Objective: This was an analysis of the effect of pimavanserin, a 5-hydroxytryptamine-2A antagonist and inverse receptor agonist, on dysregulated sleep in patients with major depressive disorder (MDD) by DSM-5 criteria and an inadequate antidepressant response.
Methods: For this analysis of CLARITY, a phase 2 study of adjunctive pimavanserin (N = 207) conducted between December 2016 and October 2018, sleep/wakefulness disturbances were measured with the 17-item Hamilton Depression Rating Scale (HDRS17) insomnia items (sum of items 4, 5, and 6) and the Karolinska Sleepiness Scale (KSS). Outcomes included change from baseline in HDRS17 insomnia factor score and KSS score, correlation between the HDRS17 insomnia factor score and KSS score, and change from baseline in the Sheehan Disability Scale (SDS) total score and Unproductive Days subscore in patients with a baseline KSS score ≥ 6.
Results: At baseline, HDRS17 insomnia factor score ≥ 3 occurred in 76% of patients receiving placebo and 85% of patients receiving pimavanserin. The overall least squares (LS) mean weighted difference (SE) was −0.5 (0.32) with a 95% CI of −1.2 to 0.1 (P = .088) at week 5. Improvement was observed with pimavanserin versus placebo at weeks 2, 3, and 4, with effect sizes (ESs) of 0.370 to 0.524 (P < .05). For KSS score, the LS mean difference (SE) at week 5 was −1.1 (0.30) (95% CI, −1.7 to −0.5; P = .0003; ES = 0.627) for pimavanserin versus placebo. Among those with a KSS score ≥ 6 at baseline (n = 120 placebo and n = 42 pimavanserin), the LS mean difference (SE) in the mean SDS score at week 5 was −1.1 (0.46) (95% CI, −2.0 to −0.2; P = .019; ES = 0.442) for pimavanserin versus placebo.
Conclusions: Adjunctive pimavanserin significantly improved sleep/wakefulness disturbance during treatment of MDD, an improvement that was associated with greater improvement in function.
Trial Registration: ClinicalTrials.gov identifier: NCT03018340
ABSTRACT
Objective: This was an analysis of the effect of pimavanserin, a 5-hydroxytryptamine-2A antagonist and inverse receptor agonist, on dysregulated sleep in patients with major depressive disorder (MDD) by DSM-5 criteria and an inadequate antidepressant response.
Methods: For this analysis of CLARITY, a phase 2 study of adjunctive pimavanserin (N = 207) conducted between December 2016 and October 2018, sleep/wakefulness disturbances were measured with the 17-item Hamilton Depression Rating Scale (HDRS17) insomnia items (sum of items 4, 5, and 6) and the Karolinska Sleepiness Scale (KSS). Outcomes included change from baseline in HDRS17 insomnia factor score and KSS score, correlation between the HDRS17 insomnia factor score and KSS score, and change from baseline in the Sheehan Disability Scale (SDS) total score and Unproductive Days subscore in patients with a baseline KSS score ≥ 6.
Results: At baseline, HDRS17 insomnia factor score ≥ 3 occurred in 76% of patients receiving placebo and 85% of patients receiving pimavanserin. The overall least squares (LS) mean weighted difference (SE) was −0.5 (0.32) with a 95% CI of −1.2 to 0.1 (P = .088) at week 5. Improvement was observed with pimavanserin versus placebo at weeks 2, 3, and 4, with effect sizes (ESs) of 0.370 to 0.524 (P < .05). For KSS score, the LS mean difference (SE) at week 5 was −1.1 (0.30) (95% CI, −1.7 to −0.5; P = .0003; ES = 0.627) for pimavanserin versus placebo. Among those with a KSS score ≥ 6 at baseline (n = 120 placebo and n = 42 pimavanserin), the LS mean difference (SE) in the mean SDS score at week 5 was −1.1 (0.46) (95% CI, −2.0 to −0.2; P = .019; ES = 0.442) for pimavanserin versus placebo.
Conclusions: Adjunctive pimavanserin significantly improved sleep/wakefulness disturbance during treatment of MDD, an improvement that was associated with greater improvement in function.
Trial Registration: ClinicalTrials.gov identifier: NCT03018340
J Clin Psychiatry 2021;82(1):20m13425
To cite: Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2021;82(1):20m13425.
To share: https://doi.org/10.4088/JCP.20m13425
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
bDepartment of Psychiatry, Massachusetts General Hospital, and Harvard Medical School, Boston, Massachusetts
cDepartment of Psychiatry, Perelman School of Medicine, University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center, Philadelphia, Pennsylvania
dDepartment of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, Birmingham, Alabama
eDepartment of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas
fACADIA Pharmaceuticals Inc, San Diego, California
*Corresponding author: Manish K. Jha, MD, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, New York, NY 10029 ([email protected]).
Sleep disturbances, including insomnia and daytime sleepiness, occur in about one-third of the general population1 and adversely affect workplace performance.2,3 As a core symptom domain of major depressive disorder (MDD),4,5 sleep disturbances affect up to 90% of MDD patients6,7 and are associated with poor treatment outcomes such as failure to achieve remission8 and increased risk of relapse6,9 and recurrence5 along with impaired psychosocial functioning10 and quality of life (QoL).6,7 Insomnia is one of the most common residual symptoms of MDD7,11,12 and may add to the economic burden of the disorder.13 Treating insomnia in patients with MDD improves mood14 and is a key factor in achieving remission.11 However, evidence for the effectiveness of antidepressants for treating insomnia is lacking.15 In fact, while select antidepressants such as doxepin, trazodone, and mirtazapine may improve insomnia, many commonly used antidepressants (such as selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and bupropion) may cause worsening of insomnia in a sizable number of patients. Worsening of insomnia in a substantial proportion (1 in 6) of patients with MDD may be associated with significantly lower likelihood of acute-phase remission.16
Pimavanserin is a 5-hydroxytryptamine-2A (5-HT2A) receptor antagonist/inverse agonist with lesser activity as a 5-HT2C antagonist and inverse agonist, but no activity at adrenergic, dopaminergic, histaminergic, or muscarinic receptors,17 and is approved in the United States for the treatment of hallucinations and delusions in patients with Parkinson’s disease psychosis. In CLARITY,18 a randomized, placebo-controlled trial of pimavanserin as adjunctive therapy in patients with MDD and an inadequate response to antidepressant treatment, pimavanserin demonstrated a significant reduction in symptoms of depression and improvement in function measured by the Sheehan Disability Scale (SDS). This secondary analysis of CLARITY was undertaken to evaluate the effects of adjunctive pimavanserin on sleep/wakefulness disturbances and whether improvement in these symptoms mediates the improvement in depression and psychosocial function associated with pimavanserin.
METHODS
An independent ethics committee or institutional review board at each study site reviewed and approved the study protocol. The study was conducted following the principles of Good Clinical Practice derived from the Declaration of Helsinki and in accordance with local regulations and International Council of Harmonization guidelines. All patients provided written informed consent prior to any study procedures. This study was registered at ClinicalTrials.gov (NCT03018340).
