Objective: To analyze the effect of adjunctive brexpiprazole on metabolic parameters and body weight in adults with major depressive disorder (MDD) based on pooled data from 4 short-term studies and 1 long-term extension study.
Methods: The short-term studies (June 2011 to November 2016) were randomized, double-blind, placebo-controlled studies in outpatients with MDD (DSM-IV-TR criteria) and inadequate response to 1-3 prior antidepressant treatments (ADTs) plus 1 prospective ADT. Patients were randomized to adjunctive brexpiprazole (fixed or flexible doses in the range of 1-3 mg/d; n = 1,032) or placebo (n = 819) for 6 weeks. The long-term study (October 2011 to May 2017) was a 52-week (amended to 26 weeks), open-label, uncontrolled study of adjunctive brexpiprazole 0.5-3 mg/d (flexible dose; n = 2,938). Mean changes from baseline and categorical shifts in fasting metabolic parameters (cholesterol, triglycerides, and glucose) and body weight were analyzed.
Results: Mean changes from baseline in metabolic parameters were small after 6 weeks (all < 2 mg/dL) and 52 weeks (all < 4 mg/dL, except triglycerides, 15.83 mg/dL) of treatment. In most cases, the incidence of unfavorable shifts in metabolic parameters was lower than the incidence of favorable shifts. Mean body weight increase at last visit in the short-term studies was 1.5 kg with ADT + brexpiprazole and 0.3 kg with ADT + placebo. During long-term treatment, mean body weight increased by 3.8 kg over 58 weeks.
Conclusions: Adjunctive brexpiprazole was associated with small changes in metabolic parameters and moderate weight gain during short- and long-term treatment.
Trial Registration: ClinicalTrials.gov identifiers: NCT01360645, NCT01360632, NCT02196506, NCT01727726, NCT01360866
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Changes in Metabolic Parameters and Body Weight in Patients With Major Depressive Disorder Treated With Adjunctive Brexpiprazole:
Pooled Analysis of Phase 3 Clinical Studies
ABSTRACT
Objective: To analyze the effect of adjunctive brexpiprazole on metabolic parameters and body weight in adults with major depressive disorder (MDD) based on pooled data from 4 short-term studies and 1 long-term extension study.
Methods: The short-term studies (June 2011 to November 2016) were randomized, double-blind, placebo-controlled studies in outpatients with MDD (DSM-IV-TR criteria) and inadequate response to 1-3 prior antidepressant treatments (ADTs) plus 1 prospective ADT. Patients were randomized to adjunctive brexpiprazole (fixed or flexible doses in the range of 1-3 mg/d; n = 1,032) or placebo (n = 819) for 6 weeks. The long-term study (October 2011 to May 2017) was a 52-week (amended to 26 weeks), open-label, uncontrolled study of adjunctive brexpiprazole 0.5-3 mg/d (flexible dose; n = 2,938). Mean changes from baseline and categorical shifts in fasting metabolic parameters (cholesterol, triglycerides, and glucose) and body weight were analyzed.
Results: Mean changes from baseline in metabolic parameters were small after 6 weeks (all < 2 mg/dL) and 52 weeks (all < 4 mg/dL, except triglycerides, 15.83 mg/dL) of treatment. In most cases, the incidence of unfavorable shifts in metabolic parameters was lower than the incidence of favorable shifts. Mean body weight increase at last visit in the short-term studies was 1.5 kg with ADT + brexpiprazole and 0.3 kg with ADT + placebo. During long-term treatment, mean body weight increased by 3.8 kg over 58 weeks.
Conclusions: Adjunctive brexpiprazole was associated with small changes in metabolic parameters and moderate weight gain during short- and long-term treatment.
Trial Registration: ClinicalTrials.gov identifiers: NCT01360645, NCT01360632, NCT02196506, NCT01727726, NCT01360866
J Clin Psychiatry 2019;80(6):18m12680
To cite: Newcomer JW, Eriksson H, Zhang P, et al. Changes in metabolic parameters and body weight in patients with major depressive disorder treated with adjunctive brexpiprazole: pooled analysis of phase 3 clinical studies. J Clin Psychiatry. 2019;80(6):18m12680.
To share: https://doi.org/10.4088/JCP.18m12680
© Copyright 2019 Physicians Postgraduate Press, Inc.
aThriving Mind South Florida, Miami, Florida
bWashington University School of Medicine, St Louis, Missouri
cH. Lundbeck A/S, Valby, Copenhagen, Denmark
dOtsuka Pharmaceutical Development & Commercialization Inc, Princeton, New Jersey
*Corresponding author: John W. Newcomer, MD, Thriving Mind South Florida, 7205 Corporate Center Dr, Ste 200, Miami, FL 33126 ([email protected]).
Despite the availability of different classes of antidepressants, many patients with major depressive disorder (MDD) fail to respond to antidepressant treatment (ADT).1 For these patients, treatment options include optimizing the dose, switching to another ADT, or adding a second agent to the current ADT.2,3 With regard to adding a second agent, adjunctive atypical antipsychotics are supported by the strongest evidence.2 A meta-analysis4 of 14 studies (approximately 3,500 patients) showed that the likelihood of response and remission was increased when an adjunctive atypical antipsychotic was added to an ADT. However, many atypical antipsychotics are associated with metabolic adverse effects, including impaired glucose metabolism, dyslipidemia, and weight gain.4-7 Metabolic adverse effects have serious implications on prognosis due to the increased risk of treatment-related diabetes and cardiovascular disease.5,6
Brexpiprazole is a serotonin-dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors and as an antagonist at serotonin 5-HT2A and norepinephrine α1B/α2C receptors, all with subnanomolar affinity.8 Relative to its D2/5-HT1A receptor affinity, brexpiprazole has a moderate to low affinity for histamine H1 receptors,8 which may limit the risk for diabetes and weight gain.6
The efficacy and safety of brexpiprazole as adjunctive treatment to ADT over 6 weeks have been demonstrated in 4 short-term studies in MDD (Pyxis,9 Polaris,10 Sirius,11 and Delphinus12). In addition, a long-term extension study in adults with MDD (Orion13) showed that adjunctive brexpiprazole was generally well tolerated for up to 52 weeks. Brexpiprazole is approved in various countries and regions, including the United States, Canada, Australia, Japan, and the European Union, for the treatment of schizophrenia in adults. Brexpiprazole is also approved in the United States, Canada, and other countries as an adjunctive therapy to antidepressants for the treatment of MDD in adults.
