Objective: To assess guanfacine extended-release (GXR) efficacy and safety in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods: This phase 3, double-blind, placebo-controlled study (conducted between October 2016 and July 2017) included Japanese patients aged ≥ 18 years with ADHD (DSM-5). Patients received GXR (n = 101) or placebo (n = 100) titrated from 2 mg/d to 4-6 mg/d (dose-optimization; 5 weeks), followed by 4-6 mg/d (dose-maintenance; 5 weeks), then tapered doses to 2 mg/d (2 weeks). Primary endpoint was change from baseline in total score on the Japanese version of the ADHD-Rating Scale IV with adult prompts (ADHD-RS-IV) at week 10. Other measures were ADHD-RS-IV subscales, Clinical Global Impression-Improvement scale (CGI-I) and Patient Global Impression-Improvement scale (PGI-I) (percentage of patients very much improved/much improved), treatment-emergent adverse event (TEAE) incidences, and TEAEs leading to discontinuation.
Results: Compared with placebo, there was statistically significantly greater improvement in ADHD-RS-IV total score reduction with GXR (least squares mean ± SE: GXR vs placebo, -11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005; effect size 0.52). There were significantly greater improvements in GXR for ADHD-RS-IV inattention (-7.39 ± 0.79 vs -4.89 ± 0.76; P = .0032) and hyperactivity-impulsivity (-3.84 ± 0.54 vs -2.10 ± 0.52; P = .0021) subscale scores, CGI-I scores (48.1% vs 22.6%; P = .0007), and PGI-I scores (25.3% vs 11.8%; P = .0283). More patients in the GXR versus the placebo group reported TEAEs (81.2% vs 62.0%) and discontinued due to TEAEs (19.8% vs 3.0%). The main TEAEs in the GXR group were somnolence, thirst, blood pressure decrease, nasopharyngitis, postural dizziness, and constipation; most TEAEs were mild to moderate in severity.
Conclusions: In Japanese adults with ADHD, GXR improved ADHD symptoms without any major safety concerns.
Trial Registration: Japan Primary Registries Network (https://rctportal.niph.go.jp/en/): JapicCTI-163231
ABSTRACT
Objective: To assess guanfacine extended-release (GXR) efficacy and safety in adults with attention-deficit/hyperactivity disorder (ADHD).
Methods: This phase 3, double-blind, placebo-controlled study (conducted between October 2016 and July 2017) included Japanese patients aged ≥ 18 years with ADHD (DSM-5). Patients received GXR (n = 101) or placebo (n = 100) titrated from 2 mg/d to 4-6 mg/d (dose-optimization; 5 weeks), followed by 4-6 mg/d (dose-maintenance; 5 weeks), then tapered doses to 2 mg/d (2 weeks). Primary endpoint was change from baseline in total score on the Japanese version of the ADHD-Rating Scale IV with adult prompts (ADHD-RS-IV) at week 10. Other measures were ADHD-RS-IV subscales, Clinical Global Impression-Improvement scale (CGI-I) and Patient Global Impression-Improvement scale (PGI-I) (percentage of patients very much improved/much improved), treatment-emergent adverse event (TEAE) incidences, and TEAEs leading to discontinuation.
Results: Compared with placebo, there was statistically significantly greater improvement in ADHD-RS-IV total score reduction with GXR (least squares mean ± SE: GXR vs placebo, -11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005; effect size 0.52). There were significantly greater improvements in GXR for ADHD-RS-IV inattention (-7.39 ± 0.79 vs -4.89 ± 0.76; P = .0032) and hyperactivity-impulsivity (-3.84 ± 0.54 vs -2.10 ± 0.52; P = .0021) subscale scores, CGI-I scores (48.1% vs 22.6%; P = .0007), and PGI-I scores (25.3% vs 11.8%; P = .0283). More patients in the GXR versus the placebo group reported TEAEs (81.2% vs 62.0%) and discontinued due to TEAEs (19.8% vs 3.0%). The main TEAEs in the GXR group were somnolence, thirst, blood pressure decrease, nasopharyngitis, postural dizziness, and constipation; most TEAEs were mild to moderate in severity.
Conclusions: In Japanese adults with ADHD, GXR improved ADHD symptoms without any major safety concerns.
Trial Registration: Japan Primary Registries Network (https://rctportal.niph.go.jp/en/): JapicCTI-163231
J Clin Psychiatry 2020;81(3):19m12979
To cite: Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979.
To share: https://doi.org/10.4088/JCP.19m12979
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Showa University School of Medicine, Tokyo, Japan
bAiiku Counselling Office, Aiiku Research Institute, Imperial Gift Foundation Boshi-Aiiku-Kai, Tokyo, Japan
cBiostatistics Center, Shionogi & Co, Ltd, Osaka, Japan
dClinical Research Department, Shionogi & Co, Ltd, Osaka, Japan
eJapan Developmental Disorders Network, Tokyo, Japan
*Corresponding author: Akira Iwanami, MD, PhD, Department of Psychiatry, Showa University School of Medicine, 6-11-11 Kita Karasuyama, Setagaya-ku, Tokyo, 157-8577, Japan ([email protected]).
Attention-deficit/hyperactivity disorder (ADHD), characterized by inattention, hyperactivity, and impulsivity, is one of the most common psychiatric disorders worldwide. Although childhood ADHD often persists through adolescence and into adulthood,1 a significant proportion of adults with ADHD have no childhood history of the disorder.2 Notably, the symptom presentation of childhood ADHD often differs from that of adult ADHD,3 potentially complicating the diagnosis. In childhood ADHD, symptoms of hyperactivity predominate, while in adult ADHD, symptoms of inattention predominate.1,4,5 Moreover, ADHD diagnosed in adulthood is often accompanied by psychiatric and/or medical comorbidities.6-9 Significant associations have been reported between ADHD and anxiety, mood, behavior (including suicidal behavior), and substance use disorders, as well as role impairments (including days out of role, impaired cognition, and social interactions).10-12 For these reasons, ADHD in adults is often underdiagnosed and undertreated,10 leading to lower quality of life,13-15 impaired social16,17 and professional functioning,18,19 susceptibility to addiction20 or mood disorders,21,22 unsafe behaviors,23 premature accidental death,24 and suicide.25,26
According to Japanese and global estimates, ADHD prevalence in adults is 1.7%27 and 1.2%-7.3%,10,28-32 respectively, and it is estimated that > 1.8 million Japanese adults very likely have ADHD.33 In Japan, ADHD significantly affects the health status and social and occupational functioning of adults with the disorder, imposing a substantial burden on patients, their family, and society.27 Thus, new treatment options are needed in Japan, as only osmotic-release oral system methylphenidate and atomoxetine are currently approved for adult ADHD.34
Guanfacine extended-release (GXR), a nonstimulant selective α2A-adrenergic receptor agonist, enhances noradrenergic activity in the dorsolateral-prefrontal cortex35 and has been demonstrated to improve ADHD symptoms in Japanese and non-Japanese children with ADHD.36-40 Although GXR monotherapy is approved for the treatment of pediatric ADHD, its efficacy and safety have not been investigated in adult ADHD. Therefore, it would be beneficial to study the effect of GXR in this patient population.
The objective of this randomized, double-blind, placebo-controlled trial was to assess the efficacy and safety of once-daily oral, dose-optimized GXR monotherapy (4-6 mg/d) in Japanese adults with ADHD. Specifically, the primary objective was to assess the efficacy of GXR on ADHD symptoms (assessed by the Japanese version of the ADHD-Rating Scale IV with adult prompts [ADHD-RS-IV]41,42) at week 10 compared with placebo.