Study Design
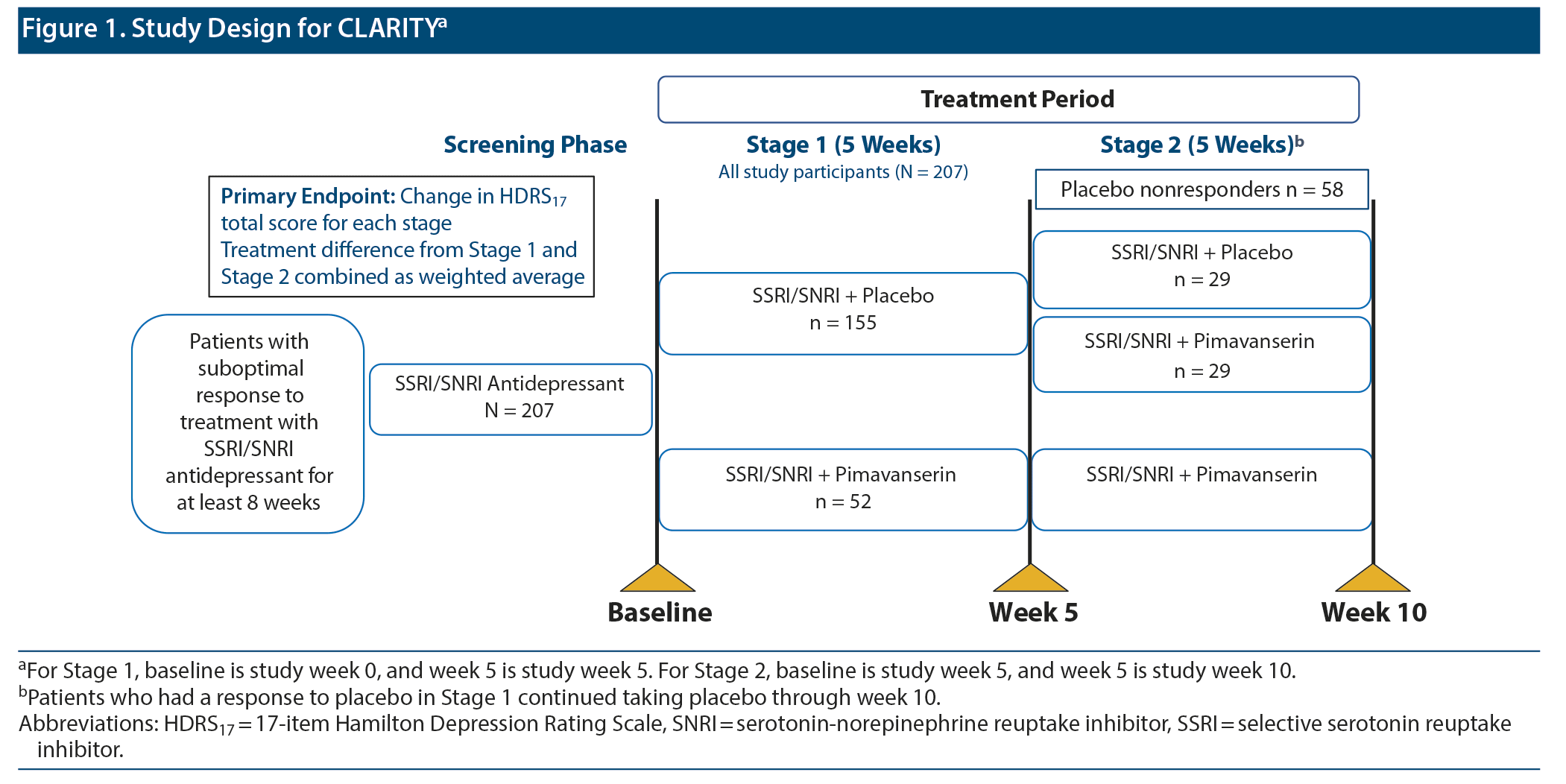
CLARITY was a multicenter, randomized, double-blind, placebo-controlled study in patients with MDD. The study was conducted between December 2016 and October 2018. The detailed study methodology was previously published.18 In brief, after an 8- to 21-day screening period, patients entered a 10-week double-blind treatment period and a 30-day safety follow-up period. A 2-stage Sequential Parallel-Comparison Design (SPCD)19 was used to randomize patients in Stage 1 in a 3:1 ratio to placebo or pimavanserin 34 mg added to current SSRI or SNRI therapy (citalopram, escitalopram, fluoxetine, paroxetine, sertraline, desvenlafaxine, duloxetine, or venlafaxine) for 5 weeks. Placebo nonresponders after 5 weeks (17-item Hamilton Depression Rating Scale [HDRS17]20 total score > 14 and < 50% reduction in score from baseline) were re-randomized to placebo or pimavanserin 34 mg (1:1 ratio) added to current therapy for an additional 5 weeks.
Patient Selection
To be eligible, patients were at least 18 years of age, with a body mass index (BMI) of 19 to 35 kg/m2, and were required to have a primary diagnosis of MDD and a current major depressive episode (MDE), defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), and confirmed by the Structured Clinical Interview for DSM-5, Clinician Version (SCID-5-CV).21 Also required was a history of MDD for ≥ 1 year prior to screening, a Montgomery-Asberg Depression Rating Scale (MADRS)22 total score > 20, a Clinical Global Impressions—Severity of Illness scale (CGI-S)23 score ≥ 4 (moderately ill or worse) at screening and baseline visits, and a history of inadequate response to 1 or 2 adequate trials with SSRI or SNRI antidepressant treatment during the current depression episode.
Study Assessments

- Adjunctive pimavanserin significantly improved depressive symptoms, reduced sleepiness, and improved functioning as measured by the Sheehan Disability Scale in patients with major depressive disorder and an inadequate response to antidepressants.
- Pimavanserin is not approved by the US Food and Drug Administration for treating major depression, and these results require further replication in a randomized clinical trial.
Clinic visits occurred weekly from baseline through week 10 (end of study). The HDRS17 was administered by trained raters at each visit as a measure of depression severity. Participants completed the SDS24 as a measure of disability and impairment in domains of work/school, social life, and family life/home responsibilities (responses summed as SDS total score) at each visit. Furthermore, participants also were asked to indicate the number of days in the past week that they felt their productivity to be reduced while at school or work (SDS Unproductive Days subscore). As a measure of daytime sleepiness, participants completed the Karolinska Sleepiness Scale (KSS)25 at each study visit, via which a retrospective assessment of the level of sleepiness during the past 7 days was recorded. For this secondary analysis, assessments of sleep/wakefulness disturbance included the insomnia items (items 4, 5, and 6) of the HDRS17 and the KSS.
