2). ANOVA did not reveal significant main effect of time course (F = 0.08, P = .97) and interaction (F = 0.19, P = .90), but revealed a tendency for main effect of treatment (F = 3.79, P = .053) on sleep-wake cycle disturbance score (item 1) of DRS-R-98-J (Supplementary eFigure 3).
Although sleep metrics in the suvorexant group were marginally better than those in the placebo group, no significant differences in any items were apparent between groups (Table 2). There were no significant differences in adverse events between groups (Table 2). No serious adverse events occurred. Significant differences in change in CRP, hs-CRP, and IGF-1 on the second morning compared to the first morning after admission between the groups were not found. Serum S100β concentrations in all samples were less than measurable levels.
Although the study drug was not given on and after the night of day 4, follow-up data up to day 7 showed that an additional 2 patients in the placebo group developed delirium, whereas 1 patient in the suvorexant group developed delirium. Consequently, development of delirium up to day 7 occurred significantly less often among patients taking suvorexant than among those taking placebo (3% [n/N = 1/36] vs 22% [8/36], P = .028). No serious adverse events occurred during the period.
In the placebo group, the mean baseline CRP level and hs-CRP level in patients who developed delirium were significantly lower than those in patients who did not develop delirium (CRP, 2.79 [standard deviation = 2.84] vs 6.41 [6.51] mg/dL, t = 2.16, P = .045; hs-CRP, 25,237 [24,744] vs 59,370 [61,603] ng/mL, t = 2.24, P = .037), but a significant difference in baseline IGF-1 level was not found (55.5 [26.3] vs 83.0 [37.5] ng/mL, t = 1.70, P = .10). There were no significant differences in mean change in CRP, hs-CRP, and IGF-1 levels between patients who developed delirium and patients who did not develop delirium (CRP, 2.02 [5.67] vs −0.11 [3.41] mg/dL, t = 0.89, P = .41; hs-CRP, 16,707 [49,286] vs −1,174 [32,040] ng/mL, t = 1.13, P = .27; IGF-1, 0.83 [10.42] vs −0.79 [14.98] ng/mL, t = 0.25, P = .80).
DISCUSSION
The present study showed that the incidence of delirium in patients taking suvorexant was significantly lower than that in patients taking placebo, suggesting preventive effects of suvorexant on delirium. Well-balanced baseline characteristics including delirium risk factors such as advanced age, dementia, and previous incidence of delirium suggest the appropriate validity of the findings. The lack of any adverse event potentially attributable to the study drug suggests the safety of suvorexant use for preventing delirium.
Although no significant differences in any items of sleep metrics were apparent between groups, analysis of variance revealed a tendency for main effect of treatment on sleep-wake cycle disturbance score (item 1) of DRS-R-98-J. To analyze sleep-wake cycle disturbance score (item 1) of DRS-R-98-J over the study period is possibly a more efficient and focused way to document the typical types of circadian disturbances seen in delirium, as reported over a 24-hour period, than the subjective sleep metrics.
Patients with moderate to severe Alzheimer’s disease reportedly presented with higher mean orexin levels compared to controls and with more impaired nocturnal sleep with respect to controls and patients with mild Alzheimer’s disease.35 A partial explanation of the marginal superiority of sleep metrics in patients given suvorexant is that suvorexant might have antagonized elevated orexin levels in patients with a CDR score ≥ 0.5. Moreover, significantly higher levels of orexin in the brain of rats submitted to acute pancreatitis were reportedly found when compared to healthy controls.36 This finding raises a possibility that effects of an increased level of orexin induced by systemic inflammation are antagonized by suvorexant. In the present study, the mean values of CRP and hs-CRP at baseline were elevated, suggesting that most patients had systemic inflammation. Therefore, the antagonism of suvorexant against the effects of an increased level of orexin induced by systemic inflammation might have partly produced delirium prevention.