METHODS
Study Design
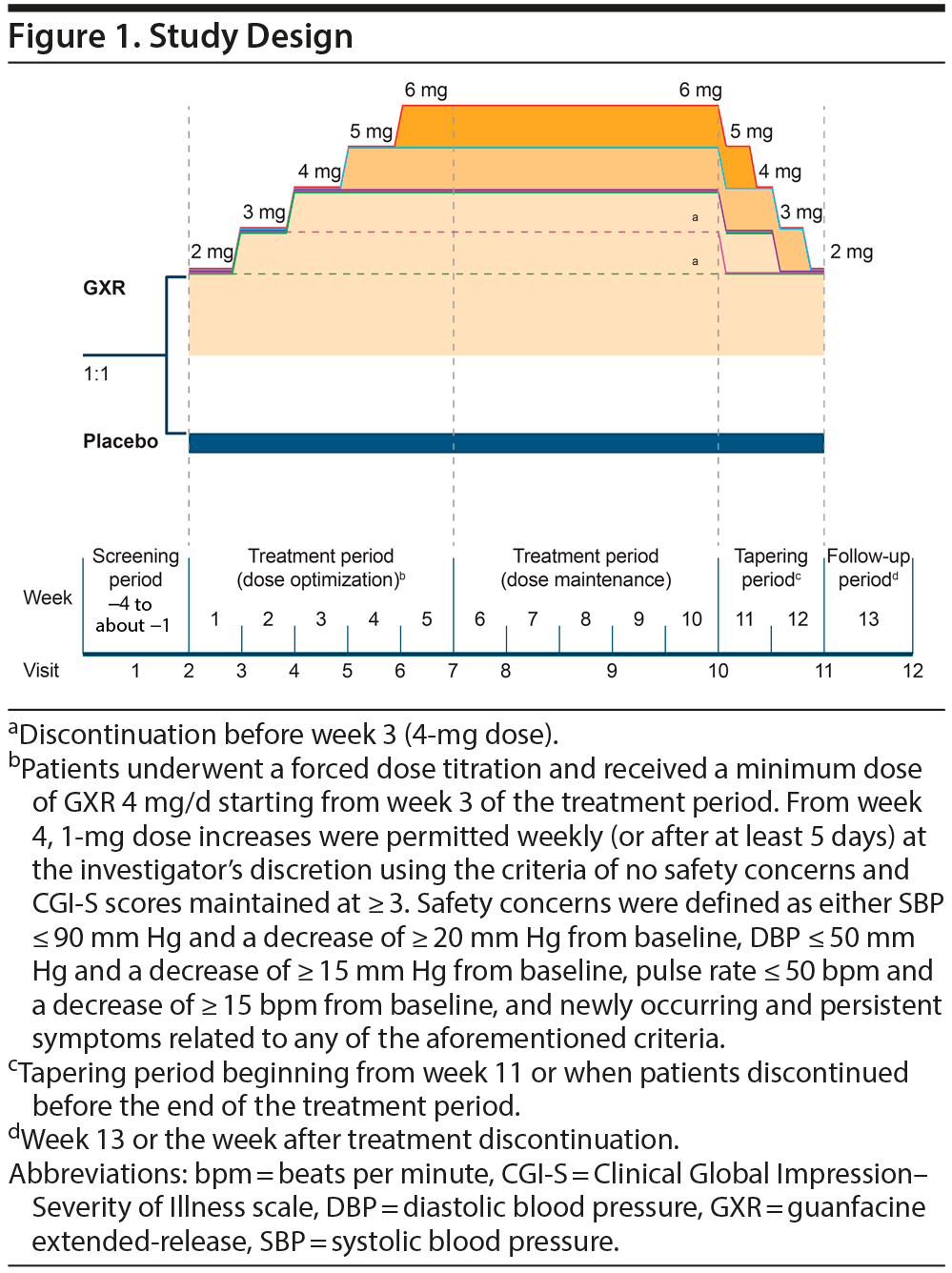
This phase 3, randomized, double-blind, placebo-controlled, dose-optimized trial compared GXR with placebo in adults with ADHD. The study was registered at Japan Primary Registries Network (identifier: JapicCTI-163231; https://rctportal.niph.go.jp/en/), approved by the local ethics committees, and conducted at 71 Japanese centers (October 2016-July 2017) in compliance with the Japanese Ethical Guideline for Clinical Studies and the Declaration of Helsinki. All patients provided written informed consent before participation.
Participants
Adults aged ≥ 18 years currently diagnosed with ADHD (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5])43 who had a total score ≥ 24 on the ADHD-RS-IV and a score ≥ 4 on the Clinical Global Impression-Severity of Illness scale (CGI-S) were eligible for inclusion.
CLINICAL POINTS
- Effective treatments for attention-deficit/hyperactivity disorder (ADHD) in adults have limitations.
- In adults with ADHD, guanfacine extended-release may be a suitable treatment option that offers improvements in core ADHD symptoms, clinician- and patient-rated health improvements, and some aspects of executive function and quality of life, in addition to having a known safety profile.
Patients were excluded if they had a diagnosis (based on DSM-5) of schizophrenia, bipolar disorder, personality disorder, or mental retardation; a documented moderate or severe psychiatric disorder (based on DSM-5) necessitating treatment (excluding counseling); documented DSM-5-defined anxiety/depression; a history of DSM-5-defined substance use disorder or seizures; or a diagnosis of a serious tic disorder (including DSM-5-defined Tourette disorder) or if they were considered at suicide risk. Additionally, patients with evidence or a history of cardiovascular disease (prolonged corrected QT interval [QTc; Fridericia-adjusted, > 450 msec], orthostatic hypotension, continuous bradycardia, abnormal electrocardiogram [ECG] or laboratory values at screening) or patients requiring medications affecting blood pressure or cardiac rate at or after enrollment were excluded.
Randomization
Eligible patients were randomized 1:1 to GXR or placebo. Computer-generated randomization codes were maintained in a sealed envelope by the study drug assignee. Equal randomization to treatment group was ensured by stratifying patients by enrollment center (by a stochastic minimization method) according to ADHD presentation (based on DSM-5 codes 314.01 [F90.2], 314.00 [F90.0], and 314.01 [F90.1]) and ADHD-RS-IV total score at baseline (< 30 or ≥ 30). The cutoff selected was based on the baseline ADHD-RS-IV total score for Japanese children with ADHD.36
Blinding
GXR and placebo tablets were indistinguishable from each other. Patients, investigators, and all study personnel remained blinded to the randomized treatment assignments until database lock.
Interventions
Selected GXR doses were based on adolescent studies and the assumption that weight-based dosing was not required in adults. The 4-mg minimum maintenance dose was selected as it was deemed sufficient to achieve an effect in Japanese patients ≤ 100 kg.
The study comprised 4 periods: screening (1-4 weeks), treatment (optimization [5 weeks], maintenance [5 weeks]), tapering (2 weeks), and follow-up (1 week). Patients received GXR or placebo once daily as a single dose at approximately the same time (either morning or afternoon). Dose titrations are described in Figure 1. Safety was assessed during the follow-up period (week 13 or the week after treatment discontinuation).
Efficacy Outcome Measures
Efficacy outcomes were assessed by the investigator using the validated Japanese version of the ADHD-RS-IV42,44 and translations of the English-language Conners’ Adult ADHD Rating Scale (CAARS),45 CGI-Improvement scale (CGI-I),46 and CGI-S scale.46 Efficacy outcomes were assessed by the patients using the Patient Global Impression-Improvement scale (PGI-I),46 Adult ADHD Quality of Life Questionnaire (AAQoL),47,48 and Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A).49 The ADHD-RS-IV was administered at every visit from screening to week 10 or discontinuation. The CGI-I, CGI-S, and PGI-I were administered at every visit from week 1 to week 10 or discontinuation. The CAARS was administered at baseline and week 10 (or discontinuation). The AAQoL and BRIEF-A were administered at baseline, week 5, and week 10 (or discontinuation).
The primary endpoint was change from baseline in ADHD-RS-IV total score at week 10. Secondary endpoints included change from baseline in ADHD-RS-IV subscale, CAARS, AAQoL, and BRIEF-A scores at all time points to week 10 and last observation (including discontinuation for all scores except CAARS, or last evaluation point for CAARS). Additionally, illness severity rate (percentage of patients with assessment of normal or borderline mentally ill) for the CGI-S and improvement rate (percentage of patients very much or much improved) for the CGI-I and PGI-I were calculated at all time points to week 10 and last observation (including discontinuation).
Safety Outcome Measures
Type and frequency of treatment-emergent adverse events (TEAEs; coded using the Medical Dictionary for Regulatory Activities, Version 19.0), serious adverse events, and vital signs (including pulse rate and systolic/diastolic blood pressure) were assessed during the treatment period. Body weight, ECG parameters, and clinical laboratory test values were recorded at baseline; weeks 5, 10, and 12; or discontinuation. Change from baseline to weeks 5, 10, and 12 in body weight, vital signs, and ECG parameters was assessed.
