Objective: To determine if adjunctive treatment with a standardized extract of Withania somnifera (WSE), with known anti-inflammatory and immunomodulating properties, improves psychopathology and stress in patients with schizophrenia or schizoaffective disorder (DSM-IV-TR).
Methods: Patients experiencing an exacerbation of symptoms were assigned to WSE (1,000 mg/d) or placebo for 12 weeks, added to their antipsychotic medication, in a random-assignment, double-blind, placebo-controlled study conducted from April 2013 to July 2016. Primary outcomes were change from baseline to end of treatment on the Positive and Negative Syndrome Scale (PANSS total, positive, negative, and general symptoms) between treatment groups. Secondary outcomes evaluated stress and inflammatory indices using the Perceived Stress Scale (PSS), S100 calcium-binding protein B (S100B), and C-reactive protein (CRP).
Results: Sixty-six randomized patients (n = 33 per group) provided efficacy data. Beginning at 4 weeks and continuing to the end of treatment, WSE produced significantly greater reductions in PANSS negative, general, and total symptoms (Cohen d: 0.83, 0.76, 0.83), but not positive symptoms, when compared to placebo. PSS scores improved significantly with WSE treatment compared to placebo (Cohen d: 0.58). CRP and S100B declined more in the WSE group but were not significantly different from placebo. Adverse events were mild to moderate and transient; somnolence, epigastric discomfort, and loose stools were more common with WSE. No significant between-treatment differences were noted in body weight, vital signs, or laboratory measures, which remained stable.
Conclusions: This early study suggests that adjunctive treatment with a standardized extract of Withania somnifera provides significant benefits, with minimal side effects, for negative, general, and total symptoms and stress in patients with recent exacerbation of schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT01793935
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Adjunctive Use of a Standardized Extract of Withania somnifera (Ashwagandha) to Treat Symptom Exacerbation in Schizophrenia:
A Randomized, Double-Blind, Placebo-Controlled Study

ABSTRACT
Objective: To determine if adjunctive treatment with a standardized extract of Withania somnifera (WSE), with known anti-inflammatory and immunomodulating properties, improves psychopathology and stress in patients with schizophrenia or schizoaffective disorder (DSM-IV-TR).
Methods: Patients experiencing an exacerbation of symptoms were assigned to WSE (1,000 mg/d) or placebo for 12 weeks, added to their antipsychotic medication, in a random-assignment, double-blind, placebo-controlled study conducted from April 2013 to July 2016. Primary outcomes were change from baseline to end of treatment on the Positive and Negative Syndrome Scale (PANSS total, positive, negative, and general symptoms) between treatment groups. Secondary outcomes evaluated stress and inflammatory indices using the Perceived Stress Scale (PSS), S100 calcium-binding protein B (S100B), and C-reactive protein (CRP).
Results: Sixty-six randomized patients (n = 33 per group) provided efficacy data. Beginning at 4 weeks and continuing to the end of treatment, WSE produced significantly greater reductions in PANSS negative, general, and total symptoms (Cohen d: 0.83, 0.76, 0.83), but not positive symptoms, when compared to placebo. PSS scores improved significantly with WSE treatment compared to placebo (Cohen d: 0.58). CRP and S100B declined more in the WSE group but were not significantly different from placebo. Adverse events were mild to moderate and transient; somnolence, epigastric discomfort, and loose stools were more common with WSE. No significant between-treatment differences were noted in body weight, vital signs, or laboratory measures, which remained stable.
Conclusions: This early study suggests that adjunctive treatment with a standardized extract of Withania somnifera provides significant benefits, with minimal side effects, for negative, general, and total symptoms and stress in patients with recent exacerbation of schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT01793935
J Clin Psychiatry 2018;79(5):17m11826
To cite: Chengappa KNR, Brar JS, Gannon JM, et al. Adjunctive use of a standardized extract of Withania somnifera (ashwagandha) to treat symptom exacerbation in schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2018;79(5):17m11826.
To share: https://doi.org/10.4088/JCP.17m11826
© Copyright 2018 Physicians Postgraduate Press, Inc.
aWestern Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
bSchool of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania
*Corresponding author: K. N. Roy Chengappa, MD, FRCPC, Comprehensive Recovery Services, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, 3811 O’ Hara St, Pittsburgh, PA 15213-2593 ([email protected]).
The immune-inflammatory dysregulation theory in schizophrenia1,2 posits an imbalance of pro- and anti-inflammatory cytokines and elevated levels of inflammatory proteins (eg, C-reactive protein, S100 calcium-binding protein B [S100B], and prostaglandin E2) in subgroups of patients with schizophrenia experiencing an exacerbation of symptoms.3-8 These immune-inflammatory alterations impact dopaminergic, glutamatergic, and cholinergic neurotransmission, which in turn are linked to the positive, negative, and cognitive symptoms noted in schizophrenia.1,9,10 Furthermore, antipsychotic medications do not mitigate immune-inflammatory disturbances.11,12 A case has been made for adjunctive treatment of symptoms of schizophrenia using anti-inflammatory drugs, such as cyclooxygenase (COX) inhibitors, since these medications shift the immune responsivity from predominantly type-2 to type-1 and inhibit prostaglandin E2 synthesis.13 A review14 of 8 randomized controlled trials involving patients experiencing symptom exacerbations indicated that adjunctive COX inhibitors (celecoxib and high-dose aspirin) demonstrated a small treatment effect for positive symptoms of schizophrenia, and trend-level significance for total psychopathology, but no effects on negative symptoms. Concerns about cardiac safety and bleeding risks associated with nonsteroidal anti-inflammatory agents (NSAIDs) call for alternative anti-inflammatory agents to be tested.
Extracts of Withania somnifera (WSE, a medicinal herb) have demonstrated immunomodulatory and anti-inflammatory actions in animal studies, enhancing type-1 immune response and cytokine production and modulating production of acute phase reactants, COX-2 inhibition, and inhibition of NF-κB inflammatory signaling pathways.15-19 WSE has also shown anxiolytic, procognitive, and antiarthritic benefits and appears to have good safety in early human studies.20-23 On the basis of animal and human immune-inflammatory data, we posited that standardized extracts of Withania somnifera would prove beneficial for recently exacerbated symptoms in patients with schizophrenia.
METHODS
Study Design and Overview
This study, a double-blind, randomized, placebo-controlled trial of a standardized extract of Withania somnifera (Sensoril; Natreon, Inc., New Jersey) added to ongoing antipsychotic treatment, was conducted from April 2013 to July 2016 at the ambulatory clinics associated with Comprehensive Recovery Services of Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. The trial was approved by the Institutional Review Board of the University of Pittsburgh; it was registered at ClinicalTrials.gov (identifier: NCT01793935) and conducted under an investigational new drug application to the US Food and Drug Administration. Subjects were recruited from June 2013 to July 2016. The study comprised a 1- to 2-week screening period and a 12-week treatment period for each patient.
