Objective: To evaluate durability of therapeutic effect of long-term treatment with aripiprazole lauroxil in patients with schizophrenia following successful treatment of an acute psychotic episode.
Methods: This post hoc analysis assessed long-term outcomes for a subgroup of patients who entered a 52-week extension study after being successfully stabilized with one of 2 doses of aripiprazole lauroxil (441 or 882 mg) in a pivotal 12-week, placebo-controlled, randomized clinical trial. Durability of therapeutic effect was measured by the proportion of patients completing the 1-year course of aripiprazole lauroxil, the trajectories of the Positive and Negative Syndrome Scale (PANSS) total and the Clinical Global Impression-Severity (CGI-S) item scores beyond the first 12 weeks, and the likelihood of remission at any follow-up point.
Results: In total, 181 patients treated with aripiprazole lauroxil entered the extension study; 73% and 66% of patients from the 441 mg and 882 mg groups, respectively, completed all 13 aripiprazole lauroxil treatments scheduled every 4 weeks over 52 weeks. Both groups continued on a positive trajectory of symptom improvements (P < .0001 for reductions in PANSS total and CGI-S scores from week 12 to end of follow-up). Most patients (74% and 68% in the aripiprazole lauroxil 441 mg and 882 mg groups, respectively) achieved remission during follow-up.
Conclusions: These post hoc analyses of a subgroup of patients demonstrate the continued therapeutic efficacy of aripiprazole lauroxil after successful treatment of an acute episode of schizophrenia. Both the 441 mg and 882 mg groups had similar retention rates, degree of symptom improvement, and likelihood of remission.
Trial Registration: ClinicalTrials.gov identifier: NCT01469039; European Clinical Trials Database (EudraCT) numbers: 2012-003445-15 and 2012-003996-20‘ ‹’ ‹’ ‹’ ‹
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Durability of Therapeutic Response With Long-Term Aripiprazole Lauroxil Treatment Following Successful Resolution of an Acute Episode of Schizophrenia

ABSTRACT
Objective: To evaluate durability of therapeutic effect of long-term treatment with aripiprazole lauroxil in patients with schizophrenia following successful treatment of an acute psychotic episode.
Methods: This post hoc analysis assessed long-term outcomes for a subgroup of patients who entered a 52-week extension study after being successfully stabilized with one of 2 doses of aripiprazole lauroxil (441 or 882 mg) in a pivotal 12-week, placebo-controlled, randomized clinical trial. Durability of therapeutic effect was measured by the proportion of patients completing the 1-year course of aripiprazole lauroxil, the trajectories of the Positive and Negative Syndrome Scale (PANSS) total and the Clinical Global Impression-Severity (CGI-S) item scores beyond the first 12 weeks, and the likelihood of remission at any follow-up point.
Results: In total, 181 patients treated with aripiprazole lauroxil entered the extension study; 73% and 66% of patients from the 441 mg and 882 mg groups, respectively, completed all 13 aripiprazole lauroxil treatments scheduled every 4 weeks over 52 weeks. Both groups continued on a positive trajectory of symptom improvements (P < .0001 for reductions in PANSS total and CGI-S scores from week 12 to end of follow-up). Most patients (74% and 68% in the aripiprazole lauroxil 441 mg and 882 mg groups, respectively) achieved remission during follow-up.
Conclusions: These post hoc analyses of a subgroup of patients demonstrate the continued therapeutic efficacy of aripiprazole lauroxil after successful treatment of an acute episode of schizophrenia. Both the 441 mg and 882 mg groups had similar retention rates, degree of symptom improvement, and likelihood of remission.
Trial Registration: ClinicalTrials.gov identifier: NCT01469039; European Clinical Trials Database (EudraCT) numbers: 2012-003445-15 and 2012-003996-20
J Clin Psychiatry 2017;78(8):1103-1109
https://doi.org/10.4088/JCP.17m11625
© Copyright 2017 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Health Behavior, Medical College of Georgia, Augusta University, Augusta, Georgia
bClinical Development, Alkermes, Inc., Waltham, Massachusetts
cLviv District Clinical Psychiatric Hospital, Lviv, Ukraine
dCurrent affiliation: NeuroRx Pharmaceuticals, Wilmington, Delaware
*Corresponding author: Joseph P. McEvoy, MD, Department of Psychiatry and Health Behavior, Augusta University, 997 St Sebastian Way, Augusta, GA 30912 ([email protected]).
Schizophrenia is a severe mental disorder characterized by a range of symptoms, including delusions, auditory hallucinations, and behavioral and cognitive dysfunction.1,2 As it is a chronic disease, continued antipsychotic treatment is crucial for the achievement and maintenance of remission, which supports the ultimate goal of recovery.2,3 However, nonadherence to antipsychotic medication is common, with a report of approximately 74% of patients discontinuing study medication (oral antipsychotics) within 18 months of starting treatment.4,5 This is a major cause for concern, as nonadherence is associated with exacerbation of psychopathology (including psychosis, aggression, and self-injury) and worsening of long-term function.6,7 Nonadherence to medication also contributes to relapse rates, leading to a "revolving door" syndrome wherein patients are frequently rehospitalized,8 resulting in increased burden on patients and their caregivers, as well as on health care systems and costs. The use of long-acting injectable (LAI) antipsychotic medication is an important treatment option, as it ensures delivery of medication and provides clinicians and caregivers with real-time, accurate information on a patient’s adherence to prescribed medication.5 As the number of LAIs being developed and approved for the treatment of schizophrenia increases, studies of long-term outcomes of LAI treatment are important to help patients, caregivers, and clinicians make informed treatment decisions.