Sample Size
Sample size was calculated using the effect size of the change from baseline in ADHD-RS-IV total score for GXR compared with placebo as reported in studies of children and adolescents with ADHD. Assuming an effect size of 0.50 for GXR versus placebo, 86 randomized patients per study arm would provide ≥ 90% power by a 2-sample t test with a 2-sided significance level of .05. Moreover, assuming that 10% of randomized patients discontinued during treatment, the target sample size per study arm was determined to be 95 patients.
Statistical Analysis
Efficacy analyses were conducted on the full analysis set (FAS), defined as all randomized patients who received ≥ 1 dose of study drug and had ≥ 1 ADHD-RS-IV value (at baseline or after study drug administration). Safety analyses were conducted on the safety analysis set (SAS), defined as all patients who received ≥ 1 dose of the study drug and had ≥ 1 value for safety endpoints.
Patient background and baseline characteristics were summarized for each treatment group with descriptive statistics. Homogeneity across treatment groups was assessed using the Welch t test for baseline value of ADHD-RS-IV or Fisher exact test for other assessments (2-sided significance was .15). The primary endpoint was analyzed using a mixed model for repeated measures (MMRM) method that included treatment group, time point, and interaction effect (treatment group and time point) as a fixed effect and baseline ADHD-RS-IV total score (< 30, ≥ 30) and ADHD presentation as a covariate in the FAS population. The effect size at week 10 was calculated using the between-group difference obtained by MMRM and the estimates of variance at week 10 produced by the model.
Secondary efficacy endpoints were analyzed using the same MMRM method with the respective baseline parameter as an alternate covariate. CAARS scores were analyzed by analysis of covariance (ANCOVA), with missing data imputed using last observation carried forward (fixed effect: treatment group; covariates: baseline score on the inattention/memory problem subscale and ADHD presentation). Total score and other subscale scores were calculated in the same manner. Illness severity and improvement (CGI-S, CGI-I, or PGI-I) rates between groups were compared using Fisher exact test and 2-sided P values.
For MMRM or ANCOVA, the least squares (LS) mean, difference between treatment groups in LS means, and its 95% CI and P value were calculated; the effect size was also calculated for the primary endpoint.
All TEAEs between the first intake of study drug and follow-up observation were analyzed. Changes from baseline in weight, vital signs, and ECG parameters were presented using descriptive statistics. No analyses for multiplicity due to multiple endpoints or time points were performed. Statistical analyses were performed using SAS v9.2 (2008; SAS Institute Inc; Cary, North Carolina).
RESULTS
Patient Disposition and Demographic and Baseline Clinical Characteristics
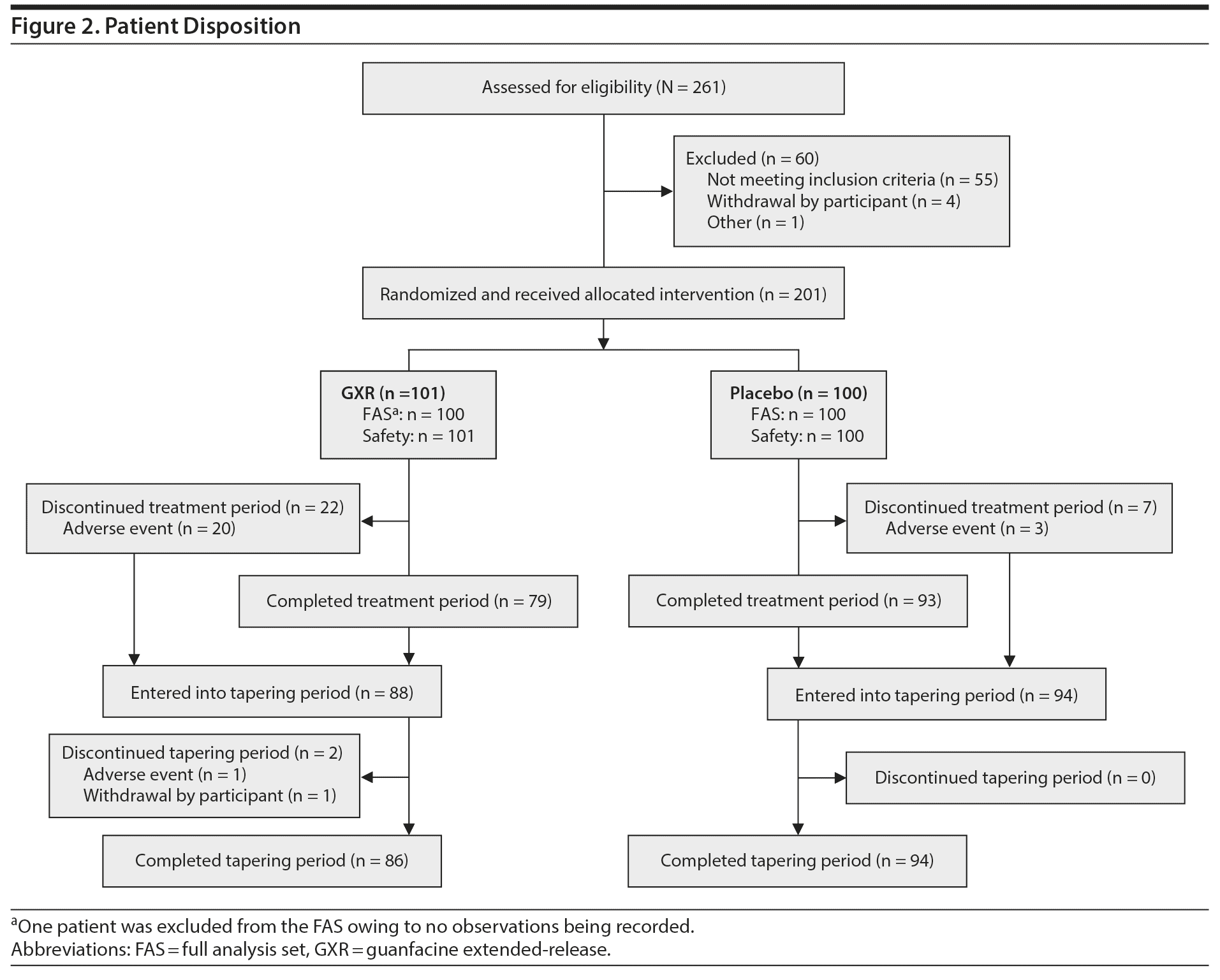
Of 261 patients screened, 201 were randomized to the treatment period (Figure 2). All randomized patients received study drug (SAS: GXR, n = 101, placebo, n = 100); however, 1 patient was excluded from the FAS population owing to no observations being recorded (FAS: GXR, n = 100, placebo, n = 100). The mean dose of GXR during the maintenance phase was 5.07 mg (4 mg, 27 patients; 5 mg, 23 patients; and 6 mg, 33 patients).
