Objective: To evaluate long-term safety and efficacy of esketamine nasal spray plus a new oral antidepressant (OAD) in patients with treatment-resistant depression (TRD).
Methods: This phase 3, open-label, multicenter, long-term (up to 1 year) study was conducted between October 2015 and October 2017. Patients (≥ 18 years) with TRD (DSM-5 diagnosis of major depressive disorder and nonresponse to ≥ 2 OAD treatments) were enrolled directly or transferred from a short-term study (patients aged ≥ 65 years). Esketamine nasal spray (28-mg, 56-mg, or 84-mg) plus new OAD was administered twice a week in a 4-week induction (IND) phase and weekly or every-other-week for patients who were responders and entered a 48-week optimization/maintenance (OP/MAINT) phase.
Results: Of 802 enrolled patients, 86.2% were direct-entry and 13.8% were transferred-entry; 580 (74.5%) of 779 patients who entered the IND phase completed the phase, and 150 (24.9%) of 603 who entered the OP/MAINT phase completed the phase. Common treatment-emergent adverse events (TEAEs) were dizziness (32.9%), dissociation (27.6%), nausea (25.1%), and headache (24.9%). Seventy-six patients (9.5%) discontinued esketamine due to TEAEs. Fifty-five patients (6.9%) experienced serious TEAEs. Most TEAEs occurred on dosing days, were mild or moderate in severity, and resolved on the same day. Two deaths were reported; neither was considered related to esketamine. Cognitive performance generally either improved or remained stable postbaseline. There was no case of interstitial cystitis or respiratory depression. Treatment-emergent dissociative symptoms were transient and generally resolved within 1.5 hours postdose. Montgomery-Åsberg Depression Rating Scale total score decreased during the IND phase, and this reduction persisted during the OP/MAINT phase (mean [SD] change from baseline of respective phase to endpoint: IND, −16.4 [8.76]; OP/MAINT, 0.3 [8.12]).
Conclusions: Long-term esketamine nasal spray plus new OAD therapy had a manageable safety profile, and improvements in depression appeared to be sustained in patients with TRD.
Trial Registration: ClinicalTrials.gov identifier: NCT02497287
ABSTRACT
Objective: To evaluate long-term safety and efficacy of esketamine nasal spray plus a new oral antidepressant (OAD) in patients with treatment-resistant depression (TRD).
Methods: This phase 3, open-label, multicenter, long-term (up to 1 year) study was conducted between October 2015 and October 2017. Patients (≥ 18 years) with TRD (DSM-5 diagnosis of major depressive disorder and nonresponse to ≥ 2 OAD treatments) were enrolled directly or transferred from a short-term study (patients aged ≥ 65 years). Esketamine nasal spray (28-mg, 56-mg, or 84-mg) plus new OAD was administered twice a week in a 4-week induction (IND) phase and weekly or every-other-week for patients who were responders and entered a 48-week optimization/maintenance (OP/MAINT) phase.
Results: Of 802 enrolled patients, 86.2% were direct-entry and 13.8% were transferred-entry; 580 (74.5%) of 779 patients who entered the IND phase completed the phase, and 150 (24.9%) of 603 who entered the OP/MAINT phase completed the phase. Common treatment-emergent adverse events (TEAEs) were dizziness (32.9%), dissociation (27.6%), nausea (25.1%), and headache (24.9%). Seventy-six patients (9.5%) discontinued esketamine due to TEAEs. Fifty-five patients (6.9%) experienced serious TEAEs. Most TEAEs occurred on dosing days, were mild or moderate in severity, and resolved on the same day. Two deaths were reported; neither was considered related to esketamine. Cognitive performance generally either improved or remained stable postbaseline. There was no case of interstitial cystitis or respiratory depression. Treatment-emergent dissociative symptoms were transient and generally resolved within 1.5 hours postdose. Montgomery-Åsberg Depression Rating Scale total score decreased during the IND phase, and this reduction persisted during the OP/MAINT phase (mean [SD] change from baseline of respective phase to endpoint: IND, −16.4 [8.76]; OP/MAINT, 0.3 [8.12]).
Conclusions: Long-term esketamine nasal spray plus new OAD therapy had a manageable safety profile, and improvements in depression appeared to be sustained in patients with TRD.
Trial Registration: ClinicalTrials.gov identifier: NCT02497287
J Clin Psychiatry 2020;81(3):19m12891
To cite: Wajs E, Aluisio L, Holder R, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020;81(3):19m12891.