Statistical Analysis
Efficacy data were analyzed for the full analysis set (FAS) for Stage 1 (placebo n = 152, pimavanserin n = 51) and for Stage 2 (placebo n = 29, pimavanserin n = 29), comprising all randomized patients who received ≥ 1 dose of blinded study drug and who had a baseline value and at least 1 postbaseline value for the HDRS17 total score within each stage. Mean change from baseline was determined for the HDRS17 insomnia factor score (sum of items 4, 5, and 6) among patients with a factor score ≥ 3 at baseline. In addition, mean change from baseline was determined for the KSS. For the SDS total score and Unproductive Days subscore, mean change from baseline was examined among the subgroup of patients with a baseline KSS score ≥ 6 (some signs of sleepiness).
The Pearson correlation coefficient was used to determine the relationship between the HDRS17 total score and (1) HDRS17 insomnia factor score and (2) KSS score for Stages 1 and 2 (and for 10 weeks in patients not randomized to pimavanserin in Stage 2) as well as association of change from baseline to week 5 in HDRS17 insomnia factor score and the KSS score. Mixed model for repeated measures (MMRM) analyses were used for comparisons with assessments of sleep/wakefulness disturbances (HDRS17 insomnia factor and KSS) as the outcome variable and treatment group, visit, and treatment-by-visit interaction as independent variables of interest. Analyses were done with baseline SDS total or Unproductive Days or baseline HDRS17 total or insomnia factor score-by-treatment interaction as factors. The treatment effect was assessed as the treatment difference in least squares (LS) mean change from baseline to the end of each stage and the corresponding 95% confidence intervals (CIs). An unstructured covariance matrix was used to model the within-patient errors. The denominator degrees of freedom were estimated using the Kenward-Rogers approximation. A 2-sided P value was calculated for the treatment difference from the stage-specific MMRM analysis. Cohen d effect size was calculated for comparisons between treatments.
Per methods proposed by Kraemer,26 the mediator effect of early improvement in sleep/wakefulness disturbances was evaluated. To be defined as a mediator, (1) the symptom domain should exhibit differential change with treatment (versus placebo), (2) this change should precede or be concurrent to the ultimate treatment outcome, and (3) this change should significantly predict treatment outcome. As described in the previous paragraph, treatment effects from the aforementioned mixed model analyses were used to ascertain whether reduction in