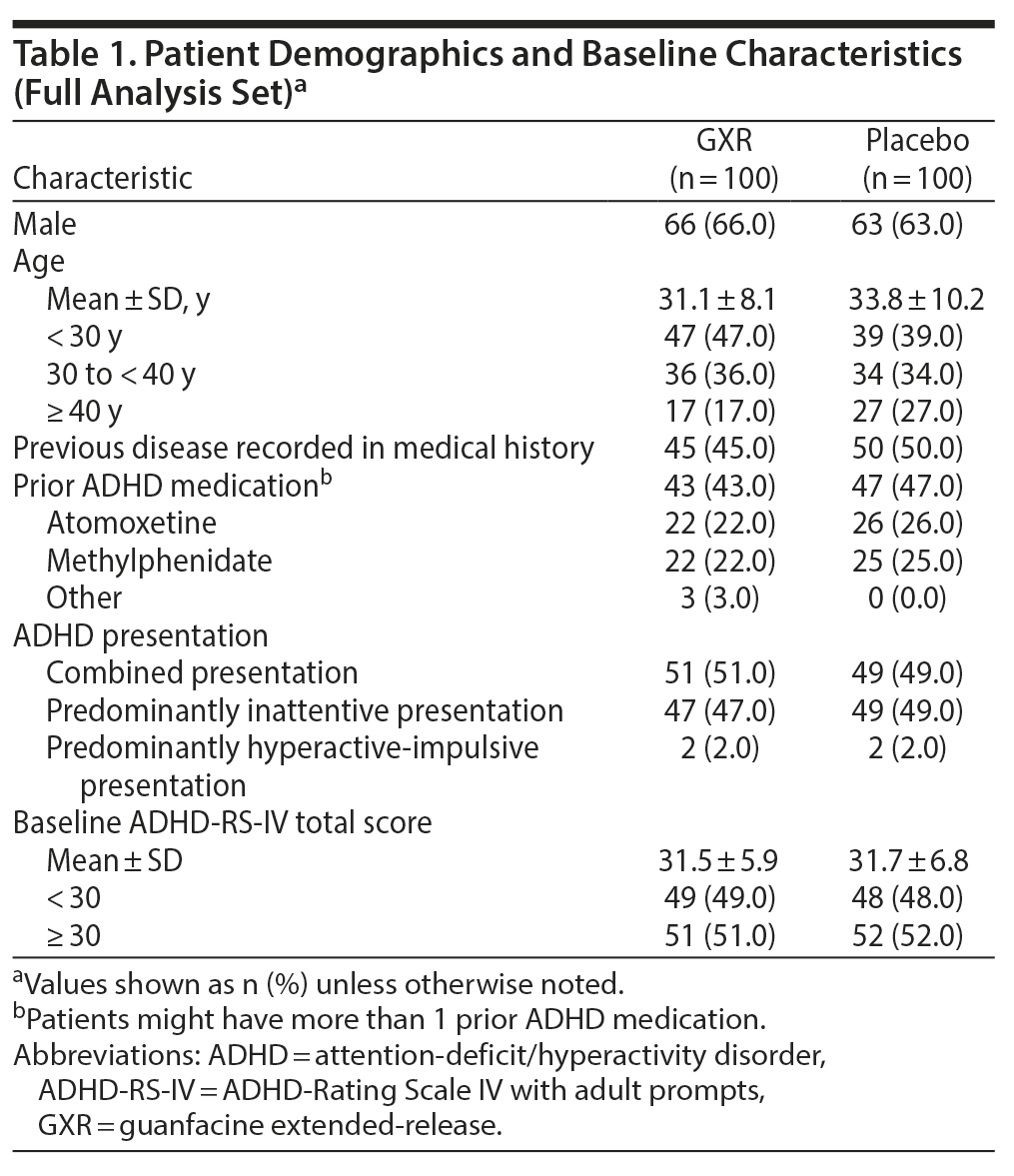
Demographic and baseline clinical characteristics were generally balanced between treatment groups (Table 1). About two-thirds of patients were male (66% vs 63% for GXR vs placebo, respectively); the mean age was 31.1 vs 33.8 years, respectively. However, there was an imbalance in age distribution between the groups. More patients were aged < 30 years (47% vs 39%) and fewer were aged ≥ 40 years (17% vs 27%) in the GXR group compared with the placebo group. About half of the patients in each treatment group had combined presentation, and nearly half in each group had predominantly inattentive presentation (combined, 51% vs 49%; predominantly inattentive, 47% vs 49%). At baseline, the mean ADHD-RS-IV total score was similar between treatment groups (31.5 vs 31.7, respectively, for GXR vs placebo).
Efficacy Measures
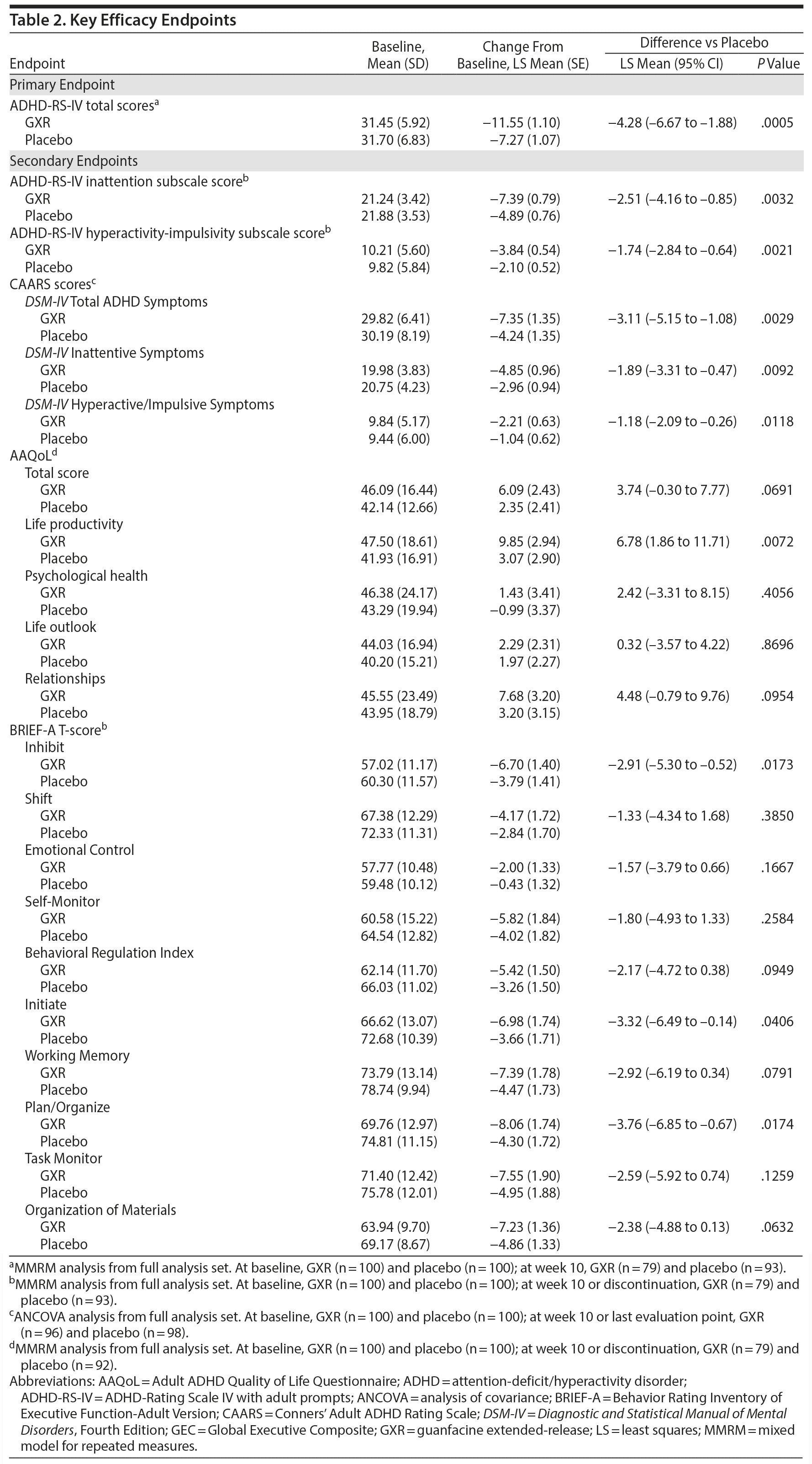
There was a significant improvement in ADHD symptoms in patients treated with GXR. At week 10, the LS mean ± SE change from baseline in ADHD-RS-IV total score was significantly greater with GXR (-11.55 ± 1.10) than with placebo (-7.27 ± 1.07); (LS mean difference -4.28; 95% CI, -6.67 to -1.88, P = .0005) (Table 2). This corresponded to an effect size of 0.52 at week 10. The greater decrease in ADHD-RS-IV total score with GXR occurred early and was sustained from weeks 4-10 of the treatment period (P < .005; Supplementary Figure 1). Regarding the ADHD-RS-IV subscale scores, treatment with GXR significantly improved symptoms of inattention and hyperactivity-impulsivity (P < .005; Table 2). These effects were sustained from week 4 (inattention) or week 5 (hyperactivity-impulsivity) to week 10 of the treatment period (P < .05, Supplementary Figure 1).