To share: https://doi.org/10.4088/JCP.19m12891
© Copyright 2020 Physicians Postgraduate Press, Inc.
aJanssen Research & Development, Beerse, Belgium
bJanssen Research & Development, San Diego, California
cJanssen Inc, Toronto, Ontario, Canada
dJanssen Research & Development, Titusville, New Jersey
eJanssen Research & Development, Pennington, New Jersey
fThe Yale Depression Research Program, Yale University, New Haven, Connecticut
gDepartment of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King’s College London & South London, London, United Kingdom
hMaudsley NHS Foundation Trust, London, United Kingdom
iDepartment of Psychiatry and Psychotherapy, Medical University, Vienna, Austria
jDepartment of Psychological Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
kDepartment of Psychiatry, Taipei Veterans General Hospital, Taipei, Taiwan; School of Medicine and Institute of Brain Science, National Yang-Ming University, Taiwan
lDepartment of Psychiatry/Kyung Hee University College of Medicine, Seoul, South Korea
mPeninsula Therapeutic and Research Group, Frankston, Victoria, Australia
nDepartment of Psychiatry, Depression Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
‡Contributed equally as senior authors
*Corresponding author: Ewa Wajs, MD, PhD, Director Neuroscience R&D, Janssen Research & Development BE, Turnhoutseweg 30, 2340 Beerse, Belgium ([email protected]).
Esketamine, the S-enantiomer of ketamine racemate and an N-methyl-d-aspartate receptor antagonist, has been recently approved as a nasal spray administration for the treatment of treatment-resistant depression (TRD).1-4 Rapid and robust reductions in symptoms of TRD along with manageable tolerability have been observed following intravenous and intranasal administration of esketamine adjunctive to oral antidepressant (OAD) therapy in short-term studies1,3-5; however, long-term safety has remained to be elucidated.