Subjects
Male or female outpatients aged 18 to 75 years, of any race, with DSM-IV-TR schizophrenia or schizoaffective disorder were recruited, and the diagnosis was affirmed by the Mini-International Neuropsychiatric Interview24 supplemented by medical records and clinicians. All subjects provided written informed consent. At study entry, the Positive and Negative Syndrome Scale (PANSS)25 total score had to be ≥ 60, with a score of ≥ 5 on any 1 item or a score of ≥ 4 on any 2 items of the positive symptom cluster or unusual thought content. Moreover, symptom exacerbation had to extend ≥ 2 weeks but ≤ 1 year, and patients had to be receiving antipsychotic agents for ≥ 4 weeks. Symptom exacerbation was defined (in weeks) using a combination of patient self-report and clinician or referring psychiatrist confirmation. Study staff confirmed this information using important chronological events or holidays to approximately date the exacerbation and/or reviewed the medical record notes. The rationale for using this type of target patient population was that, during the writing of the original grant application, the extant data indicated that patients with persistent symptoms (over the course of years) without recent exacerbations were less likely to benefit from the NSAIDs (eg, celecoxib or high-dose aspirin) that had been assessed as adjunct treatments in schizophrenia. Exclusion criteria included a positive pregnancy test or positive tests for illicit drugs (marijuana and alcohol use were allowed on a case by case basis), unstable medical disorders, pregnancy or breastfeeding, known allergy to WSE, or situations requiring imminent psychiatric hospitalization. Subjects receiving antibiotics, antiviral or antiparasitic medications, or immunosuppressive therapy and patients taking daily NSAIDs or doses of aspirin > 81 mg/d were excluded. As patients were experiencing a recent exacerbation of symptoms, referring psychiatrists were permitted to increase the dosage of an antipsychotic drug or add a second antipsychotic drug. However, switching to another antipsychotic drug resulted in participants leaving the study. Psychiatrists were also permitted dosage changes of mood stabilizers and antidepressants, and they could add sedative-hypnotic agents as clinically appropriate.

- Antipsychotic drugs are effective for psychotic symptoms but of limited utility for negative symptoms and general psychopathology (eg, depression, anxiety, stress).
- For patients with schizophrenia or schizoaffective disorder who continue to experience mild to moderate positive symptoms but also negative, general, and stress symptoms despite ongoing antipsychotic treatment, adding a standardized extract of Withania somnifera may help reduce these symptoms.
Study Procedures and Study Medications
Subjects meeting all eligibility criteria progressed to a 1:1 randomization to WSE or placebo. A computerized randomization schedule was generated by the Investigational Drug Pharmacy Service (IDS) of the University of Pittsburgh Medical Center, and IDS secured the study medications and blinded codes. All assessments were conducted double-blind. The WSE and placebo were constituted as identical capsules. The WSE capsules contained 250 mg of the standardized extract along with inactive ingredients. The placebo capsules contained the same inactive ingredients and fill weight as the WSE. To mask the smell of the WSE capsules, the placebo capsules were exposed to fully closed cloth pouches that contained WSE powder. After a couple of days, the odor permeated the placebo capsules, making them smell like the WSE capsules. The active group received 250 mg of WSE twice a day for a total daily dose of 500 mg/d for the first week, which was titrated to 500 mg twice daily (1,000 mg/d) at week 2, and this dosage was maintained for 11 weeks, unless tolerability dictated a lower dosage. Pill counts and reconciliation at each visit served as a measure of adherence. Including the screening visit, there were a total of 6 visits over 12 weeks.
Standardization of the WSE Extract
The WSE extract utilized for this study (Sensoril) ensures a minimum concentration of the critical bioactive withanolide glycosides and carrier oligosaccharides, but only traces of withaferin A (US Patent 7318938).26 Manufacturer-patented processes for WSE extraction ensure batch-to-batch reliability of critical bioactive constituents and ratios of withanolides to aglycones, as well as a lack of contaminants, toxins, microbes, and toxic heavy metals, factors that are crucial for replicating clinical results with herbal-botanical products.
Assessments
The primary outcomes were assessed using the PANSS (total and positive, negative, and general symptoms).25 Secondary outcomes included the Perceived Stress Scale27 and the Clinical Global Impressions scale (CGI).28 These assessments were conducted at every visit during the study. The cytokines interleukin (IL)-2, IL-4, interferon (IFN)-γ, and IL-6, as well as high sensitivity C-reactive protein (hsCRP) and S100B, were measured in serum at screening and at the end of treatment. The cytokines were assayed at the University of Pittsburgh Medical Center (UPMC) Immunologic Monitoring Laboratory using a multiplex Luminex bead technology assay (Invitrogen, Camarillo, California). The lower limit of detection sensitivity of the assay was < 6 pg/mL for human IL-2, < 5 pg/mL for IL-4, < 3 pg/mL for IL-6, and < 5 pg/mL for IFN-γ. Hs-CRP was measured in mg/L units using a nephelometric method that employed latex particles coated with CRP monoclonal antibodies. The lower limit of detection sensitivity of this commercially available assay (Quest Diagnostic Laboratories) is 0.2 mg/L. S100B was measured in serum in pg/mL using a commercially available sandwich enzyme-linked immunosorbent assay (EMD Millipore, Massachusetts) at the Immunopathology Laboratories of UPMC. The lower limit of detection sensitivity of the S100B assay was 2.7 pg/mL, with the intra- and interassay coefficients of variation being < 4.8% and 4.4%, respectively.
Statistical Methods and Outcome Measures
The primary efficacy measure was change from baseline to end of treatment in PANSS total and PANSS positive, negative, and general symptoms scores between the 2 treatment arms. Secondary efficacy measures included proportions of patients in each treatment group meeting ≥ 20% improvement in PANSS total, positive, negative, and general symptom scores. Baseline to end of treatment PSS total scores and proportions in each treatment group achieving ≥ 20% improvement in PSS scores were also secondary efficacy outcomes. Furthermore, time to onset of any improvements in PANSS or PSS scores (ie, change from baseline to each visit) between treatments was assessed as a secondary outcome as were proportions of patients showing improvement on the CGI-Improvement subscale at the end of treatment.
Additional secondary outcomes assessed psychotropic medication changes between treatments. Between treatments changes in levels of hsCRP, S100B, and cytokines from baseline to end of treatment were also determined.
Clinical laboratory data, review of medical history, vital signs, electrocardiograms (ECGs), body weight, and reporting of adverse events served to assess safety, and the study was conducted under the auspices of an independent Data and Safety Monitoring Board (DSMB).
In the absence of prior data for WSE in treating symptoms of schizophrenia, a guidance that a sample size of 40 to 100 subjects in adjunctive treatment trials in schizophrenia could provide reasonable power was adopted.29 Moreover, initial positive studies with celecoxib or aspirin had sample sizes ranging from 50 to 70 subjects.30,31 An intent-to-treat analysis was planned of all subjects who were randomly assigned to either WSE or placebo and who had at least 1 pre- and post-randomization primary efficacy outcome measure (PANSS total and subscale scores). For the primary efficacy analyses, a Student t test was used to examine differences between treatment groups in changes from baseline to end of treatment in PANSS (total and subscale scores) and PSS scores. This was the a priori statistical analyses plan described in the protocol. A mixed-model repeated-measures (MMRM) analysis using an autoregressive covariance matrix examined the robustness of the primary analyses and the time to improvement in PANSS and PSS scores from baseline to end of treatment. This model used treatment, visit, and treatment-by-visit interaction as fixed effects and subjects as random effects.