The aim of this article is to summarize the effects of adjunctive brexpiprazole on metabolic parameters and body weight in adults with MDD based on data from the 4 short-term studies and the long-term extension study. Via a detailed approach to inspect clinically relevant changes, rates of favorable and unfavorable shifts in metabolic parameters are investigated in the short and long term, and weight changes are investigated in patient subgroups that are of particular relevance to prescribing physicians.
METHODS
Study Design and Patients
The studies included in this analysis are described in brief in the following text, and an overview is presented in Supplementary Table 1; a full description of each study has been published.9-13 All studies were conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline and local regulatory requirements. The study protocols were approved by relevant institutional review boards and independent ethics committees. All patients provided written informed consent prior to the start of the studies, and possible side effects were fully explained. The studies were registered at ClinicalTrials.gov (identifiers: NCT01360645, NCT01360632, NCT02196506, NCT01727726, NCT01360866).
The short-term studies (conducted between June 2011 and November 2016 at sites across North America and Europe) were randomized, double-blind, placebo-controlled, phase 3 studies of adjunctive brexpiprazole in patients with MDD and inadequate response to ADTs. Each of the studies had a similar design. Briefly, adult outpatients were enrolled if they had a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)14 diagnosis of single or recurrent nonpsychotic MDD, a current depressive episode of ≥ 8 weeks in duration, an inadequate response to 1-3 prior ADTs during the current episode (defined as < 50% improved according to the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire15), and a 17-item Hamilton Depression Rating Scale (HDRS17)16,17 total score of ≥ 18 (or, in Delphinus, a Montgomery-Åsberg Depression Rating Scale [MADRS]18 total score of ≥ 26) at screening and at the start of prospective treatment. Patients entered an 8-week (or, in Delphinus, an 8- to 10-week) prospective treatment phase in which they received an investigator-determined, open-label ADT together with single- or double-blind placebo. During this phase, patients were assessed for inadequate response to prospective ADT based on HDRS17, Clinical Global Impressions-Improvement scale19 and/or MADRS total score thresholds (see Supplementary Table 1 for the definition in each study). Patients who met the criteria for inadequate response were randomized to double-blind adjunctive treatment with brexpiprazole or placebo (or quetiapine extended-release in Delphinus; these patients were not included in the present analysis) for 6 weeks. Patients received fixed doses of oral brexpiprazole in Pyxis (2 mg/d), Polaris (1 mg/d or 3 mg/d), and Sirius (2 mg/d) and flexible doses of oral brexpiprazole in Delphinus (2-3 mg/d).

- Some atypical antipsychotics used for the adjunctive treatment of major depressive disorder (MDD) are associated with metabolic adverse effects, which increase the risk of serious adverse medical outcomes.
- Brexpiprazole is an alternative adjunctive treatment option for MDD, with a favorable metabolic profile.
The long-term Orion study (conducted from October 2011 to May 2017 at sites across North America and Europe) was an open-label, uncontrolled, phase 3 study that enrolled patients who had completed the last scheduled visit of Pyxis, Polaris, or Delphinus (patients from Sirius were not enrolled), including those patients who responded to prospective ADT. Originally planned as a 52-week study, the design was amended to 26 weeks because the safety profile of brexpiprazole was considered to be well established. Patients received flexibly dosed oral brexpiprazole, adjunct to continued ADT, in the range of 0.5-3 mg/d.
Assessments
Fasting metabolic parameter assessments included serum cholesterol (total, low-density lipoprotein [LDL], and high-density lipoprotein [HDL]), triglycerides, and glucose. In Pyxis and Polaris, metabolic parameters were measured at randomization and at weeks 2, 4, and 6. In Sirius, metabolic parameters were measured at randomization and at week 6. In Delphinus, metabolic parameters were measured 2-4 weeks prior to randomization, 2-4 weeks after randomization, and 2-4 weeks after completion of randomized treatment. In Orion, metabolic parameters were measured at weeks 1, 4, 8, 14, 26, 38, and 52 (pre-amendment) or at weeks 14 and 26 (post-amendment).
In the short-term studies, body weight was measured at randomization and at weekly visits (every 2 weeks in Delphinus). In Orion, body weight was measured at weeks 1, 2, 4, 8, 14, 20, 26, 32, 38, 44, and 52 (pre-amendment) or the same schedule until week 26 (post-amendment).
Data Analysis
For this post hoc analysis, all brexpiprazole data were pooled, as were all placebo data. Analyses were performed on the safety population, defined in the short-term studies as all patients who received at least 1 dose of double-blind treatment in the randomized treatment phase, and in Orion as all patients who received at least 1 dose of open-label brexpiprazole.
In the short-term studies, the baseline value was defined as the last value obtained prior to randomization. Data from Delphinus were not included in the short-term metabolic parameter analyses because, to increase blinding in the Delphinus study, metabolic parameters were not measured at randomization or week 6. (Delphinus data were included in the short-term weight analyses, since weight was measured at each visit.)
For long-term treatment, the baseline value was the last value obtained prior to the start of brexpiprazole treatment. Specifically, for brexpiprazole-naive patients (ie, patients who did not receive brexpiprazole in the parent studies), the baseline value was the last value obtained prior to the start of treatment in Orion; for patients exposed to brexpiprazole in Pyxis or Polaris, the baseline value was the last value obtained prior to randomization in Pyxis or Polaris; and, for patients exposed to brexpiprazole in Delphinus, the baseline value was the last value obtained prior to randomization in Delphinus, except for analyses of the mean change in metabolic parameters, when the baseline value was the last value obtained prior to the start of prospective treatment in Delphinus.