Potentially serious safety concerns of long-term, ketamine/esketamine use have been posited following observations of cognitive deficits (spatial memory and pattern recognition), bladder toxicity with interstitial/ulcerative cystitis, hepatotoxicity, and dependence with prolonged, or frequent, recreational long-term use of ketamine 3 times a week or daily.6-9
As TRD is a chronic, recurrent condition with an escalating demand for pharmacotherapies that provide sustained benefit, this study aimed to assess the safety, tolerability, and efficacy of esketamine nasal spray plus a new OAD to empirically validate its long-term use in patients with TRD.
METHODS
Study Design
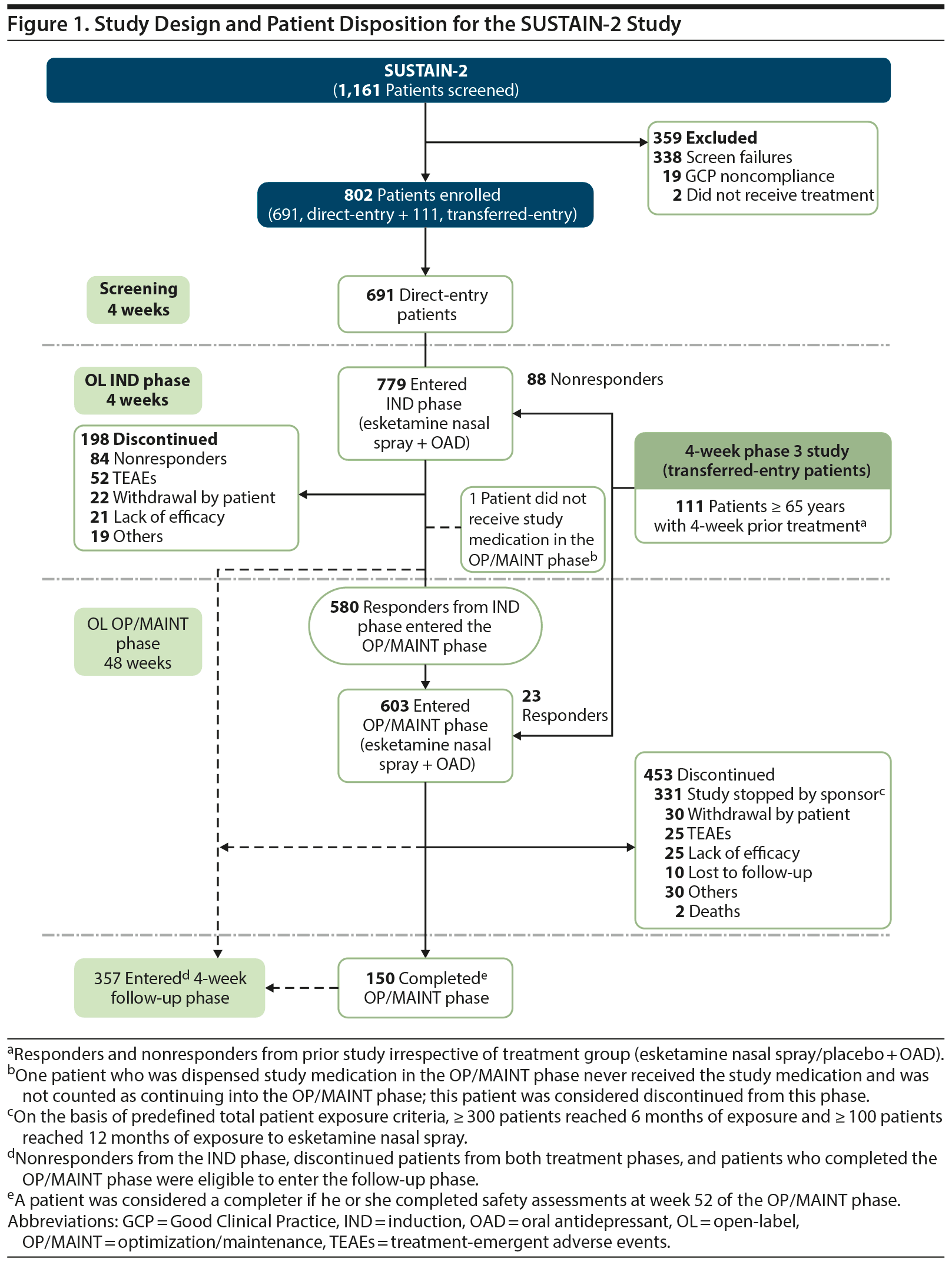
This open-label (OL), multicenter study was conducted between October 2015 and October 2017 at 114 sites in 21 countries. The study had a 4-week screening phase (direct-entry patients only), a 4-week induction (IND) phase (direct-entry patients and transferred-entry nonresponder patients), an up to 48-week optimization/maintenance (OP/MAINT) phase (all responders from the OL IND phase of the current study and the transferred-entry responders), and a 4-week follow-up phase (for all patients). By design, the study closure was set at the timepoint when the pre-specified esketamine exposure criteria were met (ie, ≥ 300 patients exposed for 6 months and ≥ 100 patients exposed for 1 year).
The appropriate ethics body at each site approved the study protocol. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent before enrollment (SUSTAIN-2; NCT02497287).
Patients
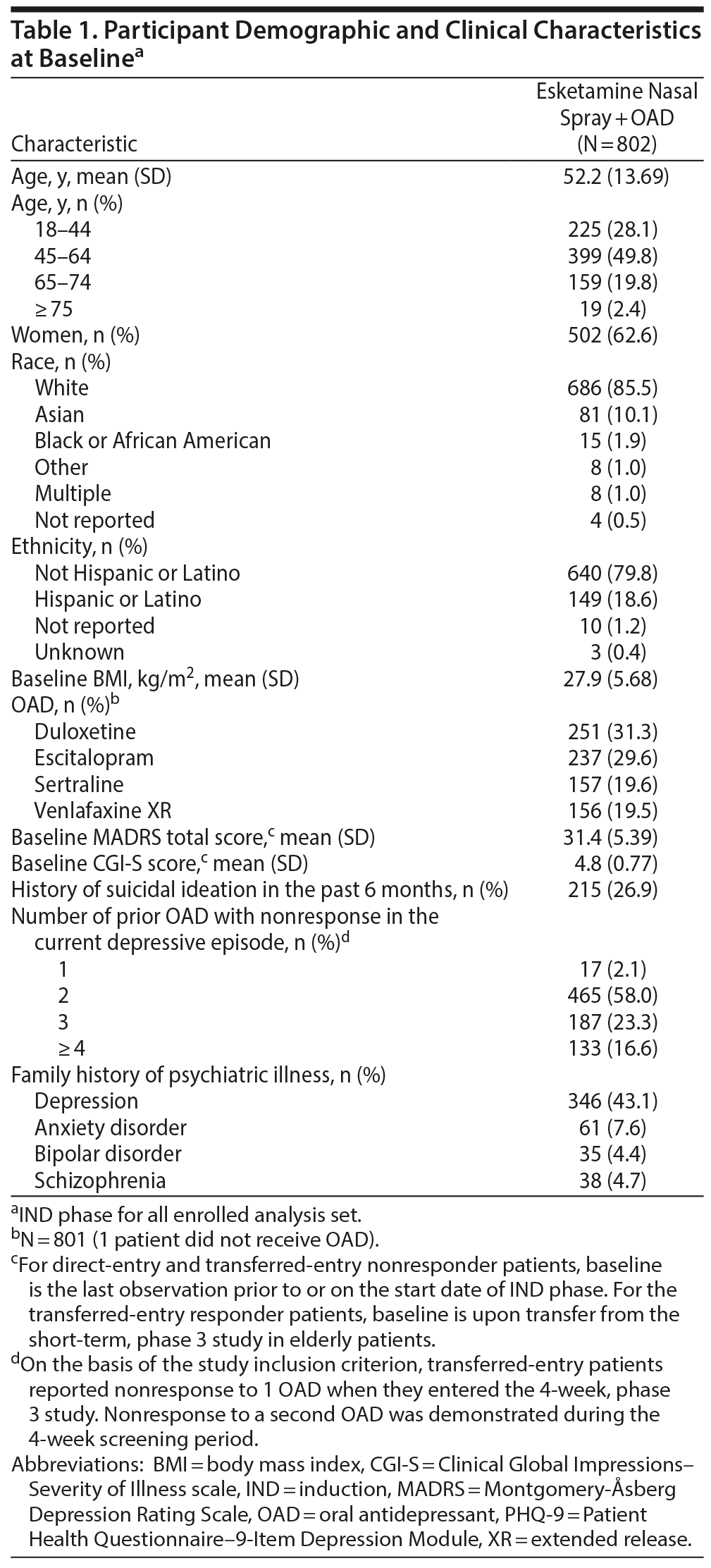
Details of patient eligibility criteria appear at https://clinicaltrials.gov/ct2/show/NCT02497287. Patients entered the study either directly (“direct-entry patients,” aged ≥ 18 years) or after completing the double-blind IND phase of a randomized, 4-week, efficacy study10 (“short-term study”) in patients aged ≥ 65 years with TRD (“transferred-entry patients”). Transferred-entry patients who were responders (≥ 50% reduction in Montgomery-Åsberg Depression Rating