Proportions of subjects in each treatment group who achieved ≥ 20% improvements were compared using a χ2 test. Cohen d (effect size) was determined using the group mean change between treatment and placebo from baseline to study endpoint, divided by the pooled standard deviation. The number needed to treat (NNT) for ≥ 20% response rates was calculated as 100/absolute risk reduction. Proportions of subjects in each treatment group who experienced antipsychotic or other psychotropic medication changes were compared using Fisher exact test. Two-tailed hypothesis testing was conducted, and a P value of ≤ .05 was considered statistically significant. Statistical analyses were undertaken blind to treatment assignment.
As distributions of cytokine, hs-CRP, or S100B levels are affected by the lower limits of the detection sensitivity of the assays or skewed, it was planned to transform the data to normalize the distribution and/or use nonparametric statistics to test statistical significance between treatment groups. Immune/inflammatory measures were compared with changes in PANSS (subscales and total) and PSS scores using Pearson or Spearman correlations. Safety analyses of vital signs, ECG, laboratory measures, and body weight were evaluated as either continuous or categorical variables (normal vs abnormal, etc). Reported treatment-emergent adverse events were grouped by organ-system and tabulated by treatment assignment, and rates between treatments were compared using χ2 or Fisher exact test.
RESULTS
Patient Recruitment and Disposition
As noted in the CONSORT flowchart (Supplementary Figure 1), 82 subjects were screened for consent and eligibility, and 68 subjects were randomly assigned to WSE (n = 34) or placebo (n = 34). One subject in each treatment group did not take the allocated treatment and provided no further data. Therefore, 66 patients (n = 33 subjects in each treatment arm) formed the efficacy-defined intent-to-treat population. Fifty-nine subjects (89.4%) completed the study, 28 (84.9%) in the WSE group and 31 (93.9%) in the placebo arm, with no statistically significant differences between treatments.
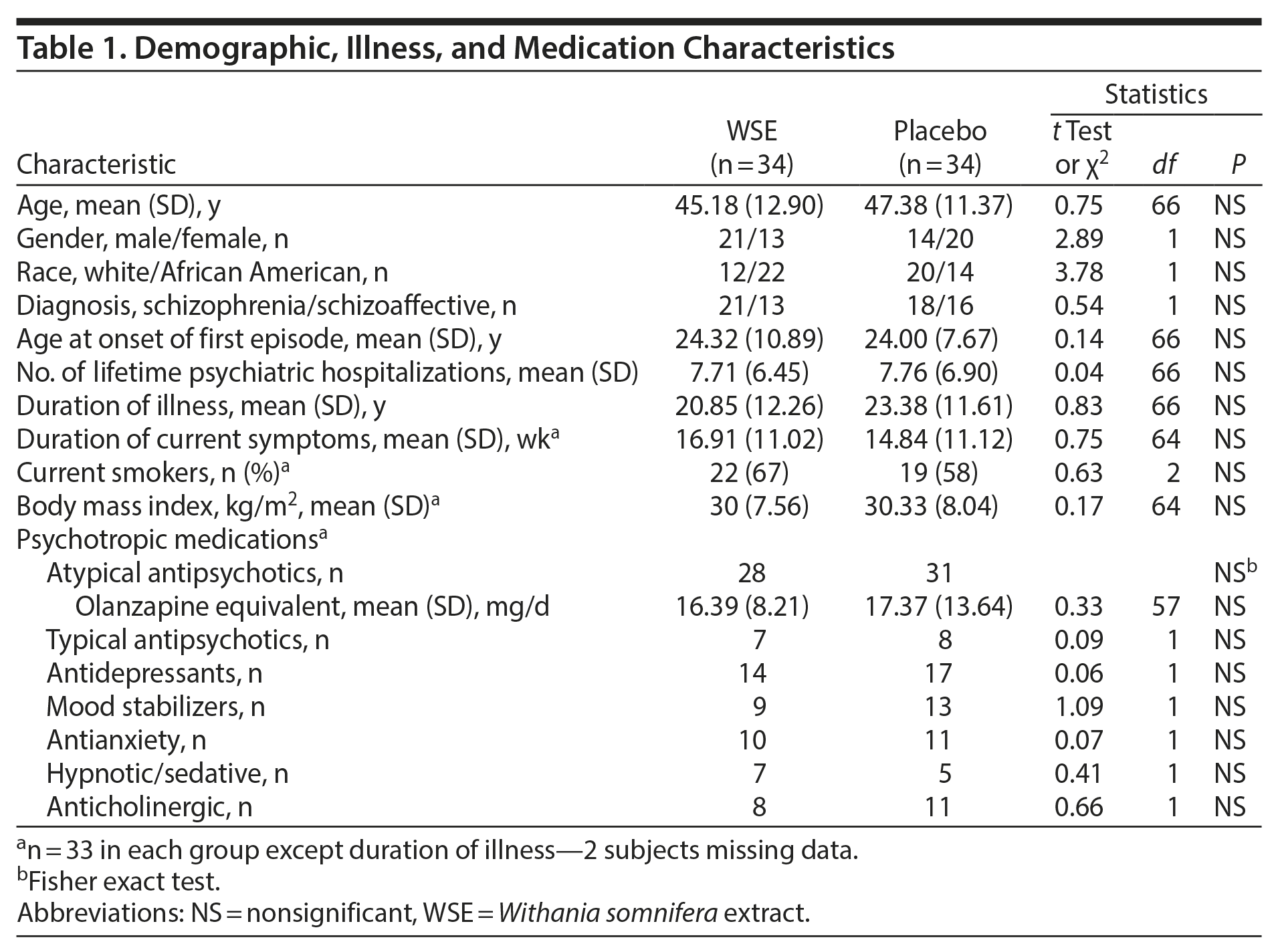
Participant Characteristics
None of the demographic, illness, or medication characteristics (Table 1) differed significantly between the treatment groups. Patients comprised a cohort in their mid to late 40s, with a DSM-IV-TR diagnosis of schizophrenia (61%) and with ≥ 20 years of illness and more than 7 lifetime hospitalizations. The mean duration of the current symptom exacerbation was nearly 16 weeks. Most patients in either treatment group (87.9% in WSE and 90.9% placebo) received atypical antipsychotic agents in similar daily olanzapine equivalents,32 and 4 subjects each in the WSE and placebo groups received more than 1 antipsychotic agent. Five WSE-assigned and 3 placebo-assigned subjects received clozapine. Antidepressants, mood stabilizers, anxiolytics, hypnotic-sedatives, and anticholinergic agents were evenly distributed between treatment groups. Adherence ranged from 82% to 100% among most patients (63/66), with no significant differences between the 2 treatment groups.