Mean change from baseline in each of the metabolic parameters and in weight was determined to week 6 for the short-term studies and to regular intervals for long-term treatment. All analyses used observed-cases data; change in body weight was also calculated to last visit in the short-term studies. For patients in Orion who were previously exposed to brexpiprazole, "study week" was calculated as the sum of the parent study week and the Orion study week (giving a maximum brexpiprazole exposure of 58 weeks), except for analyses of the mean change in metabolic parameters, when study week corresponded to the Orion study week. Mean change in body weight was also calculated in patient subgroups stratified by baseline body mass index (BMI) and triglyceride levels.
The percentages of patients with categorical shifts in metabolic parameters (definitions adapted from the National Cholesterol Education Program [NCEP] Adult Treatment Panel [ATP] III guidelines20) were determined for the following time periods: from baseline to any post-baseline visit during the first 6 weeks, from baseline to any post-baseline visit up to week 26 (ie, the first 6 months of treatment), and from week 27 to any post-week 27 visit up to week 58 (ie, the last 6 months of treatment).
All analyses were performed using SAS 9.4 software (SAS Institute Inc; Cary, NC).
RESULTS
Patients
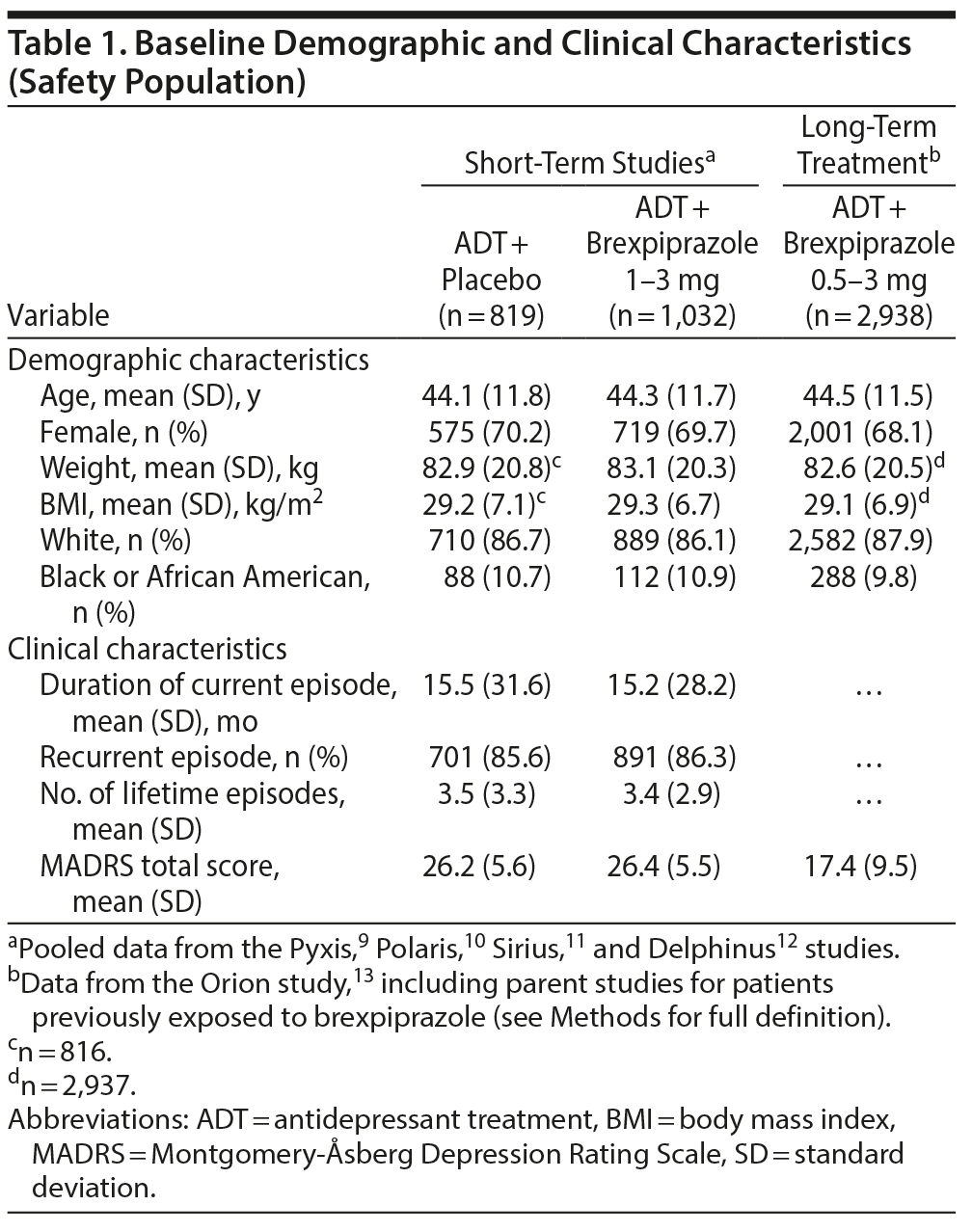
In the short-term studies, the safety population comprised 1,032 patients in the ADT + brexpiprazole 1-3 mg group and 819 patients in the ADT + placebo group. Of the patients allocated to brexpiprazole, 226 were allocated to 1 mg/d, 380 to 2 mg/d, 229 to 3 mg/d, and 197 to 2-3 mg/d (of these flexibly dosed patients, the mean dose at last visit was 2.2 mg). For long-term treatment, the safety population comprised 2,938 patients receiving ADT + brexpiprazole 0.5-3 mg/d. The mean dose of brexpiprazole at last visit of the long-term study was 1.5 mg. Baseline demographic and clinical characteristics were similar between the treatment groups (Table 1).