The present findings raise a possibility of association between orexin neurotransmission and etiologies of delirium. Critical substrates of delirium may be located in the striatum, the ventral tegmental area, or the thalamus.37 The ventral tegmental area contains both orexin-1 receptor and orexin-2 receptor messenger ribonucleic acids,38 and dopaminergic cells of the ventral tegmental area have been shown to be activated by orexins through orexin-1 receptor.39,40 An explanation for preventive effects of suvorexant on delirium can be associated with antidopaminergic activity like antipsychotics, which are clinically used for the treatment of delirium41-43 and also documented to have effects on delirium prevention despite controversy.5-11 It has been shown that administration of orexin-A increases the blood concentrations of corticotropin and corticosterone.44,45 Accumulated findings suggest that this effect takes place is via orexin-2 receptor expressed in paraventricular hypothalamic nucleus.23 It is possible that orexin-2 receptor antagonism via suvorexant inhibits the increase in the blood concentrations of corticosterone, which can be advantageous to prevent delirium as corticosteroid is a risk factor of delirium. Although orexin neurons send excitatory projections to cholinergic nuclei as well as monoaminergic nuclei with particular denseness,23 it has been reported that suvorexant is highly selective for orexin-1 receptor and orexin-2 receptor antagonism, having 6,000-fold intrinsic selectivity over 170 known receptors and enzymes, as demonstrated by in vitro assay panels.24 Accordingly, the Belsomra package insert states that suvorexant has not shown affinities for acetylcholine receptors (Ki > 10 µM).25 Even if suvorexant has a little anticholinergic property, it could be less problematic due to the timing of orexin dosing only at bedtime with T1/2 of 10 hours25 and potential to improve the circadian core domain of delirium off-setting anticholinergic effects as shown in Supplementary eFigure 3.
It has been reported that compared to patients without delirium, patients with postoperative delirium had significantly higher CRP levels at the preoperative (median paired difference [MPD], 1.56 mg/L [P < .01]), postanesthesia care unit (MPD, 2.53 mg/L [P < .01]), and postoperative day 2 (MPD, 63.76 mg/L [P < .01]) time points.46 Thus, elevated preoperative and postoperative plasma levels of CRP were reportedly associated with delirium, suggesting that a preinflammatory state and heightened inflammatory response to surgery are potential pathophysiologic mechanisms of delirium. IGF-I is believed to be neuroprotective, and low IGF-1 levels have been associated with aging and Alzheimer’s dementia, both of which are major risk factors for delirium.47 In the present study, the number of patients who developed delirium was 6, which was too small to discuss delirium prediction despite negative findings of change in CRP, hs-CRP, and IGF-1 levels. S100β protein can increase due to brain cell damage and/or increased permeability of the blood-brain-barrier, and elevated serum levels in delirium after hip fracture have been reported.48 In the present study, serum S100β concentrations in all samples were less than measurable levels, suggesting difficulties as a marker for delirium prediction in this population.
One limitation of the present study was the relatively small sample size. Especially, the sample was too small to clarify questions of sleep metrics and inflammatory markers. Another limitation was the single-blind design. However, the study physicians who were not blinded and other physicians in each emergency department were not allowed to prescribe additional psychotropic medication after admission. Accordingly, they were not involved in the study intervention. In delirium prevention, nursing care would exert influence rather than each physician, but there was blinding of nurses. Therefore, nonblinding of the study physicians would not have influenced the primary outcomes of delirium occurrence. The short follow-up period was a potential study limitation. The incidence of delirium in the placebo group (17% [6/36 patients]) is in the range of published rates for delirium studies,2 although somewhat low for intensive care units where the incidence has been reported in the range of 19% to 82%.2 The reason may have been the exclusion of patients who were intubated or had a life expectancy of less than 48 hours (574/1,419 patients admitted) and patients who declined to participate in the study (24% [23/95 eligible patients]). The lack of actigraphy or polysomnography was a limitation. Although patients included were not mildly ill as shown in relatively high mean values of serum CRP, the mean Acute Physiology Score component of the APACHE II was relatively low. Therefore, it is unclear whether preventive effects on delirium presented here would be applicable to more severely ill patients. Although a significant difference in the rate of developing delirium among patients with a CDR score ≥ 0.5 between groups was not found, the subgroup analysis may have been underpowered.
This study has several strengths, including the randomized and placebo-controlled design in emergency settings, mirroring real clinical practice, and absence of support from pharmaceutical companies. This represents the first study about preventive effects of suvorexant on delirium. Further work with a larger number of patients and a longer period of observation with more objective sleep metrics and targeting population with higher risk of delirium such as those with a CDR score ≥ 0.5 is needed. Simultaneously, such investigation will show more clearly whether suvorexant has potential to improve the circadian core domain of delirium.