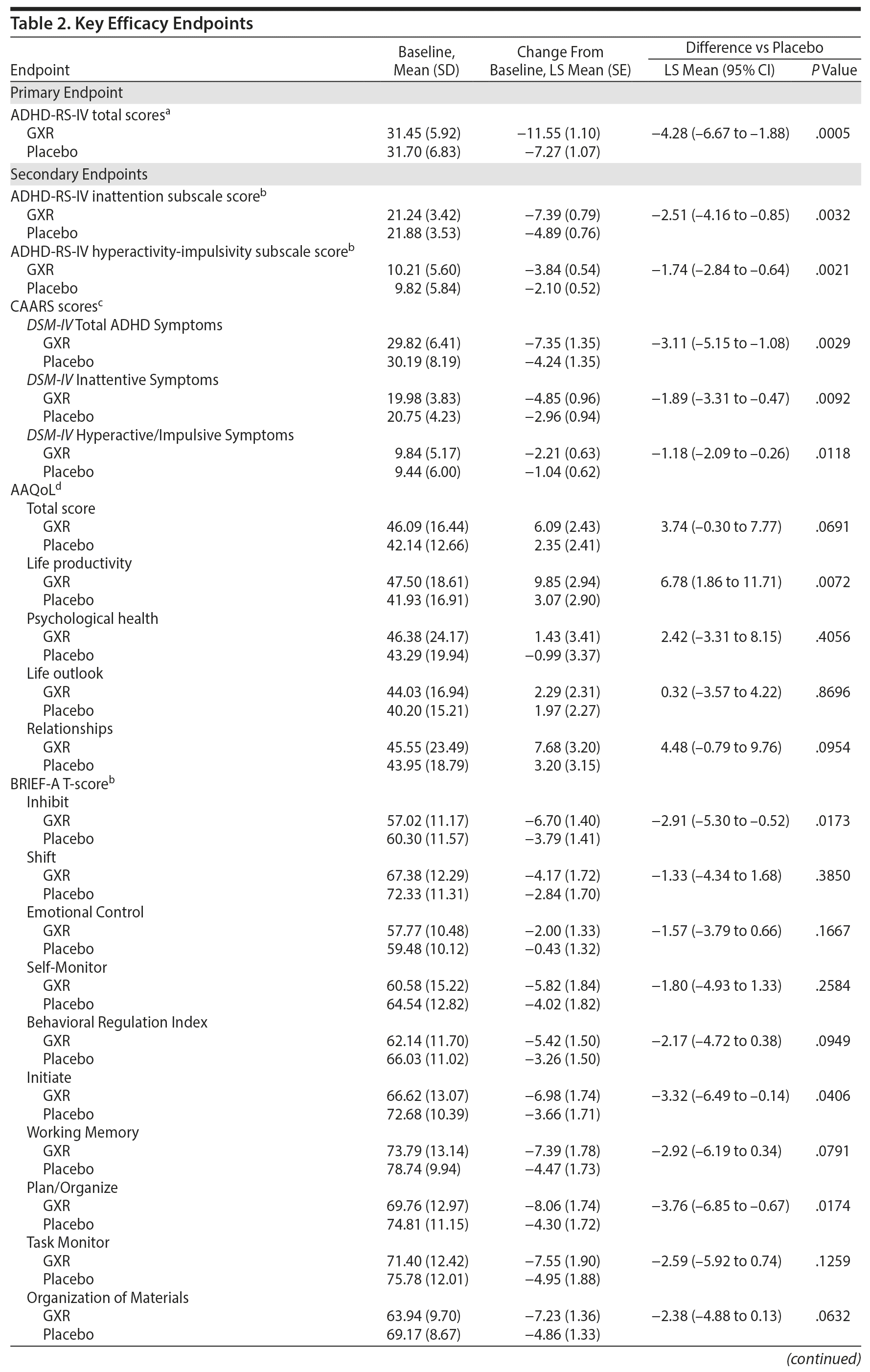
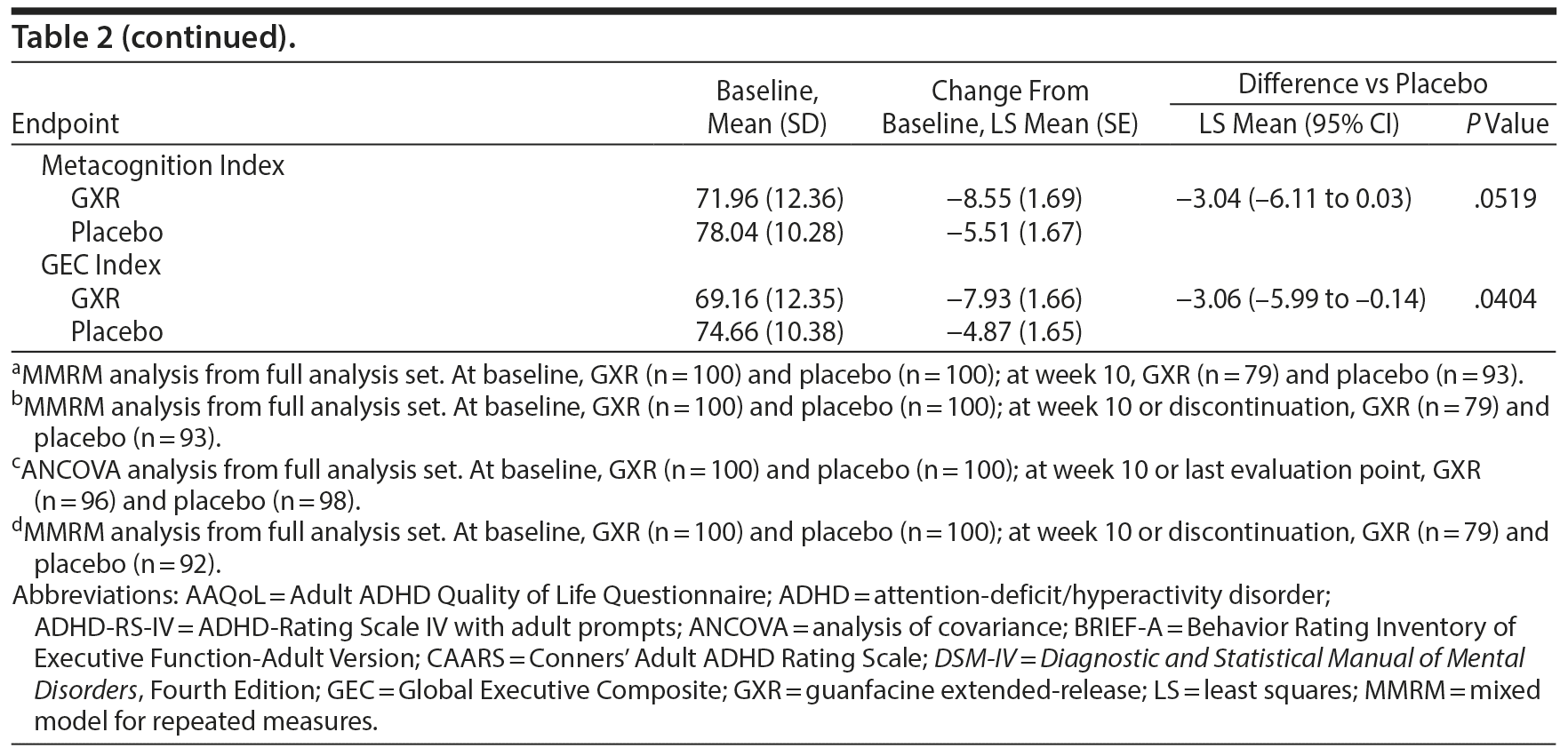
The benefits of GXR were further supported by assessments using the CAARS. Treatment with GXR significantly reduced all CAARS subscale scores (DSM-IV Total ADHD Symptoms, DSM-IV Inattentive Symptoms, and DSM-IV Hyperactive/Impulsive Symptoms) at week 10 (P < .05; Table 2).
At week 10, the improvement rate was significantly greater for GXR compared with placebo for CGI-I scores (48.1% vs 22.6%, P = .0007) and PGI-I scores (25.3% vs 11.8%, P = .0283). The improvements were statistically significant for CGI-I scores between weeks 4 and 10 and for PGI-I scores at weeks 6, 10, and last observation (data not shown). In contrast, the illness severity rate (per CGI-S scores) was similar at week 10 (3.8% vs 4.3%, P = 1.0000) and at all time points for GXR and placebo.
Some, but not all, aspects of patient-reported quality of life and executive functioning improved with GXR treatment. Compared with placebo, GXR increased the AAQoL total score and all subscale scores at week 10 (Table 2). Differences between treatment groups were significant for life productivity subscale scores (P = .0072; Table 2). Compared with placebo, GXR decreased (improved) all BRIEF-A subscale T-scores at week 10 (Table 2). Differences between treatment groups were significant for the Inhibit, Initiate, Plan/Organize, and Global Executive Composite Index subscales (P < .05; Table 2).