Aripiprazole lauroxil is a novel LAI atypical antipsychotic approved in 2015 in the United States for the treatment of adults with schizophrenia.9 The efficacy, safety, and tolerability of once-monthly aripiprazole lauroxil for the treatment of acute exacerbation of symptoms in patients with schizophrenia were previously demonstrated in a phase 3, 12-week, randomized, double-blind, placebo-controlled trial.10 A 52-week extension study was subsequently carried out to evaluate the long-term safety and durability of therapeutic effect of aripiprazole lauroxil in patients with stable schizophrenia; participants in this study included patients from the initial 12-week acute-phase study, as well as newly enrolled patients with chronic stable schizophrenia (de novo patients). The primary results of this study reporting on the long-term safety of aripiprazole lauroxil will be published in full elsewhere.
The present article reports the results of a post hoc analysis that assessed the durability of therapeutic effect of long-term aripiprazole lauroxil treatment in patients from the 12-week study receiving the same aripiprazole lauroxil regimen in the 52-week extension study.
METHODS
The initial 12-week acute-phase study (ClinicalTrials.gov identifier: NCT01469039; European Clinical Trials Database [EudraCT] number: 2012-003445-15) was conducted across 7 countries from December 2011 to March 2014. The 52-week extension study (EudraCT number: 2012-003996-20) was conducted at 92 study centers in North America, Europe, and Asia between June 2012 and April 2015. Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines by the International Conference on Harmonization, 1997. The study protocols, amendments, and informed consent forms were approved by an independent ethics committee/institutional review board for each site. All patients provided written informed consent before entering both the initial and extension studies.

- As the number of long-acting injectable antipsychotics being developed and approved for treatment of schizophrenia increases, studies of long-term treatment outcomes are important to allow clinicians to make informed treatment decisions.
- The present study provides 1-year follow-up data on treatment continuation and symptom course in a cohort of outpatients with schizophrenia who were recently stabilized with one of 2 fixed doses (441 mg or 882 mg) of aripiprazole lauroxil given every 4 weeks.
- Both doses of once-monthly aripiprazole lauroxil were associated with high retention rates and continued improvements in schizophrenia symptoms beyond the acute psychotic episode. The majority of patients achieved, and remained in, remission until the end of the study.
Patients
Patients aged 18 to 70 years who successfully completed the initial 12-week study10 were invited to enter a 52-week extension study to assess the long-term safety and durability of therapeutic effect of aripiprazole lauroxil. Study design details and results of the 12-week study are published elsewhere.10 The key eligibility criteria for transitioning from the acute-phase study to the long-term extension study were successful completion of the full 12 weeks of the study and mutual agreement between patient and investigator that continued treatment by monthly injections of aripiprazole lauroxil was indicated. The screening and consent procedures were done at the end of the 12-week study, 4 weeks after the patients’ last (blinded) study injection. In this post hoc analysis, only patients who had received active treatment (aripiprazole lauroxil) in the initial study and continued in the extension study were evaluated. Patients who had received placebo were excluded from this analysis.
Study Design and Treatments
Patients followed in this post hoc analysis had previously been randomized to receive an aripiprazole lauroxil dose of either 441 mg or 882 mg (corresponding to 300 or 600 mg aripiprazole equivalent, respectively, excluding the lauroxil component) in the initial 12-week study. Aripiprazole lauroxil was administered by intramuscular injection once every 4 weeks, and patients would have received a total of 3 fixed doses of aripiprazole lauroxil at the end of the 12-week study. On entering the 52-week extension study (at the time or within 1 week of the last visit of the 12-week study), patients received their fourth dose of aripiprazole lauroxil. Subsequent extension study visits occurred every 4 weeks thereafter, with patients receiving a total of 12 additional doses (once every 4 weeks), with the final dose administered 4 weeks before the final assessment at the 1-year point (ie, at week 60). Thus, patients who completed both the initial and extension studies received a total of 16 aripiprazole lauroxil doses over the span of 64 weeks. Patients in the extension study continued to receive the same dose as previously received in the acute-phase study (either 441 mg or 882 mg aripiprazole lauroxil) and remained blind to their dose assignment, which was unchanged throughout the study.
Study Assessments
The durability of the therapeutic effect of aripiprazole lauroxil was assessed using the following variables: time until discontinuation, treatment response trajectories (Positive and Negative Syndrome Scale [PANSS] total11 and Clinical Global Impression-Severity [CGI-S]12 item scores), and time to remission.
Overall retention and time to discontinuation were assessed from week 12 (the beginning of the 52-week extension period) in all patients who had received at least 1 dose of aripiprazole lauroxil in the extension study.
Clinical symptoms, global severity, and time to remission were assessed from the beginning of the initial acute-phase study (baseline) in patients who had at least 1 PANSS/CGI-S assessment after drug administration in the extension study. PANSS and CGI-S scales were administered at baseline and at weeks 1, 2, 3, 4, 8, 12, 16, 24, 32, 44, 60, and 64. Severity was rated (scored) using CGI-S according to the following scale: normal, not at all ill (1); borderline mentally ill (2); mildly ill (3); moderately ill (4); markedly ill (5); severely ill (6); or extremely ill (7).