Submitted: September 5, 2016; accepted December 27, 2016.
Published online: August 1, 2017.
DELIRIA-J Group members: The DELIRIA-J Group members are K.H.; Y.K.; K.W.; T.T.; S.I.; A.K.; K.M.; M.S.; C.U.; H.N.; Daisuke Jitoku, MD, PhD; Kohei Hino, MD; and Takehiro Tamura, MD.
Potential conflicts of interest: Dr Hatta has received lecture honoraria from Astellas, Daiichi-Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Meiji Seika, Otsuka, Pfizer, Takeda, and Mitsubishi-Tanabe within the last 3 years. Dr Kishi has received honoraria from Sumitomo-Dainippon, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Mochida, MSD, Otsuka, Pfizer, Shionogi, Takeda, and Tsumura and has served as a consultant for Meiji Seika. Dr Wada has received honoraria from Eisai, Eli Lilly, GlaxoSmithKline, Meiji Seika, MSD, Novartis, Otsuka, Pfizer, Shionogi, Takeda, Sumitomo-Dainippon, and Janssen. Dr Takeuchi has received honoraria from Otsuka, GlaxoSmithKline, Sumitomo-Dainippon, Janssen, Eisai, Eli Lilly, Daiichi-Sankyo, Shionogi, and Mitsubishi-Tanabe. Dr Ito has received honoraria from GlaxoSmithKline. Dr Kurata has received honoraria from MSD and Yoshitomiyakuhin. Dr Sugita has received grants from Pfizer, Taisho-Toyama, and Teijin and has received honoraria from Asahi Kasei, Astellas, Daiichi-Sankyo, Hospira Japan, Kowa, Maruishi, Otsuka, Takeda, and Taisho-Toyama. Dr Usui has received honoraria from Shionogi and Pfizer. Dr Nakamura and Mr Murakami declare no conflicts of interest.
Funding/support: This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI grant number 26461773).
Role of the sponsor: The sponsor of the study had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplementary material: See accompanying pages.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. Fifth Edition. Washington, DC: American Psychiatric Publishing; 2013.
2. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. PubMed doi:10.1016/S0140-6736(13)60688-1
3. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3(3):CD005563. PubMed
4. Hsieh SJ, Ely EW, Gong MN. Can intensive care unit delirium be prevented and reduced? lessons learned and future directions. Ann Am Thorac Soc. 2013;10(6):648-656. PubMed doi:10.1513/AnnalsATS.201307-232FR
5. Girard TD, Pandharipande PP, Carson SS, et al; MIND Trial Investigators. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38(2):428-437. PubMed doi:10.1097/CCM.0b013e3181c58715
6. Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116(5):987-997. PubMed doi:10.1097/ALN.0b013e31825153cc
7. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. PubMed doi:10.1111/j.1532-5415.2005.53503.x
8. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409-418. PubMed doi:10.1016/S0033-3182(10)70723-4
9. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515-523. PubMed doi:10.1016/S2213-2600(13)70166-8
10. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714-719. PubMed
11. Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40(3):731-739. PubMed doi:10.1097/CCM.0b013e3182376e4f
12. Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687-694. PubMed doi:10.1002/gps.2582
13. de Jonghe A, van Munster BC, Goslings JC, et al; Amsterdam Delirium Study Group. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547-E556. PubMed doi:10.1503/cmaj.140495
14. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. 2010;4(3):169-173. PubMed doi:10.4103/1658-354X.71132
15. Hatta K, Kishi Y, Wada K, et al; DELIRIA-J Group. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71(4):397-403. PubMed doi:10.1001/jamapsychiatry.2013.3320
16. Hatta K, Kishi Y, Wada K. Ramelteon for delirium in hospitalized patients. JAMA. 2015;314(10):1071-1072. PubMed doi:10.1001/jama.2015.8522
17. Azama T, Yano M, Oishi K, et al. Altered expression profiles of clock genes hPer1 and hPer2 in peripheral blood mononuclear cells of cancer patients undergoing surgery. Life Sci. 2007;80(12):1100-1108. PubMed doi:10.1016/j.lfs.2006.11.048
18. Tamiya H, Ogawa S, Ouchi Y, et al. Rigid Cooperation of Per1 and Per2 proteins. Sci Rep. 2016;6:32769. PubMed doi:10.1038/srep32769
19. Fitzgerald JM, Adamis D, Trzepacz PT, et al. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81(4):568-576. PubMed doi:10.1016/j.mehy.2013.06.032
20. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. PubMed doi:10.1016/j.jagp.2013.09.005
21. Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461-471. PubMed doi:10.1016/S1474-4422(14)70053-5
22. Ma J, Svetnik V, Snyder E, et al. Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects. Sleep. 2014;37(10):1609-1619. PubMed doi:10.5665/sleep.4068
23. Mieda M, Sakurai T. Integrative physiology of orexins and orexin receptors. CNS Neurol Disord Drug Targets. 2009;8(4):281-295. PubMed doi:10.2174/187152709788921663
24. Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61. PubMed doi:10.3109/01677063.2011.566953