Safety Measures
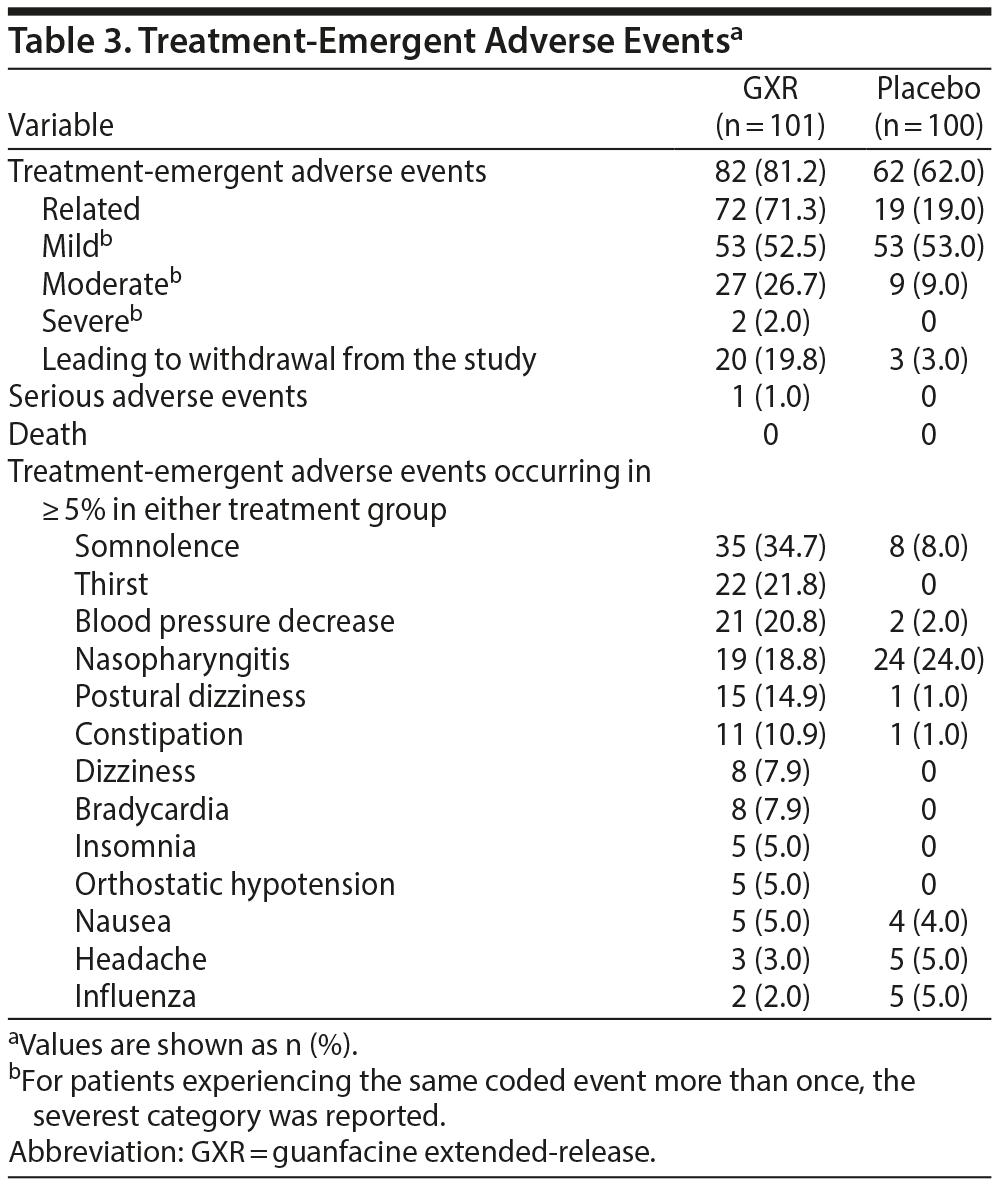
Over the 12 weeks of treatment, the overall incidences of TEAEs, drug-related TEAEs, and TEAEs leading to discontinuation were higher for GXR compared with placebo (Table 3), and most events were mild or moderate. One serious TEAE (suicide attempt occurring during the dose-optimization period, with recovery after 5 days, unrelated to the study drug), and no deaths were reported. The most common TEAEs with an incidence ≥ 10% in either group were somnolence, thirst, blood pressure decrease, nasopharyngitis, postural dizziness, and constipation; except for nasopharyngitis, most of these TEAEs were considered to be related to the study drug. Regarding somnolence events, many of them occurred within the first week of the treatment period; most were mild and resolved during treatment. More patients receiving GXR compared with placebo discontinued treatment owing to TEAEs (19.8% vs 3.0%; Table 3). The main TEAEs resulting in discontinuation from the GXR group were blood pressure decrease and somnolence; all of these events occurred during the dose-optimization period.
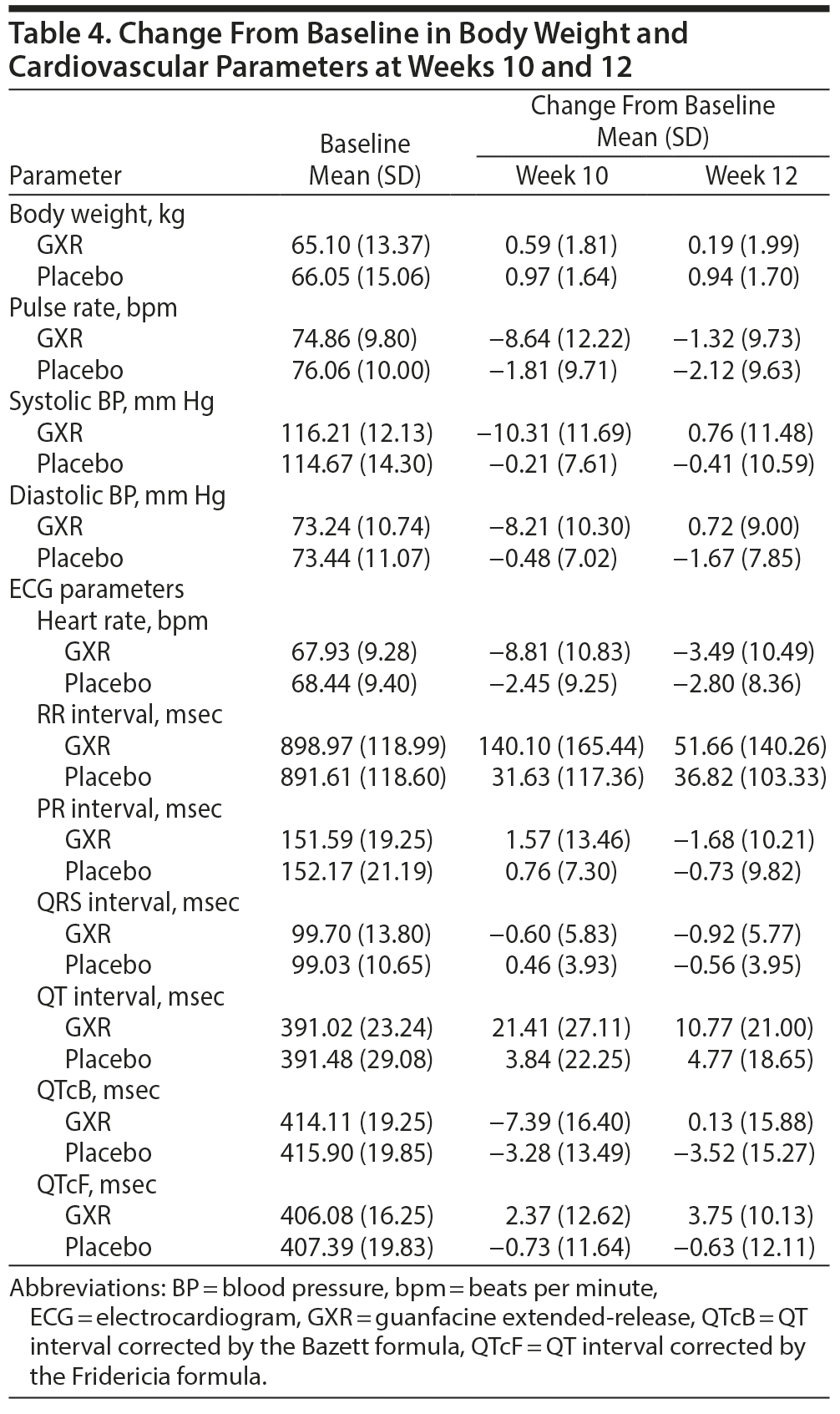
Changes from baseline in blood pressure, pulse rate, and some ECG parameters may have been affected by the pharmacologic effects of GXR, an α2A-adrenergic receptor agonist. Compared with placebo, pulse/heart rate, blood pressure, and QTc corrected by the Bazett formula were decreased with GXR at week 10 and mostly returned to baseline levels by week 12. Compared with placebo, QT and RR intervals increased with GXR at week 10 and mostly returned to baseline levels by week 12 (Table 4). Additionally, slight changes from baseline in QTc corrected by the Fridericia formula were observed for GXR at weeks 10 and 12. At last observation, 2 patients receiving GXR had abnormal ECG readings (sinus bradycardia, QT-prolonged), which were both reported as TEAEs.