Remission rates were assessed using the cross-sectional criteria established by the Remission in Schizophrenia Working Group (Andreasen criteria).13 The remission criteria required attaining a score ≤ 3 (mild or less) in 8 specified PANSS items: delusions (P1), unusual thought content (G9), hallucinatory behavior (P3), conceptual disorganization (P2), mannerisms/posturing (G5), blunted affect (N1), social withdrawal (N4), and lack of spontaneity (N6). In the current analysis, the definition of remission was modified from the original Andreasen recommendation of 6 months as the minimum duration during which the aforementioned PANSS items criteria must be maintained to achieve remission, to the requirement that patients had to have met the PANSS items criteria in 2 consecutive efficacy assessments. Durability of remission was evaluated by assessing the conditional probability of continued remission in the first assessment subsequent to the achievement of remission. The proportion of patients who achieved remission and continued to meet these criteria for the remainder of their follow-up time in the extension study was also assessed.
Statistical Analysis
Retention/discontinuation and remission rates were estimated using the Kaplan-Meier method. Log rank tests were performed for treatment comparisons, and hazard ratios (HRs) with 95% confidence intervals (CIs) were computed.
Changes from baseline in PANSS total and CGI-S scores were analyzed using a mixed effects model for repeated measures. The model included the baseline score at entry to the initial (12-week) and extension (52-week) studies, study region, visit, and treatment group, and interaction term of treatment group and visit. The within-subject variability was modeled using an unstructured covariance matrix. The least squares (LS) mean and standard error (SE) of change from baseline scores at each visit were reported and plotted for PANSS total and CGI-S scores.
RESULTS
Patient Disposition and Baseline Characteristics
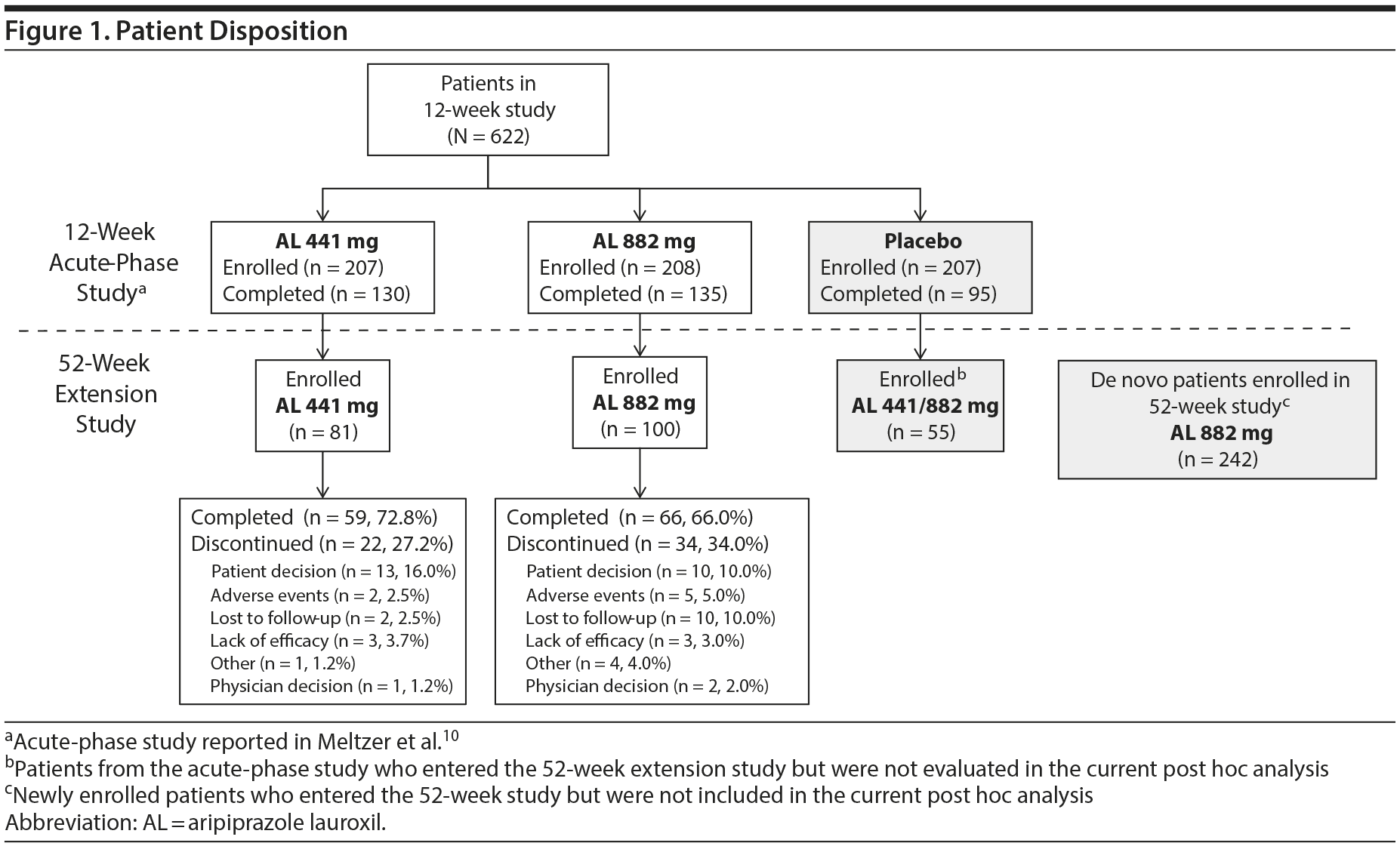
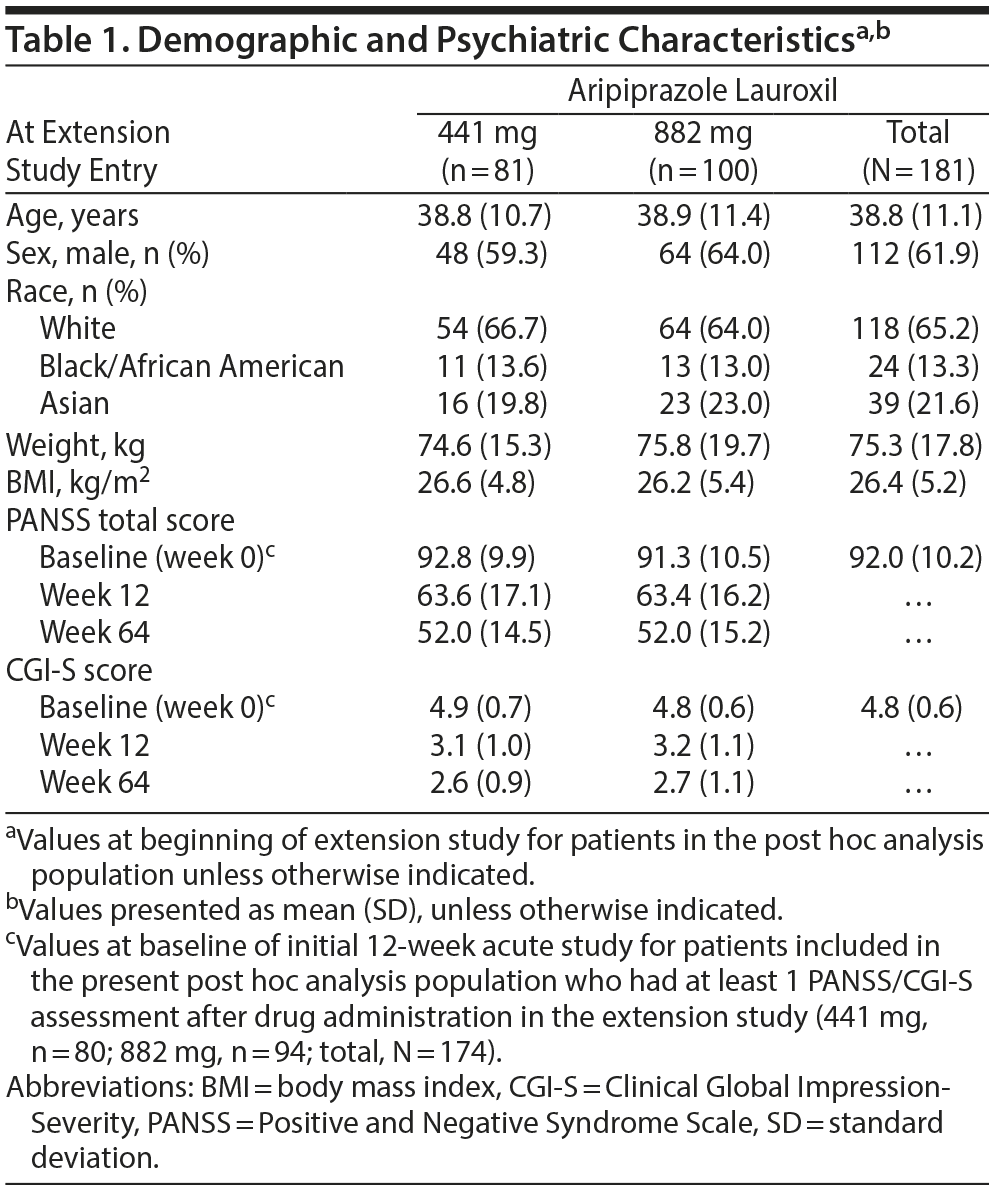
A total of 181 patients who had received aripiprazole lauroxil in the acute-phase study and received at least 1 aripiprazole lauroxil injection (their fourth injection overall) in the extension study were evaluated in this post hoc analysis (81 and 100 patients in the aripiprazole lauroxil 441 mg and 882 mg cohort, respectively) (Figure 1). Reasons for exclusion from the post hoc analysis include failure to meet eligibility criteria for the long-term study and/or initial placebo arm assignment. The mean age (standard deviation [SD]) was 38.8 (11.0) years, 61.9% of patients were male, and 65.2% were white (Table 1). Mean (SD) PANSS total scores at the beginning of the extension study (n = 80 and n = 94 in the 441 mg and 882 mg groups, respectively) were 63.6 (17.1) and 63.4 (16.2), respectively. Mean (SD) CGI-S scores at the beginning of the extension study were 3.1 (1.0) for the 441 mg group and 3.2 (1.1) for the 882 mg group. Additional PANSS total scores and CGI-S scores at the beginning of the 12-week acute phase and at the end of the extension phase (week 64) are provided in Table 1.