sleep/wakefulness disturbances from baseline to week 1 differed significantly between pimavanserin and placebo. If a significant difference was found, linear regression analyses with baseline-to-week 5 changes in SDS score as the outcome were used with baseline-to-week 1 changes in sleep/wakefulness disturbances (significantly different between pimavanserin and placebo) as the independent variable of interest. All analyses were performed using SAS version 9.3 (SAS Institute, Inc; Cary, North Carolina).
RESULTS
In the primary study, a total of 207 patients were randomized between December 2016 and October 2018.18 In Stage 1, 152 patients (98.1%) and 51 patients (98.1%) in the placebo and pimavanserin groups, respectively, were included in the FAS population. In Stage 2, 29 patients each in the placebo and pimavanserin groups were included in the FAS population (Figure 1). Treatment groups were generally comparable for demographic and clinical characteristics at baseline. Concomitant sedative/hypnotic medications were taken by < 10% of patients during the study.
HDRS17 Insomnia Factor
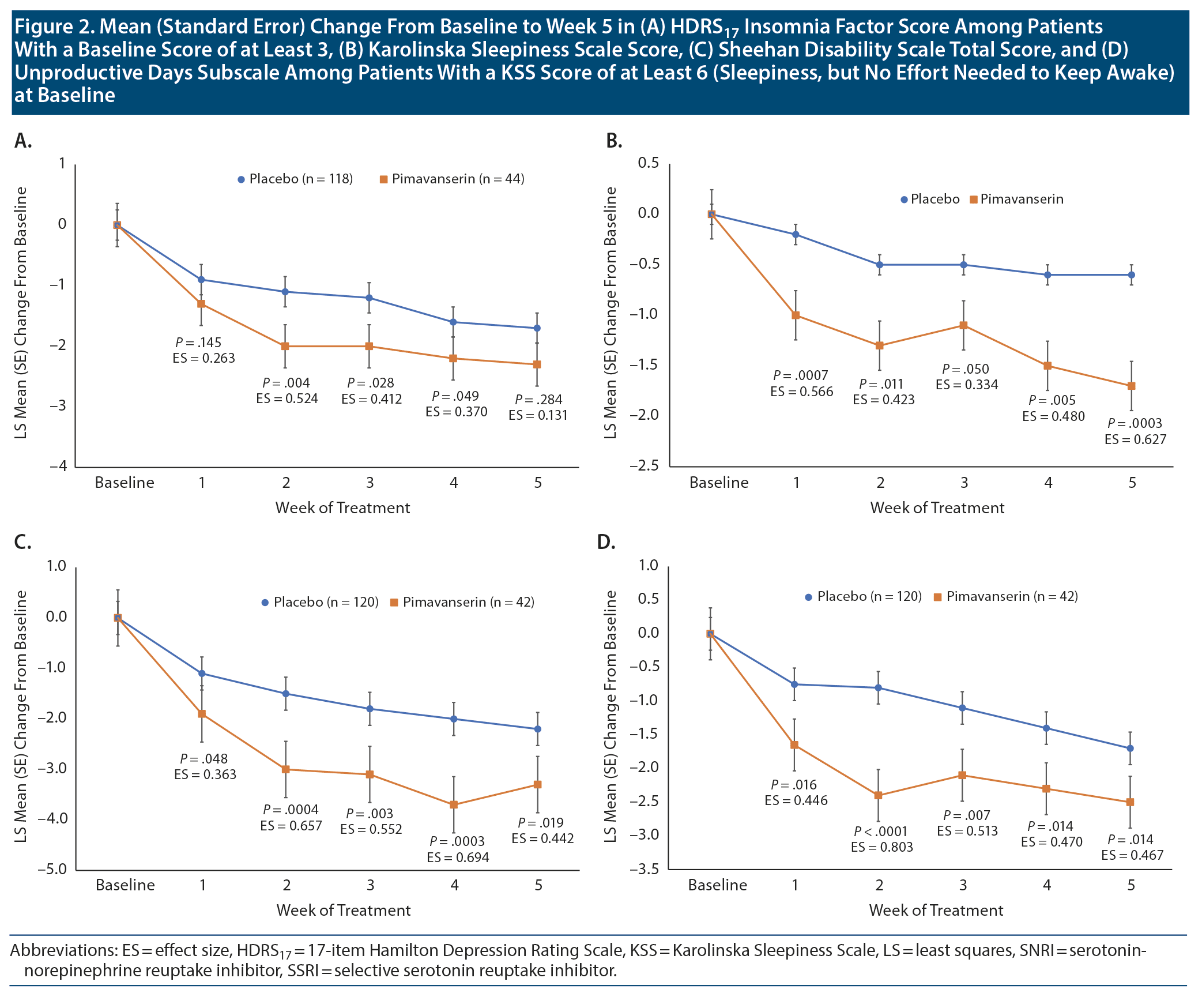
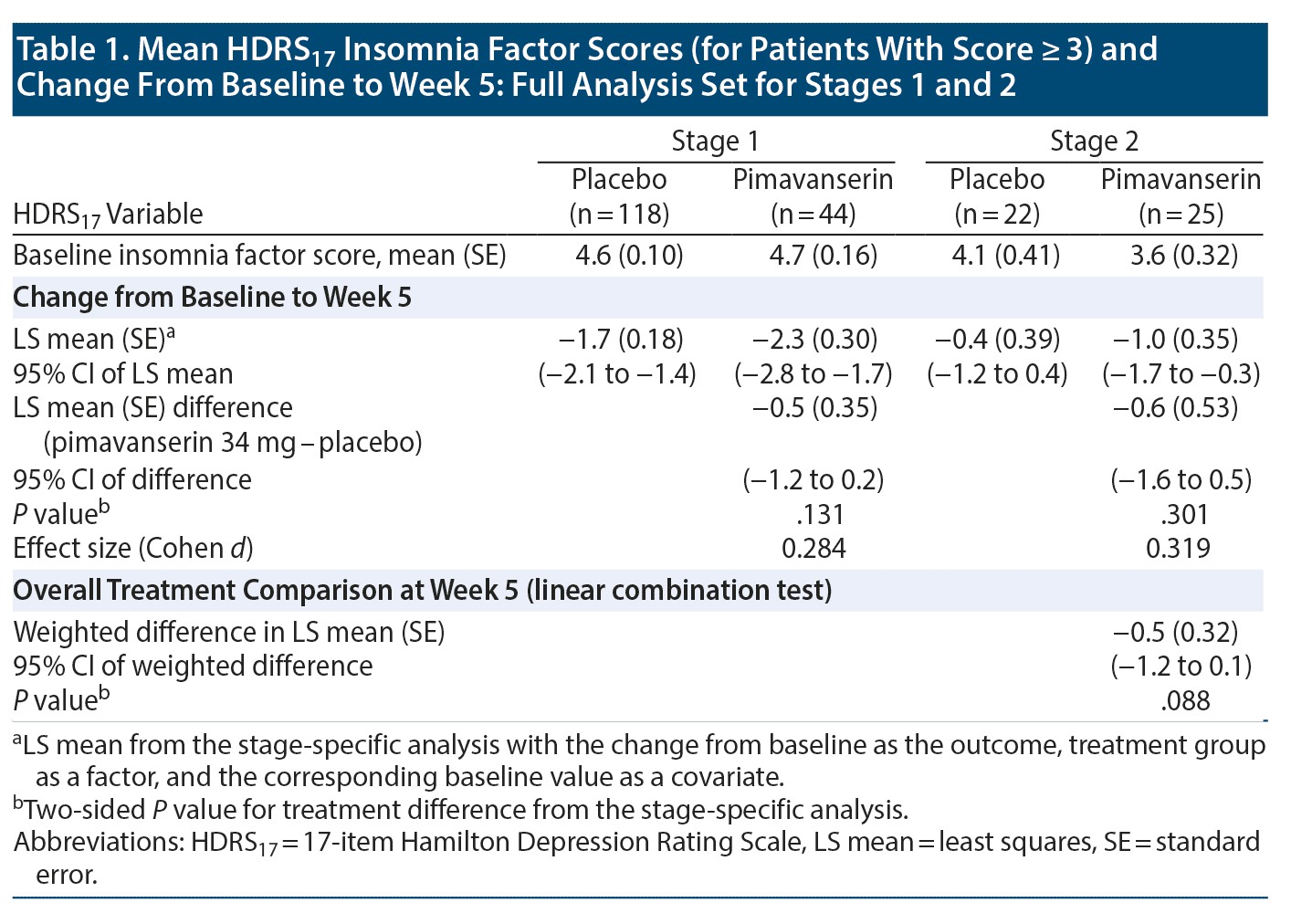
At baseline, an HDRS17 insomnia factor score ≥ 3 was recorded in 118 patients (76% of FAS population) receiving placebo and 44 patients (85% of FAS population) receiving pimavanserin. The overall LS mean (standard error [SE]) weighted difference was −0.5 (0.32) with a 95% CI of -1.2 to 0.1 (P = .088). In Stage 1, improvement was observed with pimavanserin versus placebo for the insomnia factor score at weeks 2, 3, and 4, with effect sizes of 0.370 to 0.524 (P < .05) (Figure 2A). No significant differences were observed between pimavanserin (n = 25) and placebo (n = 22) during Stage 2 or for the overall weighted difference for Stages 1 and 2 (Table 1).
Karolinska Sleepiness Scale
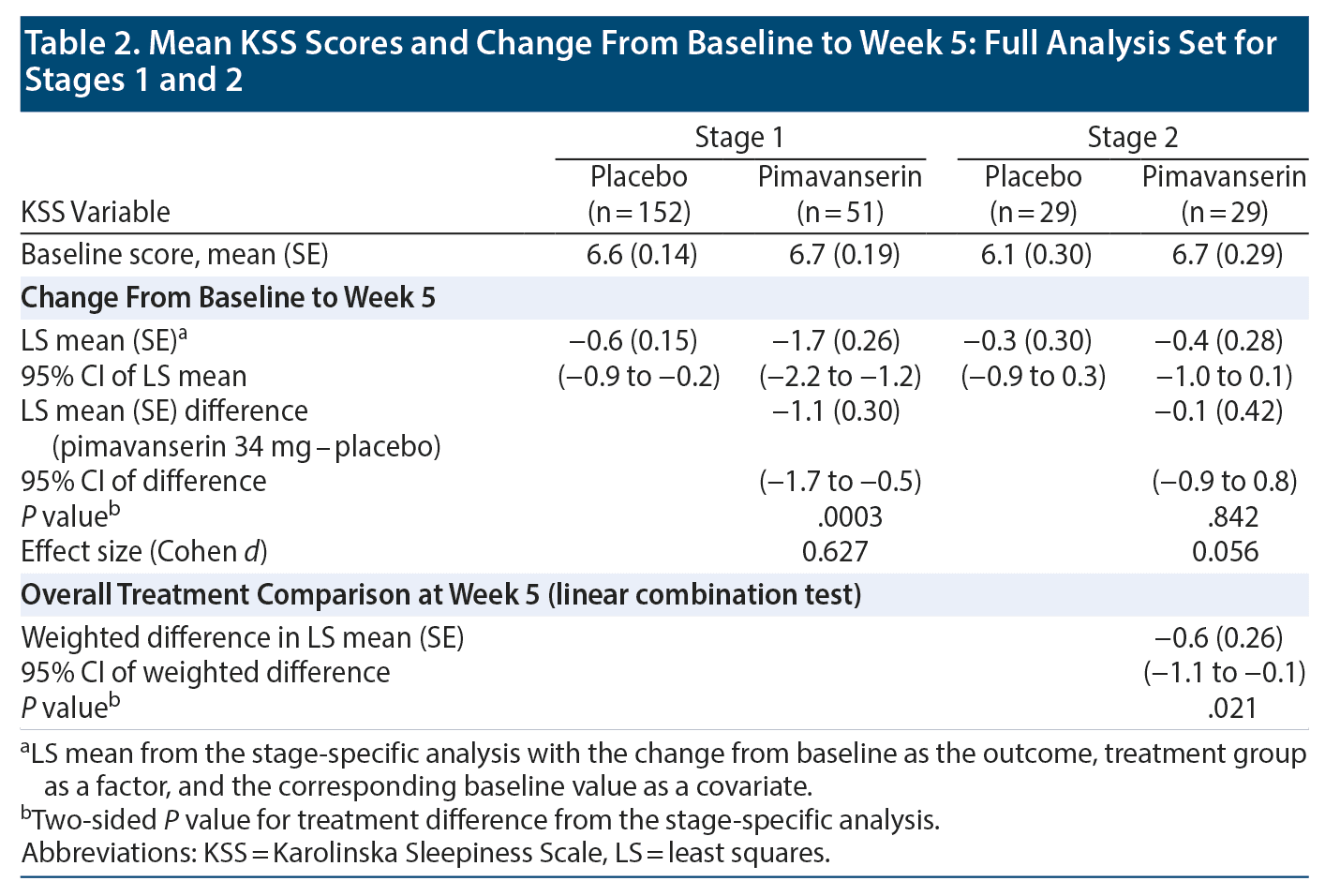
At baseline, a KSS score ≥ 6 was recorded in 120 patients (77% of FAS population) with placebo and 42 patients (81% of FAS population) with pimavanserin. LS mean (SE) baseline scores on the KSS were 6.6 (0.14), and 6.7 (0.19) for placebo (n = 152) and pimavanserin (n = 51), respectively, during Stage 1 and 6.1 (0.30) and 6.7 (0.29) for placebo (n = 29) and pimavanserin (n = 29), respectively, during Stage 2. For KSS score during Stage 1, the LS mean (SE) difference at week 5 was −1.1 (0.30) (95% CI, −1.7 to −0.5; P = .0003; ES = 0.627) for pimavanserin versus placebo. During Stage 1, a significant (P ≤ .05) reduction from baseline for the KSS score was observed with pimavanserin versus placebo from week 1 through week 5 with effect sizes of 0.4 