DISCUSSION
This randomized placebo-controlled trial is the first to demonstrate the efficacy and safety of dose-optimized monotherapy with GXR in adults with ADHD. The superiority of GXR compared with placebo was demonstrated for ADHD symptoms as measured by the ADHD-RS-IV and CAARS. Furthermore, the improvement in ADHD symptoms was associated with physician- and patient-rated clinical improvement (CGI-I and PGI-I) as well as patient-rated quality of life (life productivity on the AAQoL) and executive functioning (some BRIEF-A subscales). The most commonly observed TEAEs were mild to moderate in severity and consistent with the known safety profile of GXR. The favorable benefit-risk profile of GXR in the treatment of ADHD was established in pediatric patients, and these results support its profile in adult patients.
Currently, GXR is approved for the treatment of children with ADHD in 36 countries, including Japan. In contrast to all other ADHD medications,50 our study expands the current evidence for GXR as a treatment for ADHD in pediatric patients and demonstrates that GXR was equally efficacious in adult and pediatric patients.51 Similar to findings observed in the pediatric population, dose-optimized GXR improved multiple facets of ADHD in adults. Additionally, the effect size by ADHD-RS-IV total score in our study (0.52) was similar to the mean effect size observed in studies with pediatric patients (0.43-0.86)51 and adult patients receiving nonstimulants (0.51)51 and was lower than that observed in studies with adult patients receiving stimulants (0.89).52 Given the multidimensional impact of ADHD on an adult’s daily life, the observed benefits on not only ADHD symptoms but also general health status, life productivity, and some executive functioning subscale scores are an important finding. However, not all differences in BRIEF-A scores between GXR and placebo groups were statistically significant, potentially because the study was not sufficiently powered to verify these endpoints. Additional studies are needed to further investigate the effects of GXR on patient-reported executive function.
Although the incidence of TEAEs and the discontinuation rate due to TEAEs in this study were higher than those in most pediatric studies with GXR,36-40 the severity and absence of major safety concerns were consistent with the known safety profile of GXR in Japanese and non-Japanese children with ADHD.36-40 The higher incidence of thirst events in this study (21.8%) compared with that in previous Japanese pediatric studies (< 3% in the 0.12-mg/kg group in the double-blind study36 and < 2% in the long-term extension study37) was noted. Discontinuations in the GXR group during the dose-optimization period were mainly due to blood pressure decrease or somnolence and may have been related to the forced dose titration during this period (Figure 1). Guanfacine is a selective α2A-adrenergic receptor agonist, and its pharmacologic action may cause somnolence and blood pressure reductions. Consistent with previous reports in children and adolescents,39,53 most sedative effects were transitory and mostly occurred during the first week of treatment (optimization period), the severity of somnolence was mild to moderate, and no tolerability issues were identified. Furthermore, the ADHD-RS-IV inattention subscale scores improved significantly in the GXR group compared with the placebo group despite the increased incidence of somnolence. This finding would suggest that the increased incidence in sedation-related adverse events did not lead to increased functional impairment. Similarly, although systolic and diastolic blood pressure reductions were observed, the severity was mild to moderate and there were no issues with tolerability. Therefore, monitoring of TEAEs at initiation and dose increases of GXR may be recommended in clinical practice.
Strengths of the study include its randomized, double-blind, placebo-controlled design and the inclusion of multiple physician- and patient-specific rating instruments. Additionally, the flexible-dosing regimen allowed for individualization of the treatment dose and the potential balancing of side effects with efficacy benefits. Limitations of the study include the short duration of treatment, exclusion of patients with psychiatric or cardiovascular comorbidities, the imbalance in age distribution, the relatively large proportion of men, and possible lack of generalizability of the study to non-Japanese populations. Long-term prospective studies in a real-life clinical setting and in different races are required to confirm the efficacy and safety profile of GXR in adult ADHD treatment.
In conclusion, in Japanese adults with ADHD, 10 weeks of monotherapy with GXR improved core symptoms, physician- and patient-rated clinical impression, and some aspects of executive function and quality of life and reflected the known safety profile of GXR. Additional investigation of GXR in adults with ADHD may be needed to further support the benefits observed in this study.
Submitted: July 1, 2019; accepted November 5, 2019.
Published online: April 14, 2020.
Author contributions: Drs Iwanami and Saito were coordinating investigators in the study. Dr Ichikawa was an expert medical advisor for the study.
Potential conflicts of interest: Dr Fujiwara and Mr Okutsu are employees of and own shares in Shionogi & Co, Ltd. Dr Iwanami has received honoraria and other payments from Eli Lilly Japan, Eisai, Janssen, Kyowa, Meiji Seika, Mitsubishi Tanabe, MSD, Otsuka, Pfizer Seiyaku, Sumitomo Dainippon, and Yoshitomiyakuhin. Dr Saito has received honoraria and other payments from Eli Lilly Japan, Hisamitsu, Janssen, Meiji Seika, Mitsubishi Tanabe, Otsuka, Shionogi & Co, Shire Japan (now part of the Takeda group of companies), Sumitomo Dainippon, Taisho, and Yoshitomiyakuhin. Dr Ichikawa has received honoraria and other payments from AbbVie, Eli Lilly Japan, Hisamitsu, Janssen, Meiji Seika, Otsuka, Shionogi & Co, and Taisho.
Funding/support: This study was funded by Shire International GmbH (manufacturer/licensee of Intuniv), a member of the Takeda group of companies, and Shionogi & Co, Ltd.
Role of the sponsor: Shionogi & Co, Ltd was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Previous presentation: Posters presented at the 7th World Congress on ADHD; April 25-28, 2019; Lisbon, Portugal ▪ the 49th Annual Meeting of the Japanese Society of Neuropsychopharmacology; October 12-13, 2019; Fukuoka, Japan
Acknowledgments: The authors would like to thank Brigitte Robertson, MD, and Ming Yu, MD, Shire HGT, Inc (Lexington, Massachusetts), a member of the Takeda group of companies, for their scientific contributions to the trial and scientific review of the manuscript. Drs Robertson and Yu are employees of Shire HGT, Inc, a member of the Takeda group of companies, and own stock in the company. The authors would also like to thank all study participants. Medical writing assistance was provided by Thao Le, MD, PhD, and Tania Dickson, PhD, CMPP, of ProScribe—Envision Pharma Group, and was funded by Shionogi & Co, Ltd. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Additional information: Guanfacine extended-release is approved for the treatment of ADHD in adults in Japan and in children (< 18 years of age) throughout the world. Researchers can request access to detailed information about Shionogi’s clinical trials, including trial protocols and individual patient data, on the portal site: www.clinicalstudydatarequest.com. Sharable information includes data about Shionogi’s clinical trials conducted in patients in Japan. The information will become sharable after the medicinal products for which the trials are performed have been approved in Japan. Note that all documents will be provided in Japanese language only as they have been prepared in Japanese.
Supplementary material: See accompanying pages.