Scale [MADRS]11 total score) in the short-term study joined the current study in the OP/MAINT phase, while nonresponders joined the IND phase. Eligible patients had DSM-5 diagnosis of recurrent major depressive disorder (MDD) or single episode (≥ 2 years) MDD without psychotic features, nonresponse to ≥ 2 OADs (as assessed retrospectively using the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire [ATRQ]12) in the current depressive episode, and MADRS total score ≥ 22 at screening (Supplementary Appendix 1).
Treatment
Induction phase. Patients self-administered esketamine nasal spray (200 µL solution [14 mg esketamine/100 µL spray]) twice a week for 4 weeks as a flexible-dose regimen starting at 28 mg (≥ 65-years) or 56 mg (< 65-years) under supervision of a health care provider. Adjustments for subsequent doses (< 65-years: 56 or 84 mg; ≥ 65-years: 28, 56, or 84 mg) were allowed based on efficacy and tolerability per the investigator’s clinical judgment. Direct-entry patients simultaneously initiated a new OAD, and transferred nonresponder patients continued the OAD initiated in the short-term study (either duloxetine, escitalopram, sertraline, or venlafaxine extended-release).
Optimization/maintenance phase. During weeks 5-8, responders from the IND phase were administered esketamine once-weekly at the same dose and continued the OAD treatment. Transferred-entry responder patients started a flexible dosing regimen at 28 mg (week 5) with possible dose up-titration (56 mg or 84 mg) allowed through week 8 and continued the OAD initiated in the short-term study. Subsequently, esketamine dosing frequency was driven by an algorithm; treatment regimens were either weekly (if MADRS total score was > 12) or every-other-week (if MADRS total score was ≤ 12), with reevaluation at 4-week intervals.
Esketamine treatment was discontinued in the follow-up phase, and patients were encouraged to continue treatment with OADs if clinically appropriate (see Supplementary Appendix 1).