Durability of Therapeutic Response
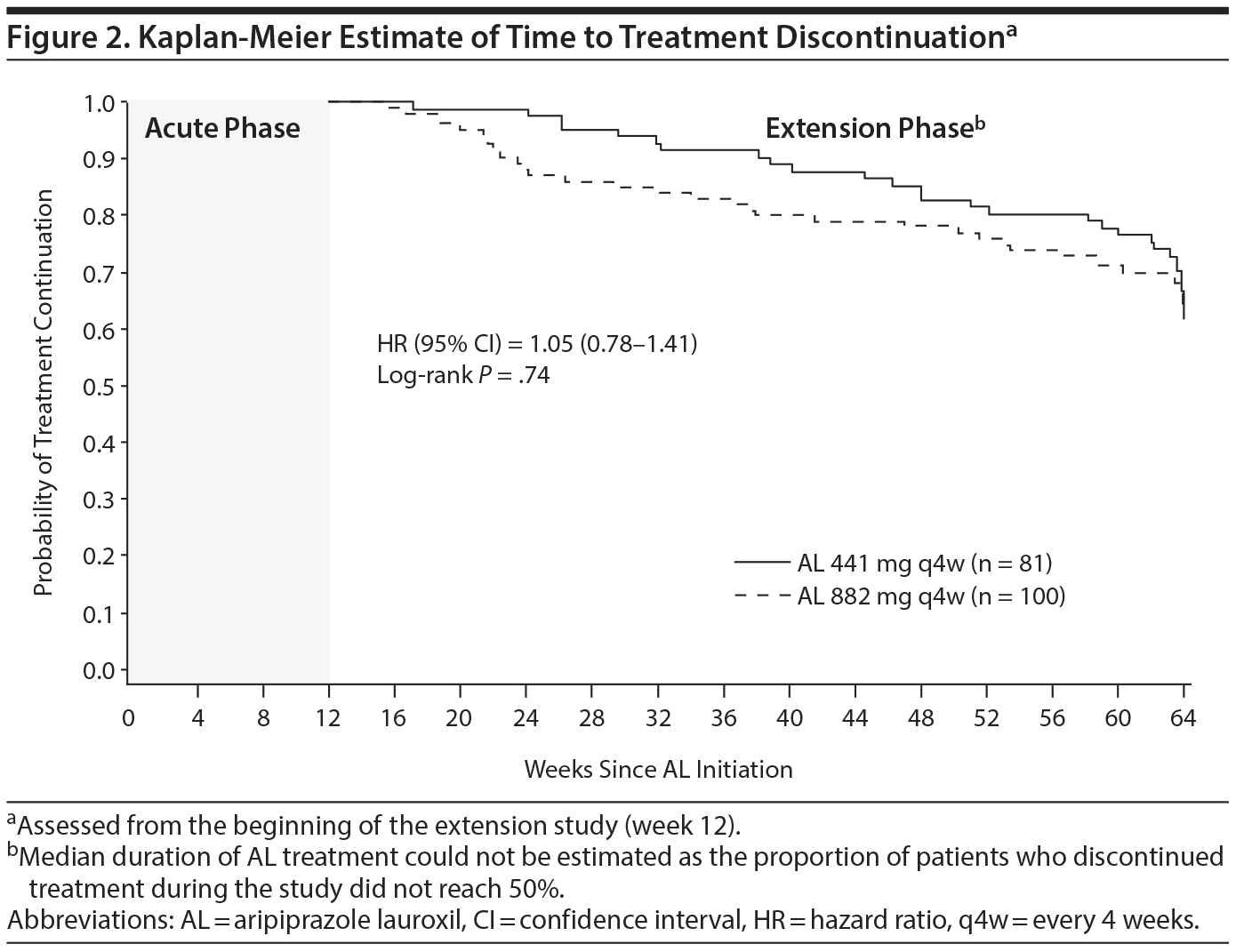
Treatment exposure and retention. Approximately 70% of patients entering the extension study received the complete regimen of 13 injections over the course of 52 weeks and completed the study (59 [72.8%] and 66 [66.0%] patients in the 441 mg and 882 mg groups, respectively) (Figure 1). The median duration of aripiprazole lauroxil treatment could not be estimated, as the proportion of patients who discontinued treatment during the study did not reach 50%. The 75th percentile durations of retention for the aripiprazole lauroxil 441 mg and 882 mg groups were estimated at 50.1 (95% CI, 36-52) and 41.3 (95% CI, 22-52) weeks, respectively. There was no significant difference in retention rates between patients who entered on the aripiprazole lauroxil 441 mg dose and those who entered on the aripiprazole lauroxil 882 mg dose (HR [95% CI] = 1.05 [0.78-1.41]; log rank test: P = .74) (Figure 2).
The primary reasons for early discontinuation were withdrawal by the patient (n = 13, 16.0%) in the 441 mg group and withdrawal by the patient and loss to follow-up (n = 10, 10.0%, each) in the 882 mg group (Figure 1). Withdrawal rates due to adverse events were low: 2 (2.5%) and 5 (5.0%) in the 441 mg and 882 mg groups, respectively (Figure 1).