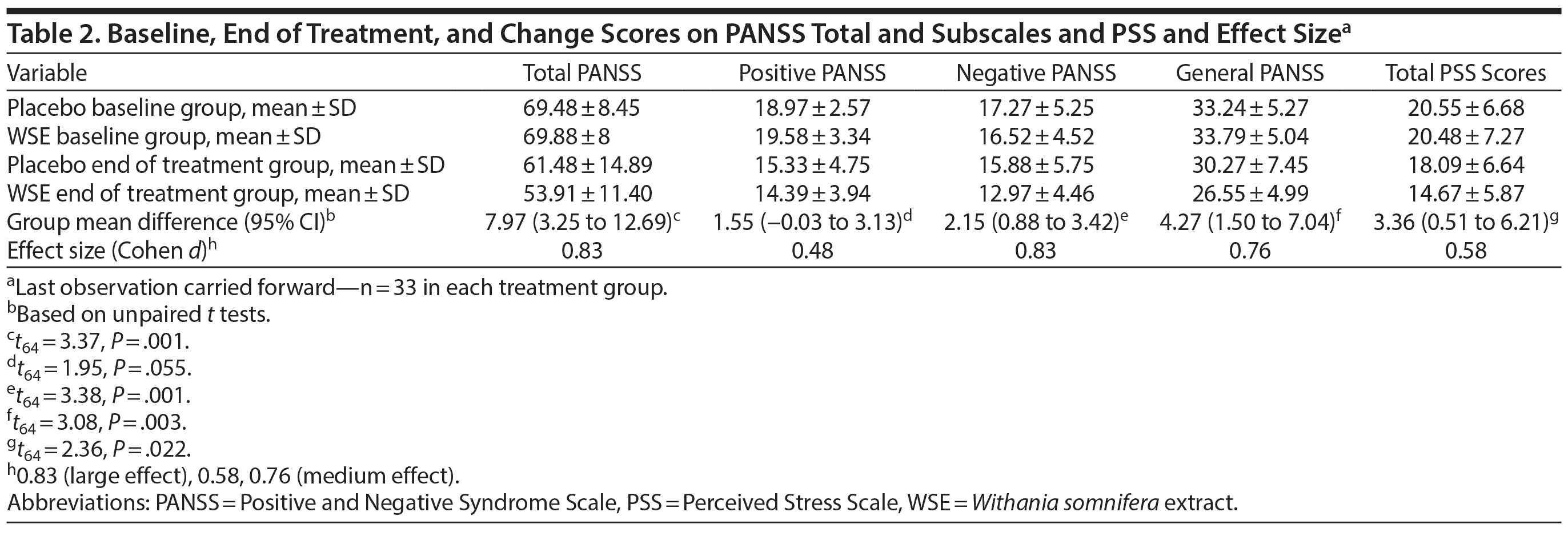
Primary Outcomes
As noted in Table 2, the WSE-treated group achieved significantly better outcomes on the PANSS negative, general, and total symptom scores compared to those assigned to placebo. Treatment effect sizes favoring WSE ranged from large (negative and total symptoms, Cohen d = 0.83) to medium (general symptoms, Cohen d = 0.76). PANSS positive symptom scores improved more in the WSE group compared to placebo but did not achieve statistical significance (Cohen d = 0.48). MMRM analyses confirmed the primary statistical analyses. Based on referee suggestions, we undertook a post hoc analysis of subjects entering the study with moderate severity PANSS negative symptom scores (defined as a score of ≥ 4, moderate or greater severity, on 2 or more items). Even though only a small number of subjects met this restrictive criterion (n = 9 WSE, n = 12 placebo), a highly significant reduction in PANSS negative symptom subscores was observed in the WSE-treated group (mean ± SD change score: 4.22 ± 2.86) vs placebo (0.25 ± 2.14) (t19 = 3.65, P = .002) with nearly double the effect size (Cohen d = 1.61, 95% CI = 0.61 to 2.6) that we had observed for the entire sample.
Secondary Outcomes
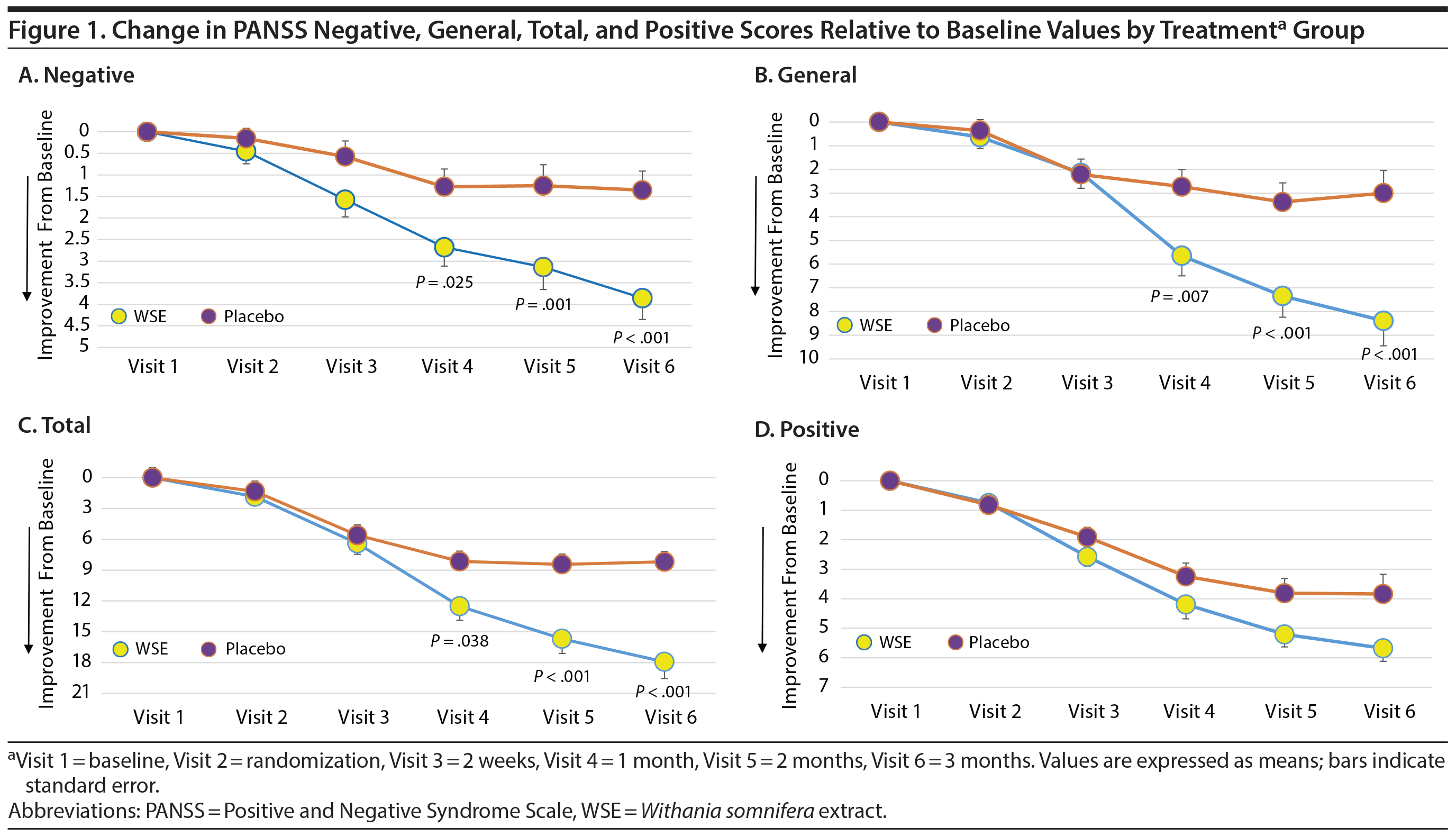
WSE-treated subjects experienced significant reductions in PSS stress scores compared to placebo (Cohen d = 0.58) (Table 2). MMRM analyses provided the results for time to onset of improvements in the PANSS and PSS scale scores between treatment groups (Supplementary Table 1). The time to significant separation in PANSS negative, general, and total symptoms and PSS scores favoring WSE over placebo began at Visit 4 (ie, at 4 weeks of treatment) and was sustained throughout the remainder of the 12-week study (Figure 1A, 1B, and 1C and Supplementary Figure 2).