Metabolic Parameters
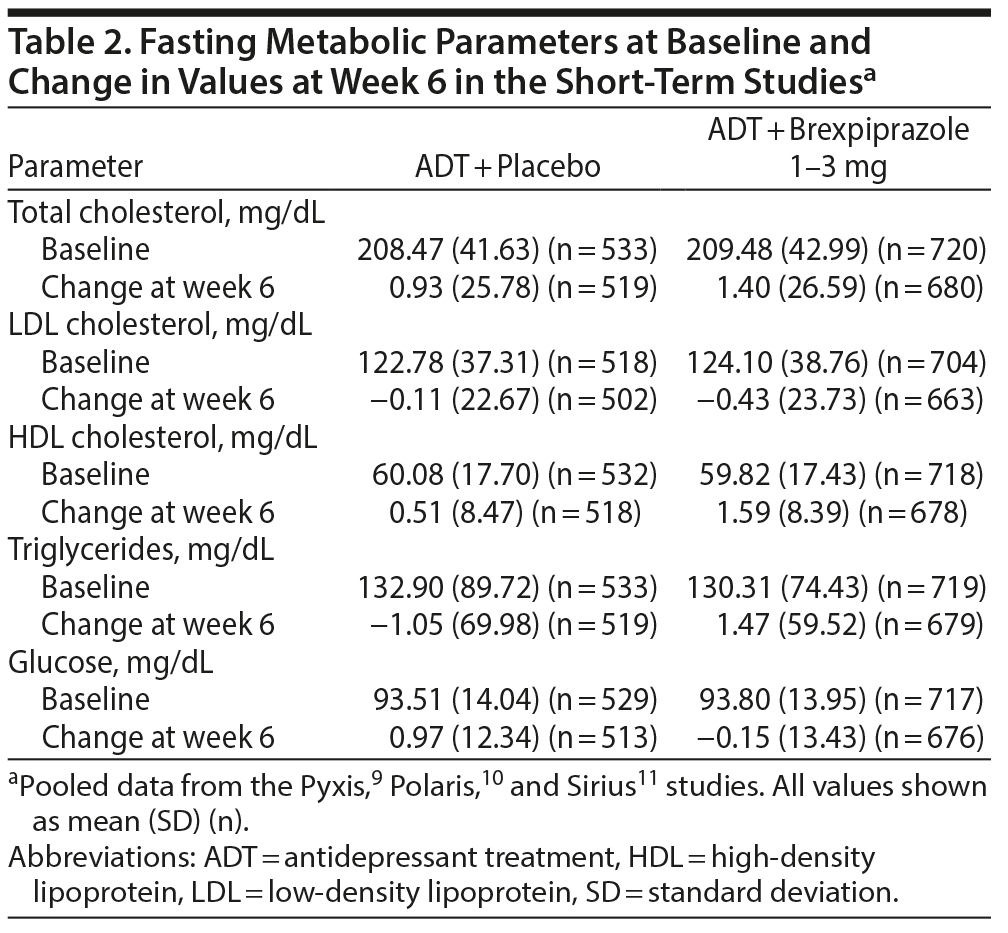
Mean change from baseline. At baseline of the short-term studies, mean fasting metabolic parameters were within the normal range for HDL cholesterol (≥ 40 mg/dL), triglycerides (< 150 mg/dL), and glucose (< 100 mg/dL) and within the borderline range for total cholesterol (≥ 200 to < 240 mg/dL) and LDL cholesterol (≥ 100 to < 160 mg/dL) in both treatment groups (Table 2). In the ADT + brexpiprazole 1-3 mg/d group (n = 1,032) compared with the ADT + placebo group (n = 819), the numbers of patients within the normal ranges at baseline were: total cholesterol, 336 (32.6%) versus 232 (28.3%); LDL cholesterol, 210 (20.3%) versus 145 (17.7%); HDL cholesterol, 673 (65.2%) versus 500 (61.1%); triglycerides, 533 (51.6%) versus 393 (48.0%); and glucose, 556 (53.9%) versus 422 (51.5%).