or greater at each week (Figure 2B). No significant differences were observed between treatments during Stage 2 (Table 2). However, the overall LS mean (SE) weighted difference was −0.6 (0.26) with a 95% CI of −1.1 to −0.1 (P = .021). Among those with a KSS score ≥ 6 (some sleepiness) at baseline (n = 120 for placebo and n = 42 for pimavanserin), the LS mean difference at week 5 was −1.1 (0.46) (95% CI, −2.0 to −0.2; P = .019; ES = 0.442) for pimavanserin versus placebo.
Sheehan Disability Scale
In Stage 1, among patients with a KSS score ≥ 6 (some sleepiness) at baseline (n = 120 for placebo and n = 42 for pimavanserin), the LS mean (SE) difference in the SDS mean score at week 5 was −1.09 (0.46) (95% CI, −2.0 to −0.2; P = .019; ES = 0.442) for pimavanserin versus placebo (Figure 2C). For SDS Unproductive Days, the LS mean difference in the mean score at week 5 was −0.86 (0.35) (95% CI, −1.6 to −0.2; P = .014; ES = 0.467) for pimavanserin versus placebo. A significant (P < .05) improvement versus placebo was observed from week 1 to week 5 for the SDS total score and Unproductive Days subscore (Figure 2D). Effect sizes for pimavanserin versus placebo were 0.363 or greater for the SDS mean score and 0.446 or greater for the SDS Unproductive Days subscore. No significant differences for the SDS mean score and Unproductive Days score were observed between treatments for Stage 2. For the SDS mean score, the overall LS mean (SE) weighted difference was −0.85 (0.34) with a 95% CI of −1.5 to −0.19 (P = .012). Among the subgroup of patients with baseline KSS score < 6, LS mean difference with pimavanserin versus placebo was significant (P = .008) only at week 4 for SDS mean score and not at any timepoint for Unproductive Days.
Correlation of Sleep/Wakefulness Disturbance With HDRS17 Total Score
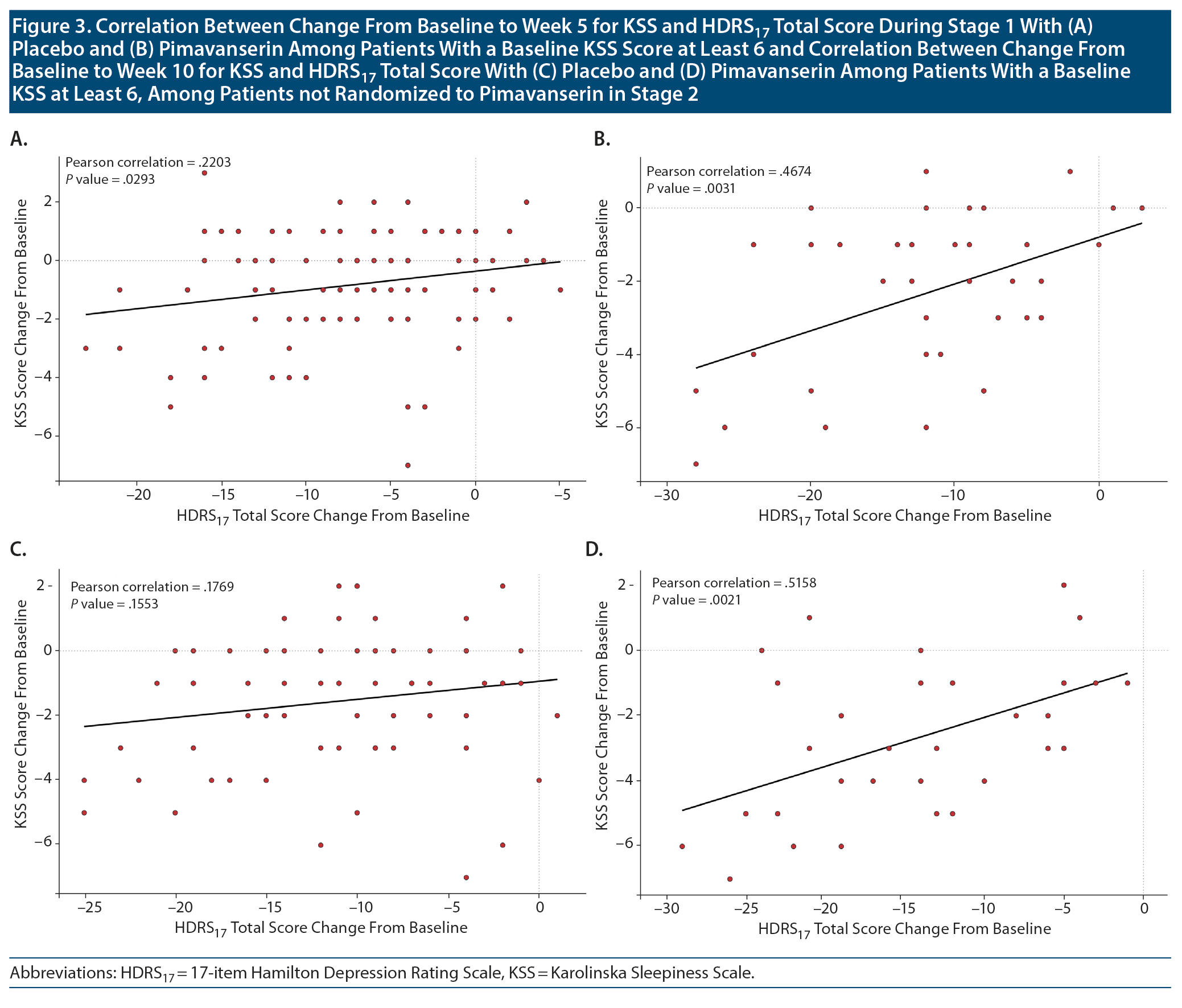
For the subgroup of patients with a baseline KSS score ≥ 6, a significant correlation was observed for improvement in the HDRS17 total score during Stage 1 for both pimavanserin (P = .003; correlation = 0.467) and placebo (P = .029; correlation = 0.220) groups (Figure 3). Among those patients not re-randomized who remained on pimavanserin treatment in Stage 2, a significant correlation between baseline KSS score and the HDRS17 total score was observed for pimavanserin (P = .002; correlation = 0.516) but not placebo (0.155; correlation = 0.177). For an analysis of mean change from baseline for the HDRS17 insomnia factor score and KSS score, a significant correlation (P = .014; correlation = 0.188) was observed for improvement in KSS score with improvement in the HDRS17 insomnia factor score.