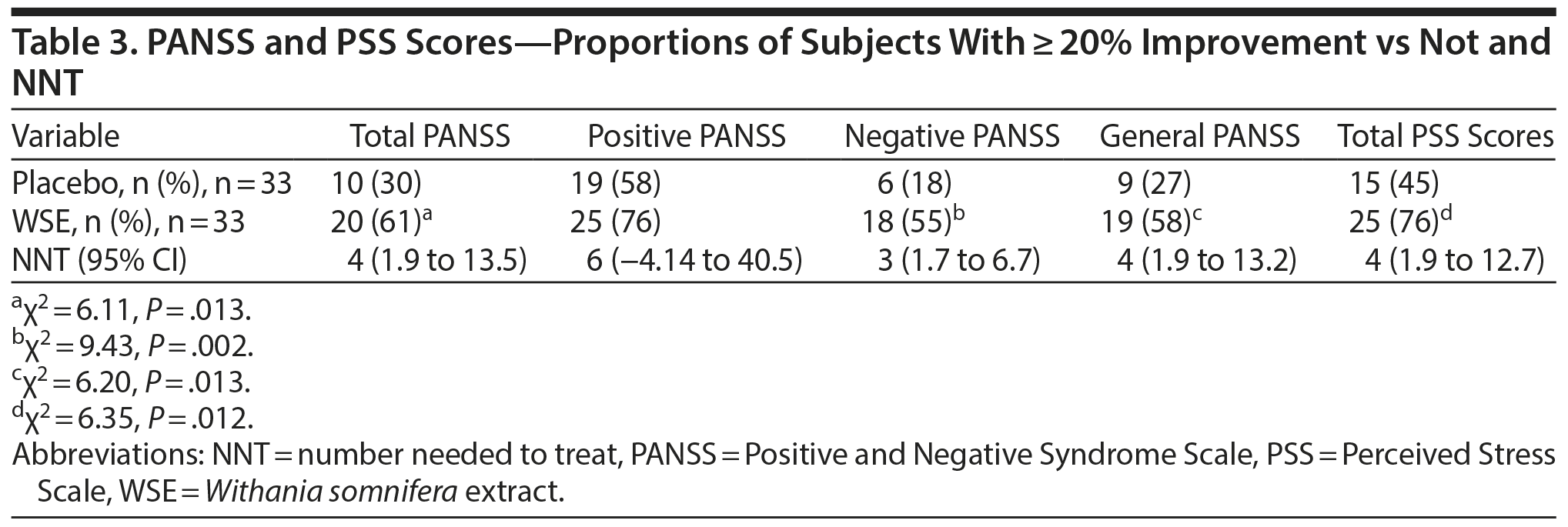
WSE-treated patients were significantly more likely to achieve ≥ 20% improvements in PANSS negative, general, and total symptom scores, but not positive symptom scores, compared to those assigned to placebo (Table 3). The NNT to achieve 1 additional such outcome with WSE ranged from 3 for negative symptoms to 4 for general and total symptoms (Table 3). Twenty percent or greater improvement in PSS stress scores also favored the WSE group compared to placebo (Table 3). The proportion of patients rated as improved on the CGI-Improvement subscale by the end of treatment did not differ significantly between treatments (WSE: 12/33 [36.4%] vs placebo: 8/33 [24.2%]).
Use of Antipsychotic and Other Psychotropic Medications
Nine (27.3%) of the placebo-assigned subjects either had their antipsychotic drug dosage increased (n = 8) or had a second antipsychotic drug added (n = 1); by comparison, 2 (6.1%) WSE-treated subjects had their antipsychotic drug dosage increased (Fisher exact P = .044). The antipsychotic drug dosage increases in both groups were with newer generation antipsychotic agents, and PANSS negative symptom change scores for the 2 WSE-assigned subjects and the 9 placebo-treated subjects were similar to the results for the entire sample presented earlier (data not shown). Other psychotropic medication changes were few and not significantly different between treatments.
Inflammation Markers
Although hsCRP and S100B levels declined in the WSE-treated group (Supplementary Figure 3A and 3B) and increased among those assigned to placebo, these data were skewed, and the Mann-Whitney U test showed no statistically significant differences between treatments. A decline in mean (SD) hsCRP levels (before treatment to end of treatment) of 1.08 (6.98) mg/L was observed in the WSE group versus an increase in the placebo group of 1.55 (5.58) mg/L (Mann-Whitney U = 372.5, P = .25). A decline in S100b levels in the WSE-treated group was seen, of 12.98 (112.89) pg/mL, versus an increase in the placebo group, of 27.54 (145.41) pg/mL (Mann-Whitney U = 469.5, P = .72). We further examined correlations between changes in hsCRP levels (from baseline to end of treatment) and corresponding changes in PANSS scores (total, positive, negative, and general psychopathology subscores) in the 2 treatment arms separately. No correlations were observed between inflammatory and clinical parameters. The nonsignificant results were confirmed using simple linear regression with change in hsCRP level as the dependent variable and change in PANSS scores as the independent variables. A similar pattern of results was obtained for S100b.
Cytokines
Over 90% (n = 60) of the subjects had detectable IL-6 levels, whereas only 12.1%, 13.6%, and 19.7% of the subjects had detectable IFN-γ, IL-4, and IL-2 levels, respectively. Therefore, we decided to analyze only IL-6. The distribution of the IL-6 levels was right skewed, and there were no statistically significant differences between the WSE and placebo groups (Mann-Whitney U test). IL-6 levels in the WSE-treated group changed from 3.37 (2.53) before treatment to 3.40 (2.51) pg/mL at the end of treatment and in the placebo group declined from 4.29 (3.34) pg/mL before treatment to 3.87 (3.5) pg/mL at the end of treatment (Mann-Whitney U = 458, P = .92).
Weight, Body Mass Index, and Vital Signs
These measures are shown in Supplementary Table 2. At the end of the treatment period, WSE-treated subjects gained a mean of 2.4 lb compared to 1.72 lb in the placebo group. Systolic and diastolic blood pressure readings, pulse, and temperature differences in the 2 treatment arms were not significantly different from baseline to end of the treatment period.