25. BELSOMRAⓇ [package insert]. http://www.info.pmda.go.jp/downfiles/ph/PDF/170050_1190023F1024_1_09.pdf.
26. Hatta K, Kishi Y, Takeuchi T, et al; DELIRIA-J Group. The predictive value of a change in natural killer cell activity for delirium. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:26-31. PubMed doi:10.1016/j.pnpbp.2013.09.008
27. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631. PubMed doi:10.1111/j.1532-5415.2012.03923.x
28. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. PubMed doi:10.1097/00003246-198510000-00009
29. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed doi:10.1016/0021-9681(87)90171-8
30. Falkson G, Von Hoff D, Klaassen D, et al. A phase II study of neocarzinostatin (NSC 157365) in malignant hepatoma: an Eastern Cooperative Oncology Group pilot study. Cancer Chemother Pharmacol. 1980;4(1):33-36. PubMed doi:10.1007/BF00255455
31. Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. PubMed doi:10.1192/bjp.140.6.566
32. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504. PubMed
33. Trzepacz PT, Maldonado JR, Kean J, et al. Delirium Rating Scale-Revised-98 (DRS-R-98) Administration Manual. Electronic ed. Indianapolis, IN: Trzepacz; 2010.
34. Kato M, Kishi Y, Okuyama T, et al. Japanese version of the Delirium Rating Scale, Revised-98 (DRS-R98-J): reliability and validity. Psychosomatics. 2010;51(5):425-431. PubMed doi:10.1176/appi.psy.51.5.425
35. Liguori C, Romigi A, Nuccetelli M, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498-1505. PubMed doi:10.1001/jamaneurol.2014.2510
36. Hamasaki MY, Barbeiro HV, Barbeiro DF, et al. "Neuropeptides in the brain defense against distant organ damage". J Neuroimmunol. 2016;290:33-35. PubMed doi:10.1016/j.jneuroim.2015.11.014
37. Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses. 2005;64(3):471-475. PubMed doi:10.1016/j.mehy.2004.08.007
38. Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29(1):70-87. PubMed doi:10.1016/j.yfrne.2007.08.001
39. Nakamura T, Uramura K, Nambu T, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873(1):181-187. PubMed doi:10.1016/S0006-8993(00)02555-5
40. Korotkova TM, Sergeeva OA, Eriksson KS, et al. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7-11. PubMed
41. Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419-427. PubMed doi:10.1097/CCM.0b013e3181b9e302
42. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. PubMed doi:10.1016/j.jpsychores.2010.05.006
43. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. PubMed doi:10.1002/gps.3999
44. Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911-10916. PubMed doi:10.1073/pnas.96.19.10911
45. Kuru M, Ueta Y, Serino R, et al. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11(9):1977-1980. PubMed doi:10.1097/00001756-200006260-00034
46. Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: a longitudinal nested case-control study. Biol Psychiatry. 2017;81(2):145-153. PubMed doi:10.1016/j.biopsych.2016.03.2098
47. Yen TE, Allen JC, Rivelli SK, et al. Association between serum IGF-I levels and postoperative delirium in elderly subjects undergoing elective knee arthroplasty. Sci Rep. 2016;6:20736. PubMed doi:10.1038/srep20736
48. van Munster BC, Korse CM, de Rooij SE, et al. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009;9:21. PubMed doi:10.1186/1471-2377-9-21
This PDF is free for all visitors!