Safety Assessments
Treatment-emergent adverse events (TEAEs) were monitored throughout the study. Cogstate Computerized Test Battery (Cogstate, Inc; New Haven, Connecticut) included the measures of processing speed (simple reaction time—detection, DET) and choice reaction time (identification, IDN), visual learning and recall (one card learning, OCL), working memory (one back, ONB), and executive function/visuospatial memory and sequencing (Groton Maze Learning Test, GMLT). In addition, episodic memory was evaluated (Hopkins Verbal Learning Test-Revised, HVLT-R13). Cognitive assessments were performed at predose. Suicidal ideation and behavior (Columbia-Suicide Severity Rating Scale [C-SSRS]14), dissociative symptoms (Clinician Administered Dissociative States Scale [CADSS]15), psychotic and affective symptoms (positive symptom subscale of the Brief Psychiatric Rating Scale [BPRS+]16), and sedation (Modified Observer’s Assessment of Alertness/Sedation [MOAA/S]17) were assessed longitudinally. Bladder symptoms were monitored using Bladder Pain/Interstitial Cystitis Symptom Score (BPIC-SS).18 The 20-item Physician Withdrawal Checklist (PWC-20)19 was used to assess potential withdrawal symptoms following cessation of esketamine at IND- or OP/MAINT-endpoint and at weeks 1, 2, and 4 of the follow-up phase. Clinical laboratory tests, vital signs assessment, electrocardiogram (ECG), nasal examination, and nasal symptom questionnaire were performed at prespecified timepoints. Respiratory rate and pulse oximetry were monitored with a Masimo Radical-7 Pulse CO-oximeter (Masimo Corporation; Irvine, California) at each dosing session. Per protocol guidance, administration of esketamine nasal spray was not recommended if the patient’s blood pressure (BP) was repeatedly > 140/90 (< 65 years) or > 150/90 (≥ 65 years) mm Hg. If the BP was ≥ 200/120 (< 65 years) or ≥ 190/110 (≥ 65 years) mm Hg, treatment with esketamine nasal spray was discontinued.

- Esketamine has shown favorable efficacy and tolerability in short-term studies of patients with treatment-resistant depression (TRD); however, data on its long-term safety have been lacking prior to the current study.
- Long-term (up to 1 year) treatment with esketamine nasal spray demonstrated acceptable tolerability and an adverse event profile comparable with that of the short-term studies in patients with TRD.
Efficacy Assessments
Changes in MADRS total score from IND and OP/MAINT baseline to respective endpoints were assessed. Response (≥ 50% reduction in MADRS total score) and remission (MADRS total score ≤ 12 and ≤ 10)20-22 rates over time were assessed from baseline to endpoint and over time in the IND and OP/MAINT phases. Symptom status was also assessed by Patient Health Questionnaire-9-Item Depression Module (PHQ-9),23 and functional disability was evaluated using Sheehan Disability Scale (SDS).24,25 Clinician-determined global severity of the patient’s illness was assessed by Clinical Global Impressions-Severity of Illness Scale (CGI-S).26
Statistical Methods
No formal sample size calculation was performed. The projected sample size of 750 direct-entry plus transferred-entry patients was considered adequate to obtain ≥ 300 patients who had received treatment with esketamine for 6 months and ≥ 100 patients for 12 months and to include ≥ 100 patients aged ≥ 65 years.
Safety and efficacy outcomes were summarized descriptively based on the full analysis sets (all patients who received ≥ 1 dose of esketamine or 1 dose of OAD) for both treatment phases. Selected safety analyses were summarized for the entire treatment period based on the all enrolled analysis set (all patients who were not screen failures and received ≥ 1 dose of esketamine or 1 dose of OAD). Efficacy was analyzed using last-observation-carried-forward data and observed data.
RESULTS
Patients and Disposition
In total, 1,161 patients were screened, and 802 patients were enrolled (Figure 1). At study closure, 364 patients were dosed for 6 months, and 136 were dosed for 12 months. A total of 357 patients entered the follow-up phase, and 463 patients continued esketamine treatment in a separate OL extension safety study (NCT02782104). The mean age at IND baseline was 52.2 years, 62.6% of patients were women, and 85.5% were white (Table 1).
Extent of Exposure
Median exposure to esketamine was 22.9 weeks. Greater proportions of patients received a final dose of 56-mg (IND: 45.8%; OP/MAINT: 45.6%) and 84-mg (IND: 48.8%; OP/MAINT: 50.2%) esketamine as compared with 28-mg esketamine (IND: 5.3%; OP/MAINT: 4.0%). During the OP/MAINT phase, 24.0% of patients received weekly dosing, 38.1% were maintained on every-other-week dosing, and 37.8% switched more than once between weekly and every-other-week dosing based on the algorithm (see Methods).