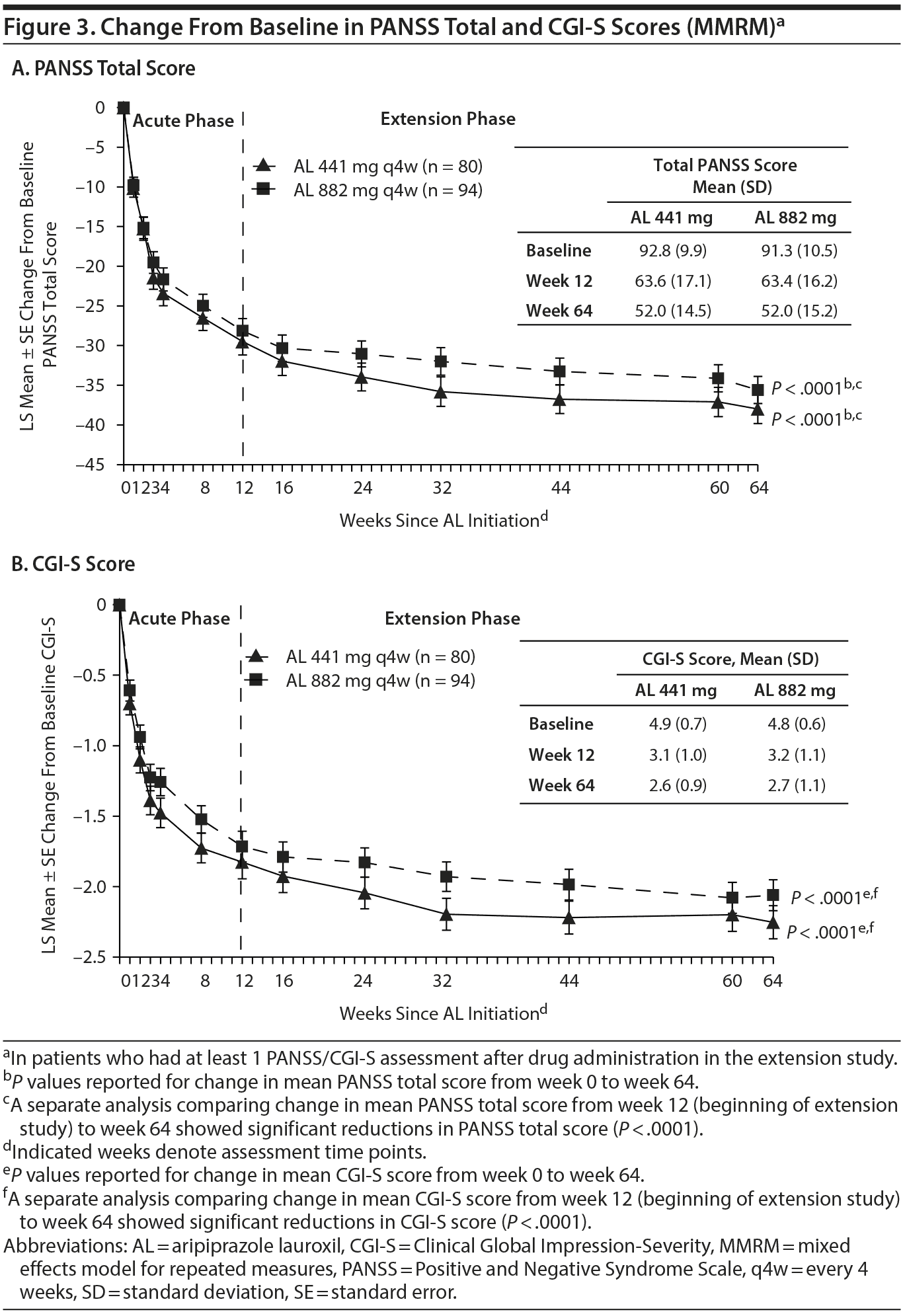
Symptoms and severity of illness. Patients from the acute-phase study who continued in the 52-week study demonstrated sustained and gradual improvements in PANSS total score for both dose groups through week 64 (Figure 3A); LS mean (SE) change from week 12 was −8.1 (1.3) (P < .0001) and −7.2 (1.2) (P < .0001) for the 441 mg and 882 mg cohorts, respectively.
Similar results were observed for patient CGI-S scores (Figure 3B); sustained improvement in CGI-S scores was observed from study baseline through the end of the 52-week study. The LS mean (SE) changes for the 441 mg and 882 mg cohorts from week 12 to week 64 were −0.4 (0.1) (P < .0001) and −0.3 (0.1) (P < .0001), respectively.