Mediator Analysis
In Stage 1, regression analysis of the mean change from baseline to week 5 for the SDS was significantly correlated with a reduction in the KSS at week 1 (F168 = 6.68, P = .011) and at week 5 (F169 = 18.18, P < .0001) in the mediator analysis. Mediator analysis was not done with the HDRS17 insomnia factor because no significant difference was observed between pimavanserin and placebo at week 1.
DISCUSSION
In this secondary analysis from CLARITY, significant improvements in sleep/wakefulness disturbances were observed with adjunctive pimavanserin when assessed with the HDRS17 insomnia factor and the KSS. Importantly, the results showed significant improvement over 5 weeks with pimavanserin versus placebo in functionality measured by the SDS among patients with a baseline KSS score ≥ 6 (some sleepiness), with robust effect sizes of 0.4 or greater, which suggests that improvement in daytime sleepiness in patients with MDD is associated with improved function. This association was further explored by assessing the effects on the SDS Unproductive Days subscore among patients with a baseline KSS score ≥ 6. Significant improvement with pimavanserin versus placebo was observed at each week with robust effect sizes that exceeded 0.4. These results suggest that patients with MDD and baseline insomnia or daytime sleepiness have improved functioning and greater productivity with pimavanserin. Improvements in the HDRS17 total score were correlated with improvements in the HDRS17 insomnia factor score and with KSS score. In total, these results suggest a statistically significant and clinically relevant effect of adjunctive pimavanserin on sleep/wakefulness disturbances in patients with MDD. These results with pimavanserin are supported by findings from a small study of patients with Parkinson’s Disease and psychosis,27 for whom a significant improvement in nighttime sleep was observed with pimavanserin among those with baseline impaired nighttime sleep.
The regression and correlation analyses attempted to explore the relationship between change in KSS score and change in SDS score as well as the correlation between change in SDS score and change in HDRS17 score. The results suggest that improvement in the mean SDS score and SDS Unproductive Days score was mediated by improvement in the KSS score. Pimavanserin had a greater effect on the KSS score versus placebo at week 1, and the reduction in KSS score from baseline to week 1 was significantly correlated with a change in SDS score from baseline to week 5. Although the trial was not designed to evaluate mediator effects, findings of this report do suggest that improvement in psychosocial function with pimavanserin is mediated by an improvement in KSS score. Future studies are needed for further confirmation.
Insomnia has been identified as a core symptom of depression7-9 and an important residual symptom of MDD.11 In a longitudinal study of depressed outpatients,28 the majority had residual symptoms of depression after 1 year of treatment, and 13.9% reported insomnia as a residual symptom. Analyses from the STAR*D trial11-13,29 found that insomnia was common in outpatients with MDD, and insomnia or sleep disturbances were among the most common residual symptoms. Presence of insomnia or sleep disturbances was associated with an increased rate of relapse.
Insomnia has a significant impact on QoL and daytime functioning10,30,31 as well as on the risk for depression. A number of studies14,30-32 have shown the effects of insomnia and sleep disturbances on MDD and the benefits of treating insomnia on MDD outcomes including QoL. In longitudinal studies,1,4 the presence of sleep disturbance in older women or men was associated with an increased risk for depression. In contrast, among patients with MDD and a baseline HDRS17 insomnia factor score ≥ 4, a reduction in the insomnia factor score was associated with improvement in symptoms of MDD.11,33 In addition to being a risk factor for MDD, insomnia also has been identified as an independent risk factor for suicide.34-36 In a 6-year longitudinal study of patients with MDD,37 the presence of insomnia and presence of suicidal ideation at baseline were risk factors for suicide attempts. Results from meta-analyses34,38 have shown an association between the presence of sleep disorders and suicidal behavior in patients with psychiatric diagnoses, including MDD.
Limitations of this study included its basis as a post hoc analysis with endpoints that were not prespecified. The sample size calculation for Stage 2 was not achieved, which most likely is the explanation for the lack of statistical significance for comparisons in Stage 2. In addition, sleep disturbances and daytime sleepiness were based on patient report, and objective sleep measures were not administered. Further, in this study, the KSS was used retrospectively to assess sleepiness, although this instrument was designed and validated for prospective use to assess the level of sleepiness at a particular time of the day and is a measure of situational sleepiness.39 Nevertheless, results showed a robust effect of adjunctive pimavanserin on sleep/wakefulness disturbances in MDD patients and suggest that an improvement in depressive symptoms accompanies improvements in sleep/wakefulness disturbances. Another limitation of this study is that it was not designed to assess BMI or obesity as an important variable in sleep dysregulation in the context of depression and treatment with pimavanserin. Notably, study eligibility was restricted to individuals with BMI under 35 kg/m2, excluding those at highest risk of obstructive sleep apnea. We also observed prospectively that pimavanserin was associated with low rates of weight gain.18 While understanding the role of obesity within the context of depression is important, we believe analyses focused on BMI are outside the scope of this report and should be considered an important variable in future studies.
In summary, adjunctive pimavanserin significantly improved sleep/wakefulness disturbances versus placebo during treatment of MDD, which appeared to be associated with greater improvements in function and productivity. Adjunctive pimavanserin may represent an option for the treatment of MDD, especially in the presence of sleep/wakefulness disturbances. Ongoing phase 3 studies of adjunctive pimavanserin in patients with MDD will provide additional findings about its beneficial effects on sleep/wakefulness disturbances.
Submitted: April 24, 2020; accepted November 5, 2020.
Published online: December 1, 2020.