Safety
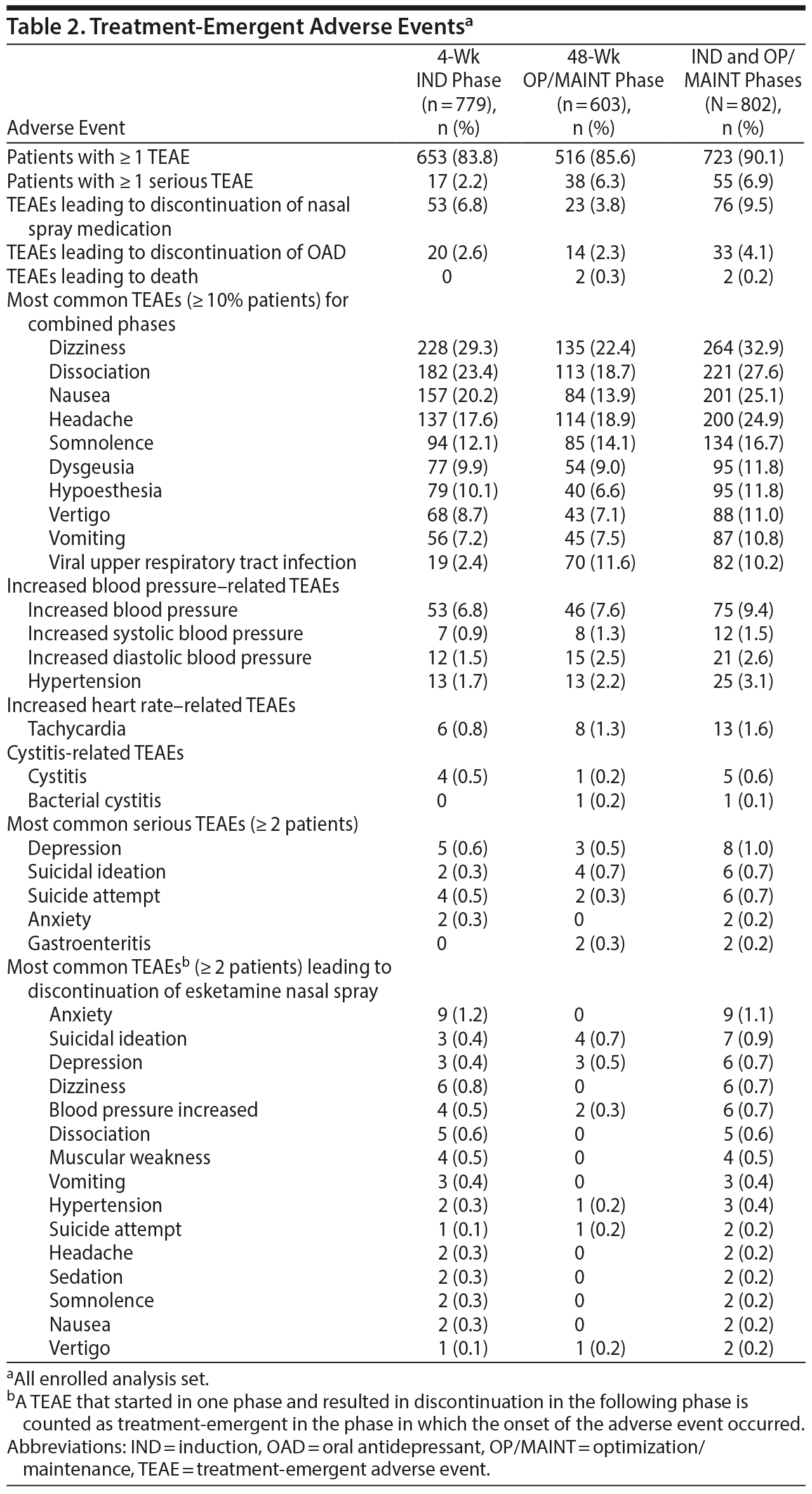
Treatment-emergent adverse events and related rating scale measures. TEAEs were reported in 723/802 patients (90.1%; Table 2). Dizziness (32.9%), dissociation (27.6%), nausea (25.1%), and headache (24.9%) were reported frequently (≥ 20% patients) during the IND and OP/MAINT phases (percentages shown are the overall combined values for the 2 study phases). Dissociative symptoms presented in a variety of ways including perceptual changes and as being described as feeling disconnected from oneself, one’s thoughts, feelings, space, and time. Overall, the types and frequencies of TEAEs were similar in the IND and OP/MAINT phases. Most esketamine-related TEAEs were mild or moderate in intensity, occurred shortly after esketamine nasal spray administration, and resolved the same day. Seventy-six patients (9.5%) had TEAEs that led to discontinuation of esketamine. Serious TEAEs were reported in 55/802 patients (6.9%), of which 5 events in 4 patients were considered to be esketamine-related by the investigator: suicidal ideation (n = 1), suicide attempt (n = 1), anxiety and delusions (both in 1 patient), and delirium (n = 1). Two deaths, assessed as doubtful or unrelated to esketamine by the investigator, were reported, both occurring in the OP/MAINT phase; 1 event due to acute cardiac and respiratory failure and the other due to suicide (see “Serious TEAEs” in Supplementary Appendix 2). Dizziness, dissociation, and somnolence were frequent (≥ 10% of patients), occurred on the day of esketamine administration, and were considered related to its mechanism of action. There were no reports of drug seeking, overdose, or abuse of esketamine (or ketamine), and there were no requests from patients to increase the frequency of dosing intervals (other than those specified in the protocol) or to increase the esketamine dose (> 84 mg). Urine drug screen tests for other drugs of abuse were conducted every 2 weeks during the IND phase and every 8 weeks in the OP/MAINT phase; 1 patient was discontinued due to protocol deviation of positive test for amphetamine and cocaine. In addition, there were 6 positive urine drug screens (+UDS; n = 2, phencyclidine; n = 2, opiates; n = 1, barbiturates; and n = 1, cocaine), which were not related to prescription drug use. The rate of positive drug screens among all 802 patients was estimated to be 0.0146 +UDS/person year.27
Postdose psychotic-like symptoms, as measured by the BPRS+, were transient and resolved on the same day (Supplementary Figure 1).
There was no case of interstitial/ulcerative cystitis during the study. A total of 136 patients (17.0%) reported TEAEs related to renal and urinary disorders (all preferred terms listed in the Medical Dictionary for Regulatory Activities [MedDRA version 20.0] for renal/urinary disorders and urinary-related preferred terms in infections and infestations). In this group, 4 patients had 6 serious TEAEs (pyelonephritis, acute pyelonephritis, and tubulointerstitial nephritis in 1 patient each, and