REFERENCES
1.Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10(1):67. PubMed CrossRef
2.Moffitt TE, Houts R, Asherson P, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? evidence from a four-decade longitudinal cohort study. Am J Psychiatry. 2015;172(10):967-977. PubMed CrossRef
3.Caye A, Rocha TB, Anselmi L, et al. Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry. 2016;73(7):705-712. PubMed CrossRef
4.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165. PubMed CrossRef
5.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816-818. PubMed CrossRef
6.Pi×±eiro-Dieguez B, Balanzá-Mart×nez V, Garc×a-Garc×a P, et al; CAT Study Group. Psychiatric comorbidity at the time of diagnosis in adults with ADHD: the CAT study. J Atten Disord. 2016;20(12):1066-1075. PubMed CrossRef
7.Instanes JT, Klungs׸yr K, Halm׸y A, et al. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. 2018;22(3):203-228. PubMed CrossRef
8.Imagawa H, Nagar SP, Montgomery W, et al. Treatment patterns, health care resource utilization, and costs in Japanese adults with attention-deficit hyperactivity disorder treated with atomoxetine. Neuropsychiatr Dis Treat. 2018;14:611-621. PubMed CrossRef
9.Clemow DB, Bushe C, Mancini M, et al. A review of the efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in children and adult patients with common comorbidities. Neuropsychiatr Dis Treat. 2017;13:357-371. PubMed CrossRef
10.Fayyad J, Sampson NA, Hwang I, et al; WHO World Mental Health Survey Collaborators. The descriptive epidemiology of DSM-IV adult ADHD in the World Health Organization World Mental Health Surveys. Atten Defic Hyperact Disord. 2017;9(1):47-65. PubMed CrossRef
11.Stickley A, Koyanagi A, Ruchkin V, et al. Attention-deficit/hyperactivity disorder symptoms and suicide ideation and attempts: findings from the Adult Psychiatric Morbidity Survey 2007. J Affect Disord. 2016;189:321-328. PubMed CrossRef
12.Stickley A, Tachimori H, Inoue Y, et al. Attention-deficit/hyperactivity disorder symptoms and suicidal behavior in adult psychiatric outpatients. Psychiatry Clin Neurosci. 2018;72(9):713-722. PubMed CrossRef
13.Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit hyperactivity disorder: a systematic review. Innov Clin Neurosci. 2012;9(5-6):10-21. PubMed
14.Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord. 2008;11(6):720-727. PubMed CrossRef
15.Mattos P, Louz×£ MR, Palmini AL, et al. A multicenter, open-label trial to evaluate the quality of life in adults with ADHD treated with long-acting methylphenidate (OROS MPH): Concerta Quality of Life (CONQoL) study. J Atten Disord. 2013;17(5):444-448. PubMed CrossRef
16.Seo JY, Lee CS, Park CS, et al. Mediating effect of depressive symptoms on the relationship between adult attention deficit hyperactivity disorder and quality of life. Psychiatry Investig. 2014;11(2):131-136. PubMed CrossRef
17.Minde K, Eakin L, Hechtman L, et al. The psychosocial functioning of children and spouses of adults with ADHD. J Child Psychol Psychiatry. 2003;44(4):637-646. PubMed CrossRef
18.Halm׸y A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187. PubMed CrossRef
19.Fredriksen M, Dahl AA, Martinsen EW, et al. Childhood and persistent ADHD symptoms associated with educational failure and long-term occupational disability in adult ADHD. Atten Defic Hyperact Disord. 2014;6(2):87-99. PubMed CrossRef
20.Capusan AJ, Bendtsen P, Marteinsdottir I, et al. Comorbidity of adult ADHD and its subtypes with substance use disorder in a large population-based epidemiological study. J Atten Disord. 2019;23(12):1416-1426. PubMed CrossRef
21.Uchida M, Spencer TJ, Faraone SV, et al. Adult outcome of ADHD: an overview of results from the MGH longitudinal family studies of pediatrically and psychiatrically referred youth with and without ADHD of both sexes. J Atten Disord. 2018;22(6):523-534. PubMed CrossRef
22.Rucklidge JJ, Downs-Woolley M, Taylor M, et al. Psychiatric comorbidities in a New Zealand sample of adults with ADHD. J Atten Disord. 2016;20(12):1030-1038. PubMed CrossRef
23.Fuermaier AB, Tucha L, Evans BL, et al. Driving and attention deficit hyperactivity disorder. J Neural Transm (Vienna). 2017;124(suppl 1):55-67. PubMed CrossRef
24.Dalsgaard S, טstergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196. PubMed CrossRef
25.Furczyk K, Thome J. Adult ADHD and suicide. Atten Defic Hyperact Disord. 2014;6(3):153-158. PubMed CrossRef
26.Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644. PubMed CrossRef
27.Nakamura S, Ohnishi M, Uchiyama S. Epidemiological survey of adult attention deficit hyperactivity disorder (ADHD) in Japan. Jpn J Psychiatr Treat. 2013;28:155-162.
28.Faraone SV, Biederman J. What is the prevalence of adult ADHD? results of a population screen of 966 adults. J Atten Disord. 2005;9(2):384-391. PubMed CrossRef
29.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723. PubMed CrossRef
30.Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190(5):402-409. PubMed CrossRef
31.Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211. PubMed CrossRef
32.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490-499. PubMed CrossRef
33.Population estimation (as of October 1, 2017)—Nationwide: Age (each age), population by gender—Prefectures: Age (5 years old), population by gender. Statistics Bureau, Ministry of Internal Affairs and Communications website. http://www.stat.go.jp/data/jinsui/2017np/index.html. Accessed December 3, 2018.
34.Matsuo Y, Okita M, Ermer J, et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of guanfacine extended-release formulation in healthy Japanese and Caucasian male adults. Clin Drug Investig. 2017;37(8):745-753. PubMed CrossRef
35.Arnsten AF, Jin LE. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J Biol Med. 2012;85(1):45-58. PubMed
36.Ichikawa H, Miyajima T, Yamashita Y, et al. Efficacy and safety of guanfacine hydrochloride extended-release tablet for children and adolescents with ADHD: a phase 2/3 placebo-controlled, double-blind study in Japan. Jpn J Clin Psychopharmacol. 2018;21(8):1093-1117.
37.Ichikawa H, Miyajima T, Yamashita Y, et al. Long-term safety and efficacy of guanfacine hydrochloride extended-release tablet for children and adolescents with ADHD: a phase 2/3 long-term, open-label extension study in Japan. Jpn J Clin Psychopharmacol. 2018;21(12):1645-1661.
38.Hervas A, Huss M, Johnson M, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. Eur Neuropsychopharmacol. 2014;24(12):1861-1872. PubMed CrossRef
39.Huss M, Dirks B, Gu J, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2018;27(10):1283-1294. PubMed CrossRef
40.Wilens TE, Robertson B, Sikirica V, et al. A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(11):916-25.e2. PubMed CrossRef
41.Adler L, Spencer T, Biederman J, et al. The internal consistency and validity of the attention-deficit/hyperactivity disorder rating scale (ADHD-RS) with adult ADHD prompts as assessed during a clinical treatment trial. J ADHD Relat Disord. 2009;1(1):14-24.
42.Ichikawa H, Saito K, Saito T, et al. Reliability and validity of the ADHD-RS-IV with adult prompts: the Japanese version. Clin Psychiatry. 2018;60(4):399-409.
43.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
44.DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press; 1998.
45.Conners K, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). New York, NY: Multi-Health Systems; 1999.
46.Guy W. Clinical global impressions. In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976:218-222.
47.Brod M, Johnston J, Able S, et al. Validation of the Adult Attention-Deficit/Hyperactivity Disorder Quality-of-Life Scale (AAQoL): a disease-specific quality-of-life measure. Qual Life Res. 2006;15(1):117-129. PubMed CrossRef
48.Matza LS, Johnston JA, Faries DE, et al. Responsiveness of the adult attention-deficit/hyperactivity disorder quality of life scale (AAQoL). Qual Life Res. 2007;16(9):1511-1520. PubMed CrossRef
49.Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function-Adult Version. Lutz, FL: Psychological Assessment Resources; 2005.
50.Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738. PubMed CrossRef
51.Huss M, Chen W, Ludolph AG. Guanfacine extended release: a new pharmacological treatment option in Europe. Clin Drug Investig. 2016;36(1):1-25. PubMed CrossRef
52.Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763. PubMed CrossRef
53.Sallee FR, Lyne A, Wigal T, et al. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):215-226. PubMed CrossRef
This PDF is free for all visitors!