Potential conflicts of interest: Dr Freeman, in the past 36 months, has conducted investigator initiated trials/research for Takeda, JayMac, and Sage; has been on advisory boards for Otsuka, Alkermes, Janssen, Sage, and Sunovion; has served on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); has performed medical editing for the GOED newsletter; has done speaking for/received honoraria from US Psychiatric Congress and Medscape; is an employee of Massachusetts General Hospital (MGH); and works with the MGH National Pregnancy Registry (Current Registry Sponsors: Teva [2019-present], Alkermes, Inc [2016-present]; Otsuka America Pharmaceutical, Inc [2008-present]; Forest/Actavis [2016-present], and Sunovion Pharmaceuticals, Inc [2011-Present]). As an employee of MGH, Dr Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and the National Institute of Mental Health [NIMH]. All (lifetime) disclosures for Dr Fava can be viewed online at https://mghcme.org/app/uploads/2020/10/MF-Disclosures-Lifetime-updated-October-2020.pdf. Research support: Abbott, ACADIA, Alkermes, American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir, Axsome Therapeutics, Biohaven, BioResearch, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, Clarus Funds, Clintara, Covance, Covidien, Eli Lilly, EnVivo, Euthymics Bioscience, Forest, FORUM, Ganeden Biotech, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen, Jed Foundation, Johnson & Johnson, Lichtwer Pharma, Lorex, Lundbeck, Marinus, MedAvante, Methylation Sciences, National Alliance for Research on Schizophrenia & Depression (NARSAD), National Center for Complementary and Alternative Medicine (NCCAM), National Coordinating Center for Integrated Medicine (NiiCM), National Institute of Drug Abuse (NIDA), NIMH, Neuralstem, NeuroRx, Novartis; Organon, Otsuka, Pamlab, Pfizer, Pharmacia-Upjohn, Pharmaceutical Research Associates, Pharmavite, PharmoRx, Photothera, Reckitt Benckiser, Roche, RCT Logic (formerly Clinical Trials Solutions) , Sanofi-Aventis, Shire, Solvay , Stanley Medical Research Institute (SMRI); Synthelabo, Taisho, Takeda, Tal Medical, VistaGen, and Wyeth-Ayerst. Advisory board/consultant:
Abbott, Acadia, Affectis, Alkermes, Amarin, Aspect Medical Systems, AstraZeneca, Auspex, Avanir, Axsome Therapeutics, Bayer, Best Practice Project Management, Biogen, BioMarin, Biovail, Boehringer Ingelheim, Boston Pharmaceuticals, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, CNS Response, Compellis, Cypress, DiagnoSearch Life Sciences (P), Dainippon Sumitomo, Dov, Edgemont, Eisai, Eli Lilly, EnVivo, ePharmaSolutions, EPIX, Euthymics Bioscience, Fabre-Kramer, Forest, Forum, GenOmind, GlaxoSmithKline, Grunenthal, Indivior, i3 Innovus/Ingenis, Intracellular, Janssen, Jazz, Johnson & Johnson, Knoll, Labopharm, Lorex, Lundbeck, Marinus, MedAvante, Merck, MSI Methylation Sciences, Naurex, Navitor, Nestle Health Sciences, Neuralstem, Neuronetics, NextWave, Novartis, Nutrition 21, Orexigen, Organon, Osmotica, Otsuka, Pamlab, Pfizer, PharmaStar; Pharmavite, PharmoRx Therapeutics, Praxis Precision Medicines, Precision Human Biolaboratory, Prexa, PPD, Purdue, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic (formerly Clinical Trials Solutions), Relmada Therapeutics, Rexahn, Ridge Diagnostics, Roche, Sanofi-Aventis, Sepracor, Servier, Schering-Plough, Shenox, Solvay, Somaxon, Somerset, Sunovion, Supernus, Synthelabo, Taisho, Takeda, Tal Medical, Tetragenex, Teva, TransForm, Transcept Pharmaceuticals, Usona Institute, Vanda, Versant Venture Management, and VistaGen. Speaking/publishing: Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis, Organon, Pfizer, PharmaStar, United BioSource, and Wyeth-Ayerst. Stock/other financial options: equity holdings in Compellis and PsyBrain. Royalty/patent, other income: patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); patent application for a combination of ketamine plus scopolamine in major depressive disorder (MDD), licensed by MGH to Biohaven; and patents for pharmacogenomics of depression treatment with folate (US_9546401, US_9540691). Copyright: the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; and World Scientific Publishing Co Pte Ltd. Dr Jha has received contract research grants from ACADIA and Janssen and honoraria for CME presentations from North American Center for Continuing Medical Education and Global Medical Education. Dr Papakostas has served as a consultant for Abbott, ACADIA,* Alkermes, Alphasigma,*, AstraZeneca, Avanir, Axsome Therapeutics,* Boston Pharmaceuticals,* Brainsway, Bristol-Myers Squibb, Cala Health,* Cephalon, Dey, Eli Lilly, Genentech,* Genomind,* GlaxoSmithKline, Evotec, H. Lundbeck, Inflabloc, Janssen Global Services,* Jazz, Johnson & Johnson,* Methylation Sciences, Mylan,* Novartis, One Carbon Therapeutics,* Osmotica,* Otsuka, Pamlab, Pfizer, Pierre Fabre, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sage Therapeutics,* Shire, Sunovion, Taisho,* Takeda, Theracos, and Wyeth; has received honoraria (for lectures or consultancy) from Abbott, ACADIA, Alkermes, Alphasigma, Asopharma America Cntral Y Caribe, AstraZeneca, Avanir, Bristol-Myers Squibb, Brainsway, Cephalon, Dey, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Inflabloc, Grunbiotics, Hypera, Jazz, H. Lundbeck, Medichem, Meiji Seika, Novartis, Otsuka, Pamlab, Pfizer, Pharma Trade, Pierre Fabre, Ridge Diagnostics, Shire, Sunovion, Takeda, Theracos, Titan, and Wyeth; and has received research support (paid to hospital) from AstraZeneca, Bristol-Myers Squibb, Forest, NIMH, Neuralstem*, Pamlab, Pfizer, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion, Tal Medical, and Theracos. (*Asterisk denotes activity undertaken on behalf of MGH). Dr Shelton reports grants and personal fees from Allergan, grants from Assurex Health, grants from Avanir, grants and personal fees from Cerecor, grants from Genomind, grants and personal fees from Janssen, personal fees from Medtronic, grants from Novartis, grants and personal fees from Otsuka, grants and personal fees from ACADIA, grants from Alkermes, grants and personal fees from Takeda, and grants from NeuroRx, outside the submitted work. Prof Thase reports the following relationships over the past 3 years: Consultant/independent contractor: Acadia, Akili, Alkermes, Allergan, Cerecor, Gerson Lehrman Group, Guidepoint Global, H. Lundbeck, MedAvante, moksha8, Novartis, Janssen (Johnson & Johnson), Otsuka, Pfizer, Sage, Sunovion, Takeda. Grant/research support: ACADIA, Agency for Healthcare Research and Quality, Alkermes, Allergan, AssureRx Health, Axome, Intracellular, Janssen, NIMH, Patient Centered Outcomes Research Instittue, Otsuka, and Takeda. Royalties: American Psychiatric Foundation, Guilford Publications, Herald House, W.W. Norton & Company, Inc. Prof Thase’s spouse, Diane Sloan, PharmD, is a Senior Medical Director of Peloton Advantage, which does business with a number of pharmaceutical companies. Dr Trivedi has been an advisor/consultant for and received fees from Alkermes, AstraZeneca, Cerecor, Eli Lilly, Lundbeck, Naurex, Neuronetics, Otsuka, Pamlab, Pfizer, Shire, and Takeda and has received grants/research support from NIMH and NIDA. Drs Dirks, Liu, and Stankovic are employees of ACADIA Pharmaceuticals Inc, San Diego, California.