the fourth patient had urinary tract infection [UTI] and additionally underwent surgery for stress urinary incontinence and vesical fistula), of which none led to discontinuation from the study or was deemed related to the treatment with esketamine. There were 5 adverse events of cystitis, which resolved while continuing esketamine treatment. The TEAE of UTI, diagnosed based on clinical symptoms and urinalysis, was reported in 65 patients (8.1%). Most cases of urinary tract symptoms were mild-to-moderate and resolved within 2 weeks. One patient had a dose interruption due to TEAE of urinary retention, assessed as doubtfully related to esketamine by the investigator; it resolved in 4 days, and treatment was re-started. Another patient had dose reduction (from 84 mg to 56 mg) due to a TEAE of pollakiuria of moderate severity, which was assessed as probably related to esketamine and resolved in 5 days. One patient discontinued due to urinary incontinence. Of a total of 14 patients who had multiple episodes of BPIC-SS scores > 18, 6 patients had the TEAE of UTI/cystitis, 2 had the TEAE of nonspecific urinary symptoms (dysuria, pollakiuria), 1 had a preexisting condition (benign prostate hyperplasia), 3 showed signs of UTI in their urinalysis, and 2 had no adverse events/laboratory changes in urinalysis reported. Overall, BPIC-SS total score over time remained low, suggesting no/minimal bladder symptoms (Supplementary Figure 2).
Vital Signs, Laboratory Tests, and ECG
Overall, mean systolic BP (SBP) and diastolic BP (DBP) increased at 40 minutes postdose, with the greatest mean (SD) change of 9.6 (11.99) mm Hg (SBP) and 5.6 (8.32) mm Hg (DBP) in the IND phase and 9.2 (11.30) mm Hg (SBP) and 5.9 (7.28) mm Hg (DBP) in the OP/MAINT phase. The BP values generally returned close to predose values at the 1.5-hour postdose timepoint, and there was no evidence of elevation in the mean predose measures over time. A total of 33/802 patients (4.1%) had treatment-emergent acute hypertension (defined as SBP ≥ 180 or DBP ≥ 110 mm Hg). Incidences of acute hypertension were numerically higher in patients with a medical history of hypertension (16/220 [7.3%]) versus patients without hypertension (17/582 [2.9%]); however, magnitude of difference from predose in mean postdose SBP and DBP levels on dosing days was similar. A new antihypertensive medication was initiated in 25/220 patients (11.4%) with hypertension and 29/582 patients (5.0%) without hypertension during the study. There was no occurrence of respiratory depression (see “Vital signs” in Supplementary Appendix 2) and no clinically relevant change in mean ECG intervals or laboratory parameters (see “Clinical laboratory tests and ECG” in Supplementary Appendix 2). On 7 occasions (in 6 patients) in the IND phase and on 3 occasions (in 3 patients) in the OP/MAINT phase, esketamine nasal spray dose was temporarily interrupted or dose was reduced due to increased BP/hypertension. Four patients met discontinuation criteria due to elevated BP and were withdrawn from the study.