Adverse Events
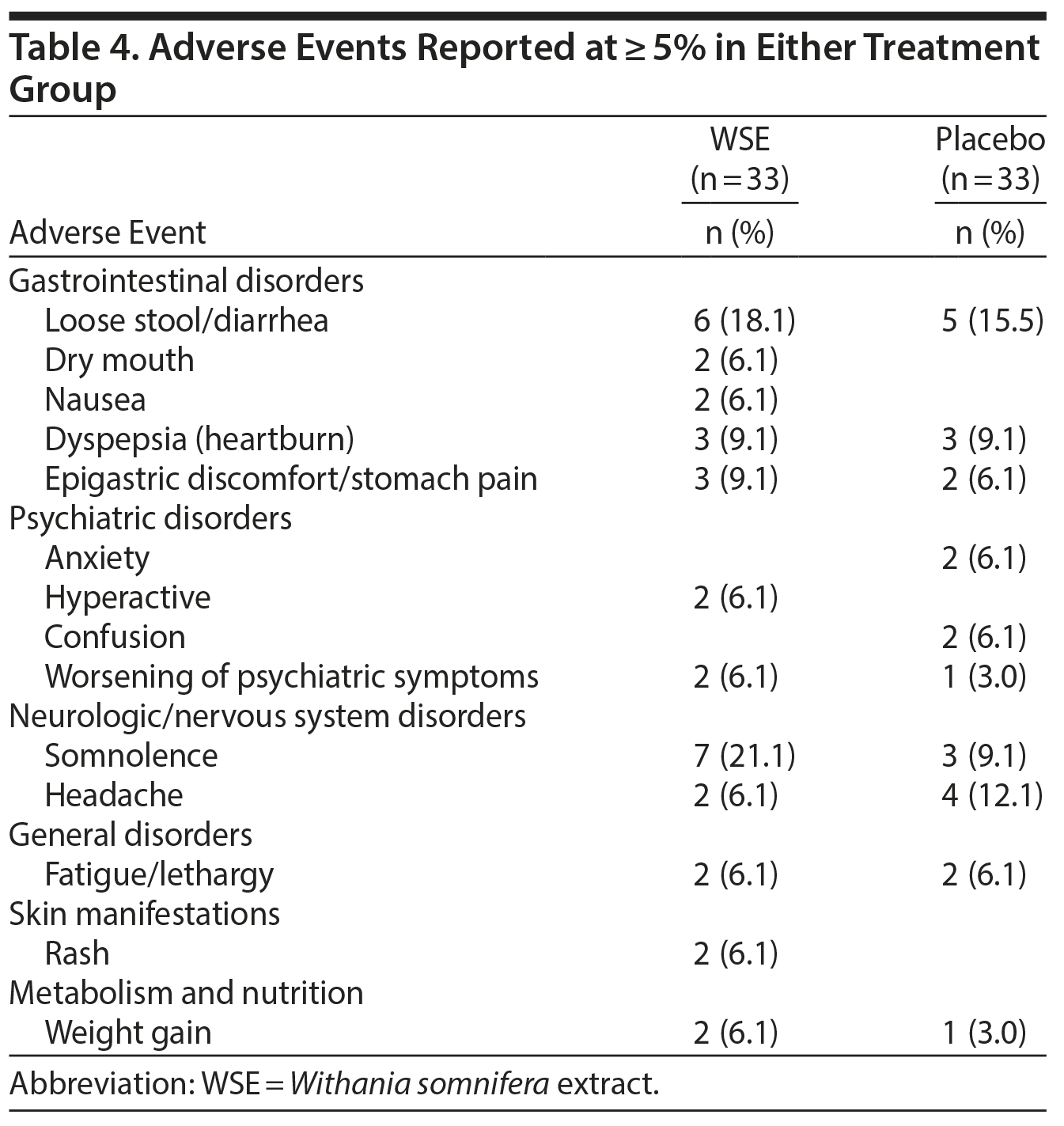
There were no statistically significant differences between treatments for adverse events that were reported at ≥ 5% in either group. The DSMB classified these adverse events as mild to moderate, and they were all reported as transient (Table 4). Somnolence (21.1%), loose stool/diarrhea (18.1%), epigastric discomfort/stomach pain (9.1%), dry mouth (6.1%), hyperactivity (6.1%), rash (6.1%), and weight gain (6.1%) were more common in the WSE group, whereas headache (12.1%), anxiety (6.1%), and confusion (6.1%) were more common in the placebo group.
DISCUSSION
Pending future replication, this early study suggests that a standardized extract of Withania somnifera, when added adjunctively to antipsychotic medications, provides significant benefits for negative, general, total, and stress symptoms in patients with schizophrenia experiencing an exacerbation of symptoms. Improvements with WSE treatment in PANSS negative, general, total, and stress symptoms were first noted at 4 weeks and continued through the 12-week study period. WSE has shown benefits for anxiety, stress, and depression in separate clinical trials,20,21,33 and the improvements in stress and PANSS general symptoms in the current study suggest similar benefits for patients with schizophrenia. The PANSS general psychopathology scale includes 16 items related to depression, anxiety, and motor activity and to phenomena such as preoccupation, social avoidance, poor impulse control or volitional disturbance, attention and concentration, judgment, and insight, among others.25 Even though positive symptom scores under WSE treatment were not significantly better than in the placebo group, there were significantly more antipsychotic medication adjustments in the placebo group.
The large treatment effects of WSE for negative, general, and total symptoms of schizophrenia are especially encouraging given that a meta-analysis14 of adjunctive anti-inflammatory agents (celecoxib and aspirin) determined a significant but small treatment effect for positive symptoms (Hedges g = −0.189), and trend-level treatment effect for total symptoms (Hedges g = −0.236), but no benefit for negative symptoms of schizophrenia. Furthermore, a recent systematic review of 42 cotreatment strategies (added to antipsychotic drugs) concluded that only the NSAID meta-analyses14 met the content-quality criteria, and that too at a modest score.34
In the present study, even though the WSE-treated group experienced greater declines in both S100B and hsCRP relative to the placebo group, the differences between treatments were not statistically significant. S100B is secreted by astrocytes and oligodendrocytes and is considered a marker of central nervous system (CNS) inflammation. WSE has known anti-inflammatory properties, including COX-2 inhibition, suppression of NF-κB activation, and attenuation of pro-oxidant and inflammatory responses in microglial and astrocytic cells and enhancement of Nrf2/ARE reporter activity in astrocytes.15,16,19,35,36 Importantly, in stressed animals, WSE enhances type-1 immunity, that is, promotes normalization of IL-2 and IFN-γ levels and significantly improves antioxidant and lipid peroxidation indices.17,18 Furthermore, human studies have shown that WSE induces the proliferation of CD4+ and CD8+ T cells.37 These separate lines of inquiry appear to point to WSE’s favorable induction of type-1 cytokines and anti-inflammatory and antioxidant actions in the CNS. Müller and Schwarz1 have argued that an imbalanced immune-inflammatory system in the CNS in persons with schizophrenia can disrupt dopaminergic-glutamatergic-cholinergic neurotransmission; this is likely mediated via increased production of kynurenic acid, an N-methyl-d-aspartate (NMDA) receptor antagonist. Over- or underactivity of dopamine, glutamate, and acetylcholine neurotransmitters has been implicated in various symptom clusters (positive, negative, cognitive, motor, sleep, etc) in schizophrenia.9,10,38 We measured cytokines in serum rather than by stimulation of blood,13,31 and 3 cytokines (IL-2, IL-4, and IFN-γ) were detected in only a minority of subjects. Therefore, we were unable to test the hypothesis that rebalancing the Th1/Th2 ratio with WSE treatment would mediate a greater reduction in psychopathology.