After 6 weeks of treatment in the short-term studies, mean changes from baseline were small (all < 2 mg/dL in magnitude) in the ADT + brexpiprazole and ADT + placebo groups (Table 2).
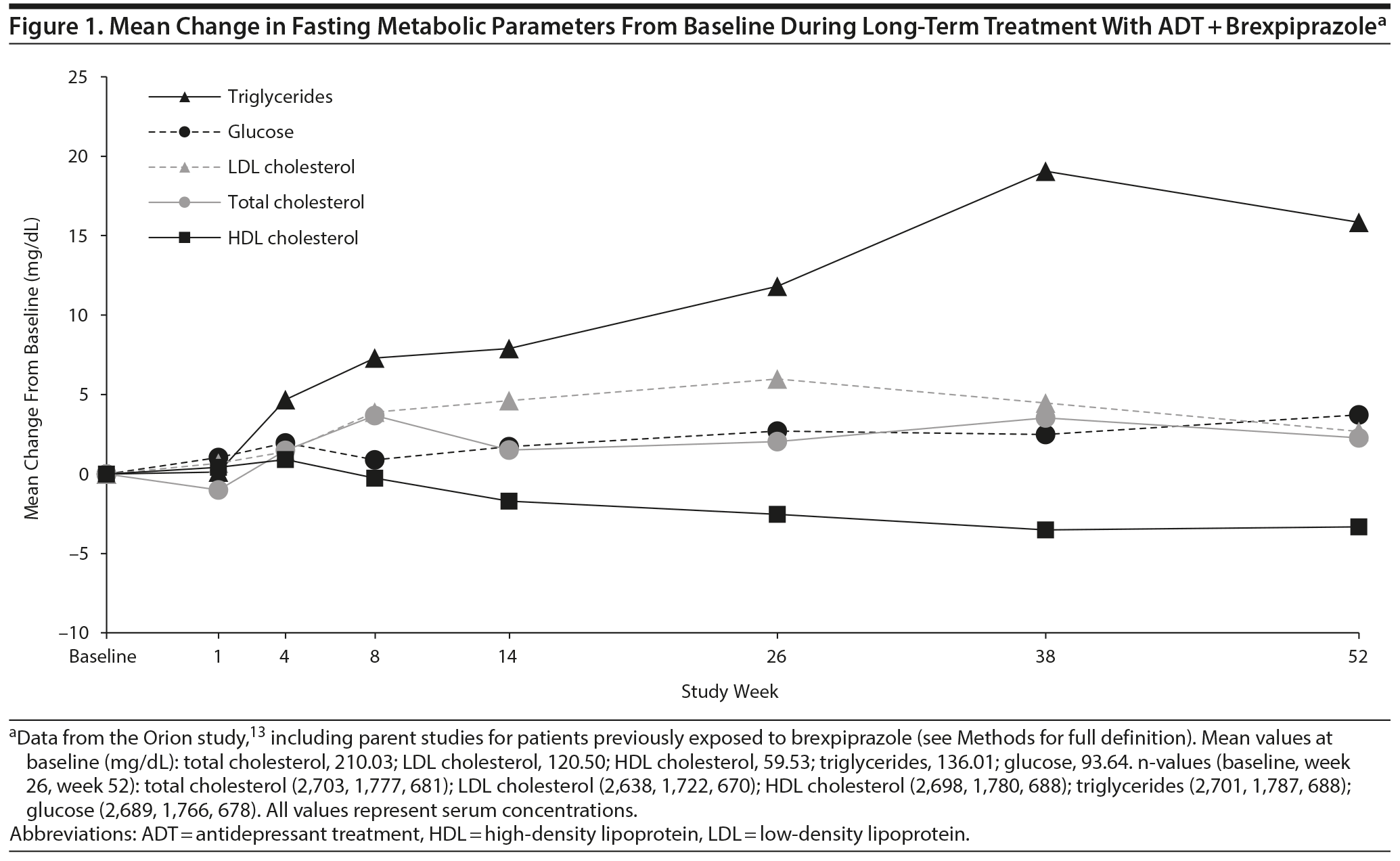
Mean changes from baseline during long-term treatment with ADT + brexpiprazole are shown in Figure 1. Triglycerides increased over time, by a mean of 15.83 mg/dL at week 52 (median increase = 9.5 mg/dL). Other parameters fluctuated over time but showed only small changes from baseline at week 52 (< 4 mg/dL in magnitude).
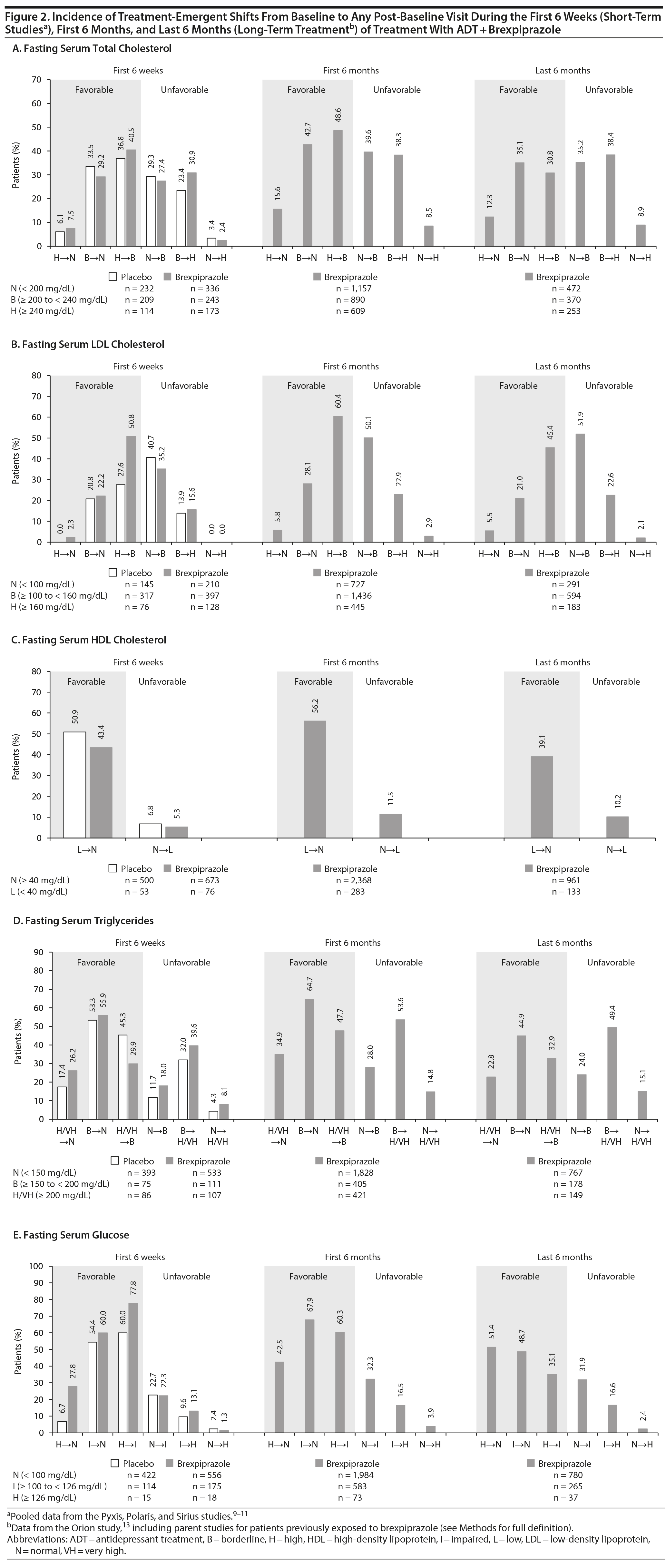
Categorical shifts. Shifts in metabolic parameter categories from baseline to any visit during the first 6 weeks and first 6 months, and from week 27 to any visit during the last 6 months, are shown in Figure 2. In most cases, the incidence of each unfavorable shift was lower than the incidence of its corresponding favorable shift (eg, normal to high versus high to normal).