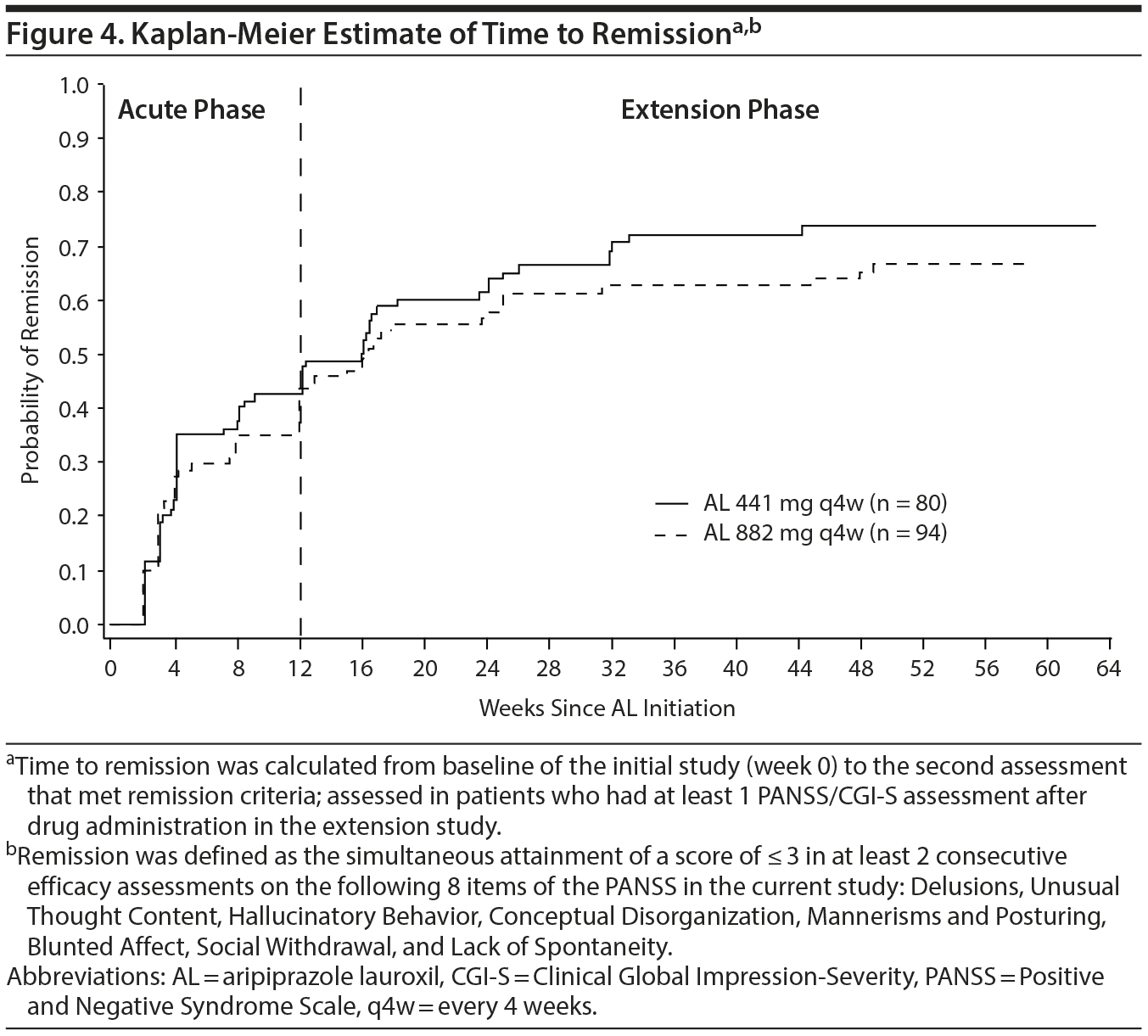
Remission. In total, 34 (42.5%) and 33 (35.1%) patients in the 441 mg and 882 mg groups, respectively, had met remission criteria by week 12, and 59 (73.8%) and 64 (68.1%) patients in the 441 mg and 882 mg groups, respectively, had achieved remission by week 64. A Kaplan-Meier plot for time to remission is shown in Figure 4. The median time to remission from the beginning of the initial study was 16.1 (95% CI, 8.1-23.6) and 16.4 (95% CI, 12.1-25.1) weeks for the 441 mg and 882 mg groups, respectively. Of the 123 patients who met the remission criteria, 112 (56 each in the 441 mg and 882 mg groups) remained in remission until the subsequent assessment; thus, the conditional probability of continued remission to a third assessment was 91% (112 of 123 patients). Most (80.5%) of the 123 patients remained in remission for the rest of the study (until week 64 or study discontinuation) (48 [81.4%] and 51 [79.7%] patients in the 441 mg and 882 mg groups, respectively).
We further explored the rate of remission using the conventionally accepted 6-month duration criteria. A subset of patients who had achieved remission (as defined in the current study) between weeks 12 and 32, inclusive, were followed up for at least 6 months to assess maintenance of remission status. In total, 48 patients achieved remission between weeks 12 and 32. Of these patients, 38 (79%) continued to meet the cross-sectional remission criteria over the next 6 months and beyond.
DISCUSSION
The continuing therapeutic benefit of aripiprazole lauroxil was observed over a 52-week extension period, as demonstrated by low rates of treatment discontinuation, which were comparable for both doses (approximately 27% and 34% in the 441 mg and 882 mg groups, respectively). The main causes of treatment discontinuation were patient withdrawal (16%) in the 441 mg group and patient withdrawal and loss to follow up (10% each) in the 882 mg group.
Withdrawals due to adverse events (AEs) were low (2.5%-5.0%), suggesting that treatment with aripiprazole lauroxil was well tolerated over the course of 52 weeks.
Mean PANSS total score and CGI-S scores were maintained throughout the course of the 52-week extension study, with a therapeutic trajectory of continued symptom improvement for both dosage groups from week 12 to week 64. Approximately 35% to 43% of the analysis population achieved remission criteria relatively early (by week 12), whereas 68% to 74% had achieved remission by the end of the study. These rates were comparable with those reported for risperidone LAI for treatment of first-episode/recent-onset schizophrenia (64%) or switched from oral antipsychotics (51.1%).14,15 Approximately 80% of patients who had achieved remission were still in remission at the end of this study, demonstrating the durability of aripiprazole lauroxil in maintaining symptom control.
We found no statistically significant or clinically meaningful differences in retention, symptom reduction, or time to remission between the 2 doses of aripiprazole lauroxil in this post hoc analysis, suggesting that the 441 mg and 882 mg doses are comparable for the maintenance of stabilization of clinical symptoms, at least over the course of a year.