In Ayurvedic medical practice, ashwagandha has been used as an “adaptogen,” ie, an agent used to increase the bodily resistance to stress and disease. Modern laboratory data indicate that bioactive constituents of WSE (eg, withanolide glycosides) have potent brain antioxidant, anti-inflammatory, and memory-enhancing activity.15-19,35-37,39-42 There is long-standing support for the notion that persons with schizophrenia show poor antioxidant defenses.43 Recent reviews that have synthesized animal and clinical data posit a multidirectional pathophysiologic central hub in schizophrenia involving a triad of poor redox capacity, neuroinflammation, and NMDA receptor hypofunction.44,45 It is argued that this pathophysiologic triad mediates dysfunction of dopaminergic, cholinergic, and glutamatergic (NMDA) neurotransmission in neural circuits involving cortical parvalbumin-containing interneurons (inhibitory via GABA) and excitatory pyramidal cells.46-48 Downstream effects of these neurotransmitter dysfunctions are thought to result in the emergence of positive and negative symptoms and cognitive impairments in schizophrenia.47,49,50 WSE is known to attenuate glutamate excitotoxicity51; protect against deleterious propoxur-induced anticholinergic brain and cognitive dysfunction52; and recover dopamine levels and attenuate glial fibrillary acidic protein, a proinflammatory marker of astrocyte activation, in a mouse model of parkinsonism.53 Furthermore, WSE was shown to have GABAergic activity on hippocampal pyramidal neurons,54 and this same group of investigators used a patch-clamp technique to show that WSE potentiated NMDA receptors on pyramidal neurons,55 an effect blocked by strychnine, indicating glycinergic activity. It is notable that agents acting on the glycine receptor (eg, glycine, d-cycloserine, sarcosine, and N-acetyl cysteine) have been assessed for improving NMDA receptor functioning,56 with the hypothesis being that such agents can improve negative symptoms and/or cognitive impairments in schizophrenia, although not all studies have been positive.57 In summary, WSE has potent anti-inflammatory, immunomodulating, and antioxidant properties and importantly, NMDA potentiating and GABAergic activity. These properties make WSE a particularly attractive candidate to test as an adjunctive treatment for persons with schizophrenia whose symptoms, particularly negative, general, and stress-related symptoms and cognitive impairments, are suboptimally responsive to standard antipsychotic drugs.
Limitations
Future work with WSE could recruit patients based on Th1/Th2 criteria or other inflammatory indices (eg, hsCRP or S100b). Subsequent studies could evaluate cognitive benefits of WSE in a more stable patient group. However, aspirin did not show cognitive benefits in symptomatic patients with schizophrenia.31 Studies of longer duration with WSE could include functional assessments. The rationale for the higher daily dosage (1,000 mg/d, as opposed to 500 mg/d in our earlier study22) was driven by acuity (ie, patients experiencing an exacerbation of symptoms) and another study that used the same WSE extract and higher dosage for cognitive benefits with no increase in side effects.58 Changes in vital signs, body weight, clinical laboratory, and ECG measures were generally unremarkable in the 12-week study, suggesting good tolerability of WSE. Nevertheless, the upper and lower bounds of the daily dosage of WSE need to be determined.
CONCLUSION
A standardized extract of Withania somnifera, when added adjunctively to antipsychotic medications in patients with schizophrenia experiencing an exacerbation of symptoms, provides benefits for negative, general, and total symptoms and stress.
Submitted: July 28, 2017; accepted December 27, 2017.
Published online: July 10, 2018.
Potential conflicts of interest: Dr Chengappa states that the University of Pittsburgh is pursuing intellectual property protection for the technology discussed in this article. Drs Brar and Gannon and Ms Schlicht have no conflicts of interest to disclose with regard to this study.
Funding/support: This clinical trial was funded by the Stanley Medical Research Institute, Maryland, under grant award 12T-001. Natreon, Inc., New Jersey, provided the standardized extract of Withania somnifera and placebo.
Role of the sponsor: Neither the Stanley Medical Research Institute nor Natreon, Inc. had any role in conducting the clinical trial, data gathering, or analyses or reporting of the study results.
Previous presentation: Portions of this trial were presented at the Annual Meeting of the American Psychiatric Association, May 20-24, 2017, San Diego, California, and at the Annual Meeting of the American Society of Clinical Psychopharmacology, May 29-June 1, 2018, Miami, Florida.
Acknowledgments: The authors gratefully acknowledge Dr Samuel Gershon, Emeritus Professor of Psychiatry, University of Pittsburgh, for chairing the DSMB; Dr Scott Turkin, former Medical Director, Penn Highlands Medical Center, Dubois, Pennsylvania, and Joan Buttenfield Rea, RN, Retired Clinical Research Coordinator, Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania, for contributing their time and effort as DSMB members and monitoring the study; Ms Joan Spinogatti for contributions to IRB submission, data entry, extraction, and verification and helping to coordinate author collaboration and journal submission; Ms Amy Johnson, RPh, of the IDS Pharmacy Services at the University of Pittsburgh Medical Center, for coordinating randomization, securing the blinded assignment codes, and working closely with the study team to organize study drug receipt, storage, prescriptions, and accounting; pharmacists at Forbes Pharmacy, including Ms Jamie Montgomery PharmD, BCPP, and Tanya Fabian, PharmD, PhD, BCPP, for dispensing as well as for administrative support throughout the study tenure; the staff of the IND office (within the University of Pittsburgh IRB) that audited this study; Ms Vidya Jayaraman, Ms Kelly Wood-Etherington, BA, and Ms Courtney Radliffe, MA, for carefully reviewing and tabulating the adverse events by body system, cross-checking the descriptions and DSMB declarations for each adverse event, and converting the medications to daily olanzapine equivalents, all while blind to treatment assignment; Dr Theresa Whiteside’s laboratory for conducting the cytokine assays; and Drs Michael and Galina Shurin for conducting and reporting the S100B results. Finally, they gratefully thank the patients volunteering for this study and the therapists, service coordinators, and psychiatrists for referring patients and coordinating care with the study team. None of the acknowledged persons have any conflicts of interest to disclose.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Müller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10(2):131-148. PubMed CrossRef
2. Müller N. COX-2 inhibitors as antidepressants and antipsychotics: clinical evidence. Curr Opin Investig Drugs. 2010;11(1):31-42. PubMed
3. Kaiya H, Uematsu M, Ofuji M, et al. Elevated plasma prostaglandin E2 levels in schizophrenia. J Neural Transm (Vienna). 1989;77(1):39-46. PubMed CrossRef
4. Ganguli R, Brar JS, Chengappa KR, et al. Mitogen-stimulated interleukin-2 production in never-medicated, first-episode schizophrenic patients; the influence of age at onset and negative symptoms. Arch Gen Psychiatry. 1995;52(8):668-672. PubMed CrossRef
5. Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132(1):96-110. PubMed CrossRef
6. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. PubMed CrossRef
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230. PubMed CrossRef
8. Schümberg K, Polyakova M, Steiner J, et al. Serum S100B is related to illness duration and clinical symptoms in schizophrenia—a meta-regression analysis. Front Cell Neurosci. 2016;10:46. PubMed CrossRef
9. O’ Donnell P, Grace AA. Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophr Bull. 1998;24(2):267-283. PubMed CrossRef
10. Krystal JH, D’ Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4):215-233. PubMed CrossRef
11. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808. PubMed CrossRef
12. Meyer JM, McEvoy JP, Davis VG, et al. Inflammatory markers in schizophrenia: comparing antipsychotic effects in phase 1 of the clinical antipsychotic trials of intervention effectiveness study. Biol Psychiatry. 2009;66(11):1013-1022. PubMed CrossRef
13. Müller N, Ulmschneider M, Scheppach C, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):14-22. PubMed CrossRef
14. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39(6):1230-1241. PubMed CrossRef
15. Anbalagan K, Sadique J. Influence of an Indian medicine (ashwagandha) on acute-phase reactants in inflammation. Indian J Exp Biol. 1981;19(3):245-249. PubMed
16. Begum VH, Sadique J. Long term effect of herbal drug Withania somnifera on adjuvant induced arthritis in rats. Indian J Exp Biol. 1988;26(11):877-882. PubMed
17. Khan B, Ahmad SF, Bani S, et al. Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int Immunopharmacol. 2006;6(9):1394-1403. PubMed CrossRef
18. Malik F, Singh J, Khajuria A, et al. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007;80(16):1525-1538. PubMed CrossRef
19. Mulabagal V, Subbaraju GV, Rao CV, et al. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother Res. 2009;23(7):987-992. PubMed CrossRef
20. Andrade C, Aswath A, Chaturvedi SK, et al. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42(3):295-301. PubMed
21. Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS One. 2009;4(8):e6628. PubMed CrossRef
22. Chengappa KNR, Bowie CR, Schlicht PJ, et al. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J Clin Psychiatry. 2013;74(11):1076-1083. PubMed CrossRef
23. Ramakanth GSH, Uday Kumar C, Kishan PV, et al. A randomized, double blind placebo controlled study of efficacy and tolerability of Withania somnifera extracts in knee joint pain. J Ayurveda Integr Med. 2016;7(3):151-157. PubMed CrossRef
24. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. PubMed
25. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
26. Ghosal S. inventor; Natreon, Inc. assignee. Withania somnifera composition, method for obtaining same and pharmaceutical, nutritional and personal care formulations thereof. US patent 7,318,938. January 15, 2008.
27. Cohen S, Kamarck T, Mermelstein R. Perceived Stress Scale (PSS). In: Rush JA, First MB, Blacker D, eds. Handbook of Psychiatric Measures. 2nd ed. Washington, DC: American Psychiatric Publishing, Inc; 2008:204-206.
28. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
29. Stern RG, Schmeidler J, Davidson M. Limitations of controlled augmentation trials in schizophrenia. Biol Psychiatry. 1997;42(2):138-143. PubMed CrossRef
30. Müller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159(6):1029-1034. PubMed CrossRef
31. Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520-527. PubMed CrossRef
32. Leucht S, Samara M, Heres S, et al. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull. 2016;42(suppl 1):S90-S94. PubMed CrossRef
33. Auddy B, Hazra J, Mitra A, et al. A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. JANA. 2008;11(1):51-57.
34. Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675-684. PubMed CrossRef
35. Ajit D, Simonyi A, Li R, et al. Phytochemicals and botanical extracts regulate NF-κB and Nrf2/ARE reporter activities in DI TNC1 astrocytes. Neurochem Int. 2016;97:49-56. PubMed CrossRef
36. Gupta M, Kaur G. Aqueous extract from the Withania somnifera leaves as a potential anti-neuroinflammatory agent: a mechanistic study. J Neuroinflammation. 2016;13(1):193. PubMed CrossRef
37. Mikolai J, Erlandsen A, Murison A, et al. In vivo effects of Ashwagandha (Withania somnifera) extract on the activation of lymphocytes. J Altern Complement Med. 2009;15(4):423-430. PubMed CrossRef
38. Barak S. Modeling cholinergic aspects of schizophrenia: focus on the antimuscarinic syndrome. Behav Brain Res. 2009;204(2):335-351. PubMed CrossRef
39. Ghosal S, Kaur R, Srivatsava RS. Sitoindosides IX and X, two new withanolide glycosides from Withania somnifera. Indian J Nat Prod. 1988;4:12-13.
40. Panda S, Kar A. Evidence for free radical scavenging activity of ashwagandha root powder in mice. Indian J Physiol Pharmacol. 1997;41(4):424-426. PubMed
41. Jain S, Shukla SD, Sharma K, et al. Neuroprotective effects of Withania somnifera Dunn. in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15(6):544-548. PubMed CrossRef
42. Naidu PS, Singh A, Kulkarni SK. Effect of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunction. Phytother Res. 2006;20(2):140-146. PubMed CrossRef
43. Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996;55(1-2):33-43. PubMed CrossRef
44. Leza JC, Garc×a-Bueno B, Bioque M, et al. Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev. 2015;55:612-626. PubMed CrossRef
45. Steullet P, Cabungcal JH, Monin A, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016;176(1):41-51. PubMed CrossRef
46. Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234-242. PubMed CrossRef
47. Lewis DA, Curley AA, Glausier JR, et al. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57-67. PubMed CrossRef
48. Barron H, Hafizi S, Andreazza AC, et al. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci. 2017;18(3):E651. PubMed CrossRef
49. Krystal JH, Karper LP, Seibyl JP, et al. Dose related effects of the NMDA antagonist, ketamine, in healthy humans. Schizophr Res. 1993;9(2-3):240-241. CrossRef
50. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199-214. PubMed CrossRef
51. Kataria H, Wadhwa R, Kaul SC, et al. Water extract from the leaves of Withania somnifera protect RA differentiated C6 and IMR-32 cells against glutamate-induced excitotoxicity. PLoS One. 2012;7(5):e37080. PubMed CrossRef
52. Yadav CS, Kumar V, Suke SG, et al. Propoxur-induced acetylcholine esterase inhibition and impairment of cognitive function: attenuation by Withania somnifera. Indian J Biochem Biophys. 2010;47(2):117-120. PubMed
53. Prakash J, Chouhan S, Yadav SK, et al. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39(12):2527-2536. PubMed CrossRef
54. Bhattarai JP, Han SK. Phasic and tonic type A γ-aminobutyric acid receptor mediated effect of Withania somnifera on mice hippocampal CA1 pyramidal neurons. J Ayurveda Integr Med. 2014;5(4):216-222. PubMed CrossRef
55. Bhattarai JP, Park SJ, Han SK. Potentiation of NMDA receptors by Withania somnifera on hippocampal CA1 pyramidal neurons. Am J Chin Med. 2013;41(3):503-513. PubMed CrossRef
56. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-885. PubMed CrossRef
57. Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593-1602. PubMed CrossRef
58. Pingali U, Pilli R, Fatima N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacognosy Res. 2014;6(1):12-18. PubMed CrossRef
This PDF is free for all visitors!