In the short-term studies, there were no notable differences between the ADT + brexpiprazole and ADT + placebo groups in terms of the incidence of each favorable and unfavorable shift (Figure 2). In the ADT + brexpiprazole group over the first 6 weeks, the incidence of normal to high/very high shifts (normal to low for HDL cholesterol) ranged from 0.0% (LDL cholesterol) to 8.1% (triglycerides), while normal to borderline/impaired shifts ranged from 18.0% (triglycerides) to 35.2% (LDL cholesterol). In the ADT + placebo group over the first 6 weeks, the incidence of normal to high/very high shifts (normal to low for HDL cholesterol) ranged from 0.0% (LDL cholesterol) to 6.8% (HDL cholesterol), while normal to borderline/impaired shifts ranged from 11.7% (triglycerides) to 40.7% (LDL cholesterol).
Considering trends in the long term with ADT + brexpiprazole, the incidence of favorable shifts generally appeared to increase from the first 6 weeks to the first 6 months and then to decrease over the last 6 months (Figure 2). The incidence of unfavorable shifts generally appeared to increase from the first 6 weeks to the first 6 months and then to remain stable between the first 6 months and the last 6 months.
During long-term treatment (Figure 2), the incidence of normal to high/very high shifts (normal to low for HDL cholesterol) over the first 6 months ranged from 2.9% (LDL cholesterol) to 14.8% (triglycerides) and over the last 6 months ranged from 2.1% (LDL cholesterol) to 15.1% (triglycerides). Corresponding normal to borderline/impaired shifts ranged from 28.0% (triglycerides) to 50.1% (LDL cholesterol) in the first 6 months and from 24.0% (triglycerides) to 51.9% (LDL cholesterol) in the last 6 months.
Shifts in metabolic parameter categories by dose in the short-term, fixed-dose studies are shown in Supplementary Tables 2-6. The incidence of shifts in metabolic parameters showed no consistent relationship with the dose of brexpiprazole.
Weight
Mean change from baseline. In the short-term studies, the mean (SD) increase in body weight from baseline to last visit was 1.5 (2.2) kg in the ADT + brexpiprazole 1-3 mg group and 0.3 (1.8) kg in the ADT + placebo group. The corresponding increases from baseline to week 6 were 1.4 (2.1) kg and 0.3 (1.7) kg. The mean changes in body weight from baseline to weeks 26 and 58 with long-term ADT + brexpiprazole treatment were 2.9 (4.2) kg and 3.8 (5.4) kg, respectively.
Mean changes in body weight for patients receiving ADT + brexpiprazole, stratified by baseline BMI and triglyceride level, are shown in Supplementary Figure 1. All subgroups showed similar increases in weight from baseline to week 6 (range, 0.9-1.7 kg), to week 26 (range, 2.7-3.0 kg), and to week 58 (range, 3.1-5.0 kg).
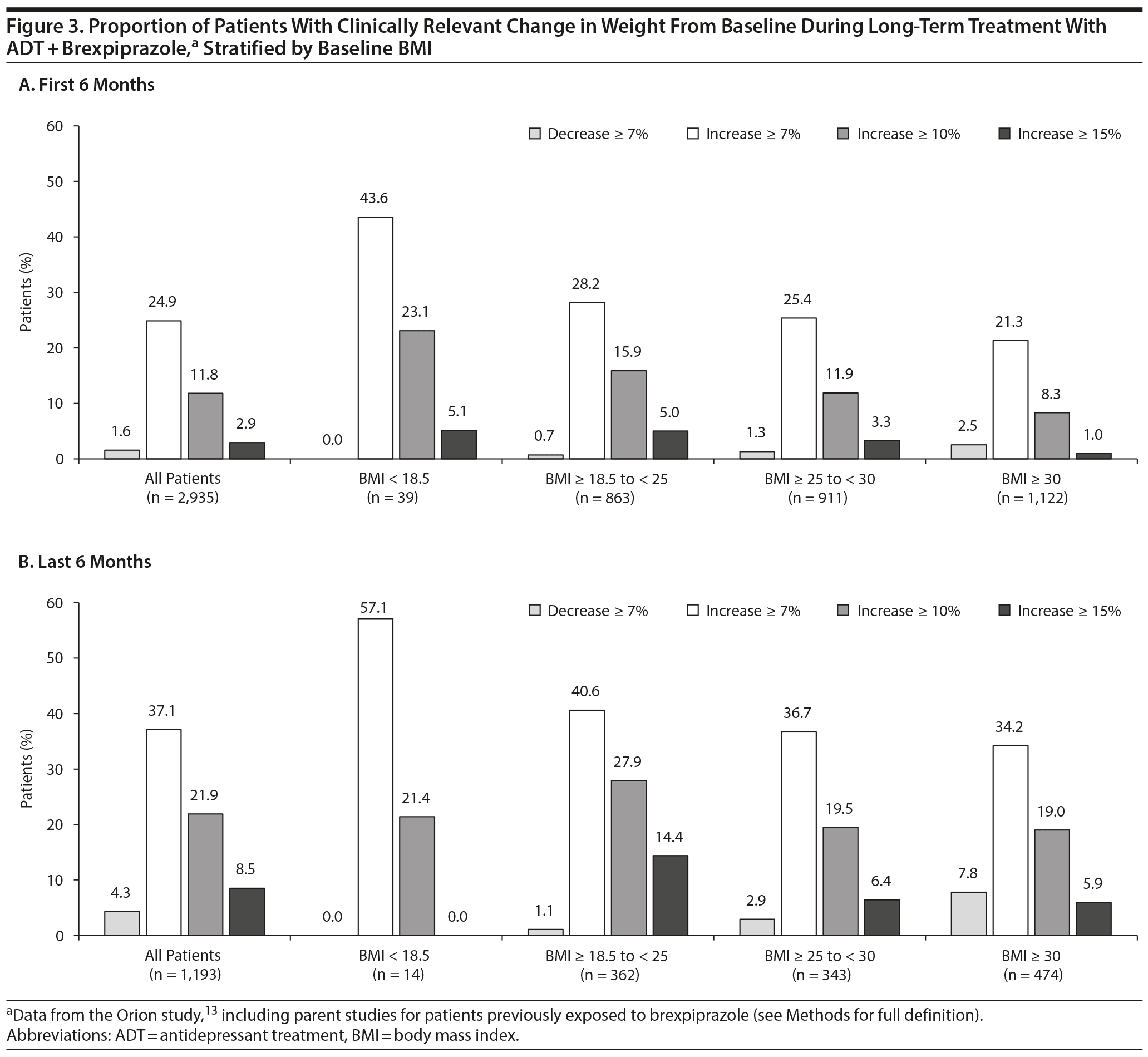
Clinically relevant change. The proportion of patients with clinically relevant change in weight from baseline, stratified by baseline BMI, is shown in Figure 3. Overall, weight increase ≥ 7% was seen in 730 (24.9%) of 2,935 patients over the first 6 months and 443 (37.1%) of 1,193 patients over the last 6 months. The proportion of patients with clinically relevant weight gain generally decreased with increasing baseline BMI.