A limitation of this post hoc analysis was the requirement that patients must have successfully completed the initial study to continue into the extension study, which would have resulted in the preferential selection of a study population who derived benefit from aripiprazole lauroxil treatment, while excluding patients who did not respond or had adverse reactions to aripiprazole lauroxil treatment in the first 12 weeks. As such, the remission and retention data reported in this post hoc analysis are likely to be more favorable in this cohort of patients compared with unselected patients in a real-world setting. A further limitation to the remission analysis was that the interval between PANSS evaluations changed over the course of the study, with the shortest assessment intervals (weekly) at the beginning of the 12-week acute study and the longest intervals (every 12 weeks) at the end of the 52-week extension study. The stepwise increase in duration between assessments precluded using a set time interval to define remission. However, because the likelihood of remaining in remission for the next adjacent assessment was > 90%, we feel that the overall finding of high remission rates over the course of the follow-up was not affected by the differing assessment periods.
Another limitation was that the efficacy of the 2 fixed doses of aripiprazole lauroxil was evaluated with no opportunity for dose adjustments over the course of the study, making it difficult to identify patients who might benefit from higher versus lower doses. It is possible that some patients might have benefited more from dose adjustment and flexible dosing. In addition, the lack of comparators made it difficult to draw any conclusions about the relative efficacy of aripiprazole lauroxil.
In conclusion, the results of this post hoc analysis demonstrated the continued therapeutic effect of aripiprazole lauroxil in the maintenance of clinical stability over a period of more than 1 year. The low discontinuation and high retention rates observed support the use of aripiprazole lauroxil as an efficacious long-term treatment option in patients with schizophrenia.
Submitted: April 3, 2017; accepted July 19, 2017.
Published online: September 19, 2017.
Potential conflicts of interest: Dr McEvoy has received consulting fees, honoraria, and/or grants from Alkermes Inc, Avanir, Boehringer Ingelheim, Neurocrine, Teva, and Otsuka. Dr Mykhnyak has received honoraria or consulting fees from Alkermes Inc, AstraZeneca, Dainippon Sumitomo Pharma, Forest Research, GlaxoSmithKline, ICON plc, Johnson & Johnson PRD, H. Lundbeck A/S, Novartis Pharmaceuticals, PAREXEL International, Roche Laboratories, Servier, Sunovion, and Targacept. Drs Weiden, Du, and Stanford are employees of and hold stock in Alkermes, Inc. Drs Risinger and Liu are former employees of Alkermes, Inc.
Funding/support: This study was sponsored by Alkermes, Inc., Waltham, Massachusetts. Funding for editorial support was provided by Alkermes, Inc.
Role of the sponsor: The study sponsor was involved in the design, collection, and analysis of the data. Interpretation of the results was by the authors, and the decision to submit the manuscript for publication in the Journal of Clinical Psychiatry was made by the authors.
Previous presentation: Part of the study results were presented as a poster at the American Psychiatric Association Meeting; May 14-18, 2016; Atlanta, Georgia.
Acknowledgments: The authors thank all the patients and investigators who contributed to this study. The authors also thank Dr Amy Claxton, PhD, of Alkermes, Inc., for clinical insights and critical reading of the manuscript. Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) was provided by Karen Yee, PhD (ApotheCom, London, United Kingdom). Dr Claxton is an employee of Alkermes, Inc. Dr Yee has no conflict of interest to declare.
REFERENCES
1. Freeman R. Schizophrenia. N Engl J Med. 2003;349(18):1738-1749. PubMed doi:10.1056/NEJMra035458
2. Leucht S, Lasser R. The concepts of remission and recovery in schizophrenia. Pharmacopsychiatry. 2006;39(5):161-170. PubMed doi:10.1055/s-2006-949513
3. San L, Ciudad A, Alvarez E, et al. Symptomatic remission and social/vocational functioning in outpatients with schizophrenia: prevalence and associations in a cross-sectional study. Eur Psychiatry. 2007;22(8):490-498. PubMed doi:10.1016/j.eurpsy.2007.06.005
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
5. Ca×±as F, Alptekin K, Azorin JM, et al. Improving treatment adherence in your patients with schizophrenia: the STAY initiative. Clin Drug Investig. 2013;33(2):97-107. PubMed doi:10.1007/s40261-012-0047-8
6. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315. PubMed doi:10.4088/JCP.v64n1105
7. Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453-460. PubMed doi:10.4088/JCP.v67n0317
8. Weiden P, Glazer W. Assessment and treatment selection for "revolving door" inpatients with schizophrenia. Psychiatr Q. 1997;68(4):377-392. PubMed doi:10.1023/A:1025499131905
9. USA Food and Drug Administration. FDA approves new injectable drug to treat schizophrenia. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465801.htm. 2015.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090. PubMed doi:10.4088/JCP.14m09741
11. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
12. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976.
13. Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. PubMed doi:10.1176/appi.ajp.162.3.441
14. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386. PubMed doi:10.1016/j.clinthera.2008.12.020
15. Smeraldi E, Cavallaro R, Folnegović-Šmalc V, et al. Long-term remission in schizophrenia and schizoaffective disorder: results from the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Ther Adv Psychopharmacol. 2013;3(4):191-199. PubMed doi:10.1177/2045125313479127
This PDF is free for all visitors!