Funding/support: This study was supported by ACADIA Pharmaceuticals Inc, San Diego, California.
Role of the sponsor: The funder of the study had a role in study design, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and had full responsibility for the content of the manuscript for publication. The corresponding author was responsible for the final review and had final responsibility for the decision to submit for publication.
Disclaimer: Dr Freeman, Editor in Chief of The Journal of Clinical Psychiatry, was not involved in the editorial review or decision to publish this article.
Acknowledgements: The authors acknowledge the editorial assistance of Richard S. Perry, PharmD, a professional medical writer, in the preparation of this manuscript, which was supported by ACADIA Pharmaceuticals Inc, San Diego, California.
REFERENCES
1.Paudel ML, Taylor BC, Diem SJ, et al; Osteoporotic Fractures in Men Study Group. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56(7):1228-1235. PubMed CrossRef
2.Geiger-Brown J, Rogers VE, Trinkoff AM, et al. Sleep, sleepiness, fatigue, and performance of 12-hour-shift nurses. Chronobiol Int. 2012;29(2):211-219. PubMed CrossRef
3.Wilson M, Permito R, English A, et al. Performance and sleepiness in nurses working 12-h day shifts or night shifts in a community hospital. Accid Anal Prev. 2019;126:43-46. PubMed CrossRef
4.Maglione JE, Ancoli-Israel S, Peters KW, et al; Study of Osteoporotic Fractures Research Group. Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep. 2014;37(7):1179-1187. PubMed CrossRef
5.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. J Am Geriatr Soc. 2012;60(4):635-643. PubMed CrossRef
6.Paterson LM, Nutt DJ, Wilson SJ. NAPSAQ-1: National Patient Sleep Assessment Questionnaire in depression. Int J Psychiatry Clin Pract. 2009;13(1):48-58. PubMed CrossRef
7.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329-336. PubMed CrossRef
8.Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(suppl 16):27-32. PubMed
9.Mendlewicz J. Sleep disturbances: core symptoms of major depressive disorder rather than associated or comorbid disorders. World J Biol Psychiatry. 2009;10(4):269-275. PubMed CrossRef
10.Cutler AJ. The role of insomnia in depression and anxiety: its impact on functioning, treatment, and outcomes. J Clin Psychiatry. 2016;77(8):e1010. PubMed CrossRef
11.Fava M, Ball S, Nelson JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257. PubMed CrossRef
12.Sakurai H, Suzuki T, Yoshimura K, et al. Predicting relapse with individual residual symptoms in major depressive disorder: a reanalysis of the STAR*D data. Psychopharmacology (Berl). 2017;234(16):2453-2461. PubMed CrossRef
13.Sunderajan P, Gaynes BN, Wisniewski SR, et al. Insomnia in patients with depression: a STAR*D report. CNS Spectr. 2010;15(6):394-404. PubMed CrossRef
14.Gebara MA, Siripong N, DiNapoli EA, et al. Effect of insomnia treatments on depression: a systematic review and meta-analysis. Depress Anxiety. 2018;35(8):717-731. PubMed CrossRef
15.Everitt H, Baldwin DS, Stuart B, et al. Antidepressants for insomnia in adults. Cochrane Database Syst Rev. 2018;5:CD010753. PubMed
16.Jha MK, Minhajuddin A, South C, et al. Worsening anxiety, irritability, insomnia, or panic predicts poorer antidepressant treatment outcomes: clinical utility and validation of the Concise Associated Symptom Tracking (CAST) Scale. Int J Neuropsychopharmacol. 2018;21(4):325-332. PubMed CrossRef
17.Vanover KE, Weiner DM, Makhay M, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N‘ ²-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006;317(2):910-918. PubMed CrossRef
18.Fava M, Dirks B, Freeman MP, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928. PubMed CrossRef
19.Fava M, Evins AE, Dorer DJ, et al. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72(3):115-127. PubMed CrossRef
20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed CrossRef
21.First MB, Williams JBW, Karg RS, et al. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV). Arlington, VA: American Psychiatric Association; 2016.
22.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
23.Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. DHEW Publication No. ADM 76-338. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:218-222.
24.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed CrossRef
25.Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117(7):1574-1581. PubMed CrossRef
26.Kraemer HC. A mediator effect size in randomized clinical trials. Int J Methods Psychiatr Res. 2014;23(4):401-410. PubMed CrossRef
27.Patel N, LeWitt P, Neikrug AB, et al. Nighttime sleep and daytime sleepiness improved with pimavanserin during treatment of Parkinson’s disease psychosis. Clin Neuropharmacol. 2018;41(6):210-215. PubMed CrossRef
28.Nil R, Lütolf S, Seifritz E. Residual symptoms and functionality in depressed outpatients: a one-year observational study in Switzerland with escitalopram. J Affect Disord. 2016;197:245-250. PubMed CrossRef
29.Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50. PubMed CrossRef
30.McCall WV, Blocker JN, D’ Agostino R Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010;6(4):322-329. PubMed CrossRef
31.McCall WV, Reboussin BA, Cohen W. Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res. 2000;9(1):43-48. PubMed CrossRef
32.Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10(1):17-23. PubMed CrossRef
33.Trivedi MH, Bandelow B, Demyttenaere K, et al. Evaluation of the effects of extended release quetiapine fumarate monotherapy on sleep disturbance in patients with major depressive disorder: a pooled analysis of four randomized acute studies. Int J Neuropsychopharmacol. 2013;16(8):1733-1744. PubMed CrossRef
34.Malik S, Kanwar A, Sim LA, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014;3(1):18. PubMed CrossRef
35.McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15(9):389. PubMed CrossRef
36.McCall WV, Blocker JN, D’ Agostino R Jr, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11(9):822-827. PubMed CrossRef
37.Eikelenboom M, Beekman ATF, Penninx BWJH, et al. A 6-year longitudinal study of predictors for suicide attempts in major depressive disorder. Psychol Med. 2019;49(6):911-921. PubMed CrossRef
38.Wang X, Cheng S, Xu H. Systematic review and meta-analysis of the relationship between sleep disorders and suicidal behaviour in patients with depression. BMC Psychiatry. 2019;19(1):303. PubMed CrossRef
39.Kaida K, Akerstedt T, Kecklund G, et al. Use of subjective and physiological indicators of sleepiness to predict performance during a vigilance task. Ind Health. 2007;45(4):520-526. PubMed CrossRef
This PDF is free for all visitors!