No patients discontinued due to weight increase in the short-term studies. In the long-term study, 60 (2.0%) of 2,938 patients discontinued due to weight increase.
DISCUSSION
Using a detailed approach to investigate favorable and unfavorable shifts in metabolic parameters, the present analysis of 4 short-term studies and 1 long-term study in adults with MDD showed that adjunctive brexpiprazole has only small adverse effects on risk-related metabolic parameters. Other atypical antipsychotics have varying risks for different metabolic adverse effects; a meta-analysis4 of adjunctive atypical antipsychotics in MDD found that quetiapine and olanzapine-fluoxetine combination were the most strongly associated with abnormal metabolic laboratory results. Across psychiatric diagnoses, all atypical antipsychotics are associated with metabolic dysfunction to some extent, with clozapine, olanzapine, and quetiapine being the most likely to cause metabolic abnormalities, and amisulpride, aripiprazole, asenapine, brexpiprazole, cariprazine, lurasidone, and ziprasidone having the lowest risk.21-23 In a naturalistic study24 of outpatients with psychosis, metabolic syndrome (a combination of metabolic factors that increases the risk of developing type 2 diabetes mellitus and cardiovascular disease) was present in 44% of patients receiving antipsychotic maintenance treatment. From a clinical perspective, metabolic adverse effects increase the complexity and cost of patient management and—most importantly—increase the risk of serious adverse medical outcomes.6 Thus, there is a need for efficacious antipsychotics with favorable metabolic profiles.
Fasting triglycerides was the only studied metabolic parameter that increased with 52 weeks of adjunctive brexpiprazole treatment. From a mean value within the normal range at baseline (136.01 mg/dL), triglycerides increased by a mean of 15.83 mg/dL to week 52 (median increase = 9.5 mg/dL). A comparable increase in fasting triglycerides (median = 8.0 mg/dL) was observed in a 52-week, open-label study25 of adjunctive aripiprazole, from a mean value that was borderline at baseline (156.5 mg/dL). Open-label quetiapine extended-release monotherapy in MDD is also associated with increases in triglycerides.26
With regard to body weight, the mean increase of 1.5 kg observed in the short-term adjunctive brexpiprazole studies is comparable to those previously reported for aripiprazole (1.1 kg), quetiapine (0.9 kg), and risperidone (1.3 kg) in a meta-analysis4 of adjunctive atypical antipsychotics in MDD and lower than that reported for olanzapine-fluoxetine combination (4.2 kg). In a post hoc study27 that compared weight data for adjunctive brexpiprazole and aripiprazole in patients with MDD, a similar effect on body weight was found over the course of 1 year (mean gains of 3.2 kg with brexpiprazole and 4.0 kg with aripiprazole). In the present analysis, the mean increase in body weight over 58 weeks with adjunctive brexpiprazole was 3.8 kg. The proportion of patients with clinically relevant weight gain was greater among those with a lower BMI at baseline. While the subgroup with baseline BMI < 18.5 kg/m2 was small (n = 39), and results should therefore be interpreted with caution, the weight gain observed in these patients may include the effect of recovery from depression, as has been seen previously in a 1-year study28 of the antidepressant fluoxetine.
Across psychiatric diagnoses, almost all approved antipsychotics have been associated with an increase in body weight over time.5,29 Atypical antipsychotics with the lowest potential for inducing weight gain are amisulpride, aripiprazole, brexpiprazole, cariprazine, lurasidone, and ziprasidone.22 Clozapine and olanzapine have the highest potential for weight gain, and paliperidone, quetiapine, risperidone, and sertindole have an intermediate risk.22 In a focus group, patients with MDD considered weight increase to be among the most "bothersome" of antipsychotic adverse events.30 Thus, the potential effect of treatment on body weight is an important consideration when selecting a particular antipsychotic for the treatment of psychiatric disorders.
In the present analysis, there was no consistent effect of adjunctive brexpiprazole dose on metabolic parameter shifts in the short-term studies (within the range of 1-3 mg/d). Previous analyses31-33 have shown that mean weight gain, the incidence of weight increase ≥ 7%, and the incidence of weight increase as a treatment-emergent adverse event (TEAE) are not affected by adjunctive brexpiprazole dose.
The present investigation used observed-cases data, meaning that patients who dropped out of the studies did not contribute data to analyses at timepoints after the time of their discontinuation. Potentially, if patients dropped out due to metabolic issues, this approach could minimize the impact of brexpiprazole on metabolic parameters. However, the rate of discontinuation due to weight increase was minimal, and the overall rate of discontinuation due to TEAEs was low in the adjunctive brexpiprazole clinical program,32 suggesting that the use of observed-cases data did not impact the results of this analysis.
With regard to the overall adverse event profile of adjunctive brexpiprazole, the only TEAEs with incidence ≥ 5% across short-term studies were akathisia, weight increase, and headache (all with incidence < 10%).32 In the long term, weight increase was the most common TEAE with adjunctive brexpiprazole,32 but was generally not associated with discontinuation. Compared with other atypical antipsychotics, brexpiprazole has a low propensity for activating side effects (akathisia, restlessness, agitation, anxiety, insomnia) and sedating side effects (somnolence, sedation, fatigue).34 Brexpiprazole has no clinically relevant effects on prolactin, electrocardiograms, vital signs, or other laboratory parameters.32
A limitation of this analysis is that it is not possible to discern the relative contributions to weight gain of the adjunctive therapy, the background ADT, and improvement from the depressive episode. It is also difficult to generalize the results of clinical trials to real-world populations. It should be noted that, on average, patients were overweight at baseline, with a mean BMI of 29, and thus these results may not be generalizable to regions associated with lower BMIs. Long-term, head-to-head data are required to fully understand and compare the effects of different treatments on metabolic parameters and weight gain. A strength of the analysis is that it is based on a large, high-quality data set that has been subjected to rigorous review by several regulatory agencies.
In conclusion, treatment with brexpiprazole, adjunct to ADT, was associated with small changes in metabolic parameters and moderate weight gain in short- and long-term settings among patients with MDD.
Submitted: December 4, 2018; accepted May 3, 2019.
Published online: October 1, 2019.
Potential conflicts of interest: Dr Newcomer has received grant support from the National Institutes of Health, Substance Abuse and Mental Health Services Administration, and Otsuka America Pharmaceutical Co Ltd; has served as a consultant to Indivior, Auris, Sunovion, Otsuka, and Alkermes; has been involved in patent litigation on behalf of Sunovion; and serves on a Data Safety Monitoring Board for Amgen. Drs Eriksson and Meehan are full-time employees of H. Lundbeck A/S. Drs Zhang and Weiss are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc.
Funding/support: This work was supported by Otsuka Pharmaceutical Development & Commercialization Inc (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark).
Role of the sponsor: Authors affiliated with the sponsors were involved in the design of the study, the analysis and interpretation of data, and the writing and reviewing of this article.
Acknowledgments: Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc and H. Lundbeck A/S. The authors would like to thank the investigators at the trial sites as well as the patients who participated in the trials.
Supplementary material: See accompanying pages.
REFERENCES
1.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed CrossRef
2.Connolly KR, Thase ME. If at first you don’ t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43-64. PubMed CrossRef
3.Patkar AA, Pae CU. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(suppl 1):S29-S37. PubMed CrossRef
4.Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed CrossRef
5.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93. PubMed CrossRef
6.Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13(1):27-35. PubMed CrossRef
7.Nicol GE, Yingling MD, Flavin KS, et al. Metabolic effects of antipsychotics on adiposity and insulin sensitivity in youths: a randomized clinical trial. JAMA Psychiatry. 2018;75(8):788-796. PubMed CrossRef
8.Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. PubMed CrossRef
9.Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231. PubMed CrossRef
10.Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232-1240. PubMed CrossRef
11.Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry. 2018;79(4):17m12058.
12.Hobart M, Skuban A, Zhang P, et al. Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: a randomized, active-referenced, placebo-controlled study. Curr Med Res Opin. 2018;34(4):633-642. PubMed CrossRef
13.Hobart M, Zhang P, Skuban A, et al. A long-term, open-label study to evaluate the safety and tolerability of brexpiprazole as adjunctive therapy in adults with major depressive disorder. J Clin Psychopharmacol. 2019;39(3):203-209. PubMed CrossRef
14.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
15.Chandler GM, Iosifescu DV, Pollack MH, et al. Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. PubMed CrossRef
16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed CrossRef
17.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. PubMed CrossRef
18.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
19.Guy W. ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD: National Institute of Mental Health; 1976.
20.National Cholesterol Education Program. ATP III Guidelines At-A-Glance Quick Desk Reference. Publication No. 01-3305. Bethesda, MD: National Institutes of Health (NIH), National Heart, Lung, and Blood Institute; 2001.
21.De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114-126. PubMed CrossRef
22.Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757-777. PubMed CrossRef
23.Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339-347. PubMed CrossRef
24.Bodén R, Edman G, Reutfors J, et al. A comparison of cardiovascular risk factors for ten antipsychotic drugs in clinical practice. Neuropsychiatr Dis Treat. 2013;9:371-377. PubMed CrossRef
25.Berman RM, Thase ME, Trivedi MH, et al. Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder. Neuropsychiatr Dis Treat. 2011;7:303-312. PubMed CrossRef
26.Liebowitz M, Lam RW, Lepola U, et al. Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: a randomized, placebo-controlled trial. Depress Anxiety. 2010;27(10):964-976. PubMed CrossRef
27.Weiss C, Weiller E, Baker RA, et al. The effects of brexpiprazole and aripiprazole on body weight as monotherapy in patients with schizophrenia and as adjunctive treatment in patients with major depressive disorder: an analysis of short-term and long-term studies. Int Clin Psychopharmacol. 2018;33(5):255-260. PubMed CrossRef
28.Michelson D, Amsterdam JD, Quitkin FM, et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry. 1999;156(8):1170-1176. PubMed
29.Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9(4):e94112. PubMed CrossRef
30.Llorca PM, Lançon C, Hartry A, et al. Assessing the burden of treatment-emergent adverse events associated with atypical antipsychotic medications. BMC Psychiatry. 2017;17(1):67. PubMed CrossRef
31.Rexulti (brexpiprazole) tablets, for oral use [prescribing information]. Tokyo, Japan: Otsuka Pharmaceutical Co Ltd; 2018. https://www.otsuka-us.com/media/static/Rexulti-PI.pdf. Accessed April 26, 2019.
32.Nelson JC, Zhang P, Skuban A, et al. Overview of short-term and long-term safety of brexpiprazole in patients with major depressive disorder and inadequate response to antidepressant treatment. Curr Psychiatry Rev. 2016;12(3):278-290. CrossRef
33.Thase ME, Zhang P, Skuban A, et al. Efficacy of adjunctive brexpiprazole in patients with major depressive disorder: a clinical overview. Curr Psychiatry Rev. 2016;12(3):291-301. CrossRef
34.Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138-147. PubMed CrossRef
This PDF is free for all visitors!