Cognitive Effects
Group mean performance on all tests (Cogstate and HVLT-R), including measures of simple and choice reaction time, visual and verbal learning and memory, working memory, and executive function, either demonstrated improvement from baseline in cognitive function or remained stable through week 44 in all patients (Supplementary Table 1). To investigate whether this pattern of results was consistent across different age groups, patients were classified into subgroups based on age: < 65 years and ≥ 65 years. In patients aged < 65 years, performance on all cognitive tests remained stable or slightly improved from baseline during long-term treatment. In patients ≥ 65 years, the mean performance on all 4 tests of higher cognitive functioning (ie, visual learning and memory, working memory, executive function, and verbal learning and memory) improved or remained stable, while the simple and choice reaction time (DET and IDN on the Cogstate battery) showed slowing that began at week 20 of the OP/MAINT phase (Supplementary Table 2). There was high intraindividual variability in reaction time among patients ≥ 65 years. Among patients with reaction times at baseline that were within age-adjusted norms, 7 showed consistent slowing as the study progressed, and no patient manifested impaired reaction time (z score < −1.5 on detection or identification tasks) at study endpoints and follow-up (Supplementary Tables 3 and 4).
Suicidal Ideation and Behavior
In the C-SSRS assessment, 114/784 patients (14.5%) reported new occurrences of suicidal ideation during the study. A total of 8 patients reported suicidal behavior, 2 of whom had a score of 6 (“preparatory acts or behavior”) and 6 a score of 9 (“non-fatal suicide attempt”). One death due to suicide was reported in a 55-year-old woman on day 188 of the study. The patient had a family history of depression and no prior history of suicidal behavior or intent. The patient was clinically in remission of depressive symptoms (MADRS score of 7 and 9 on the last 2 assessments) prior to the event; however, there were environmental circumstances present.
Dissociative and Perceptual Symptoms
Changes in CADSS total score were observed shortly after administration of esketamine, peaked at 40 minutes postdose, and generally resolved within 1.5 hours on the same dosing day. A post hoc, longitudinal analysis showed that the mean maximum postdose CADSS total score declined over time (see “Longitudinal analysis of maximum post-dose CADSS total score” in Supplementary Appendix 2 and Supplementary Figures 3 and 4).
Sedation
Clinically-relevant sedation, defined by MOAA/S score ≤ 3, occurred in 8.4% of patients in the IND phase and 7.0% of patients in the OP/MAINT phase. Of all patients who completed the scale, 5 had MOAA/S scores of 0 (corresponding to no reaction to painful trapezius squeeze) or 1 (purposeful reflexive withdrawal in response to trapezius squeeze). MOAA/S ≤ 3 was reported in 1.8% and 0.5% of dosing sessions in the IND and OP/MAINT, respectively. The longest period of sedation was 1 hour 30 minutes (starting 45 minutes postdose). Another patient was nonresponsive to pain for a shorter period; however, MOAA/S was not completed. Patients who experienced deep sedation did not require ventilation or resuscitation and woke up spontaneously.
Nasal Examination and Nasal Symptom Questionnaire
Taste disturbance (IND: 10.2%; OP/MAINT: 11.0%), postnasal drip (IND: 9.9%; OP/MAINT: 11.0%), and stuffy nose (IND: 5.9%; OP/MAINT: 9.1%) were the most common moderate or severe symptoms on the nasal symptom questionnaire, and < 1% of patients had findings (epistaxis and nasal crusts, discharge, or erythema) on nasal examination.
Withdrawal Symptoms
The mean (SD) PWC-20 total scores (range 0-60; 0-3-point scale [not present = 0, mild = 1, moderate = 2, severe = 3]) were 8.0 (7.61) (at end of treatment) and 7.9 (6.91) (week 1), 8.0 (7.42) (week 2), and 7.7 (7.07) (week 4) during follow-up. In patients who discontinued from the OP/MAINT phase, the most common (> 20%) new or worsening “withdrawal” symptom reported at week 1 was fatigue-lethargy/lack of energy (25.0%) and at endpoint was insomnia (22.7%) (Supplementary Table 5).
Efficacy
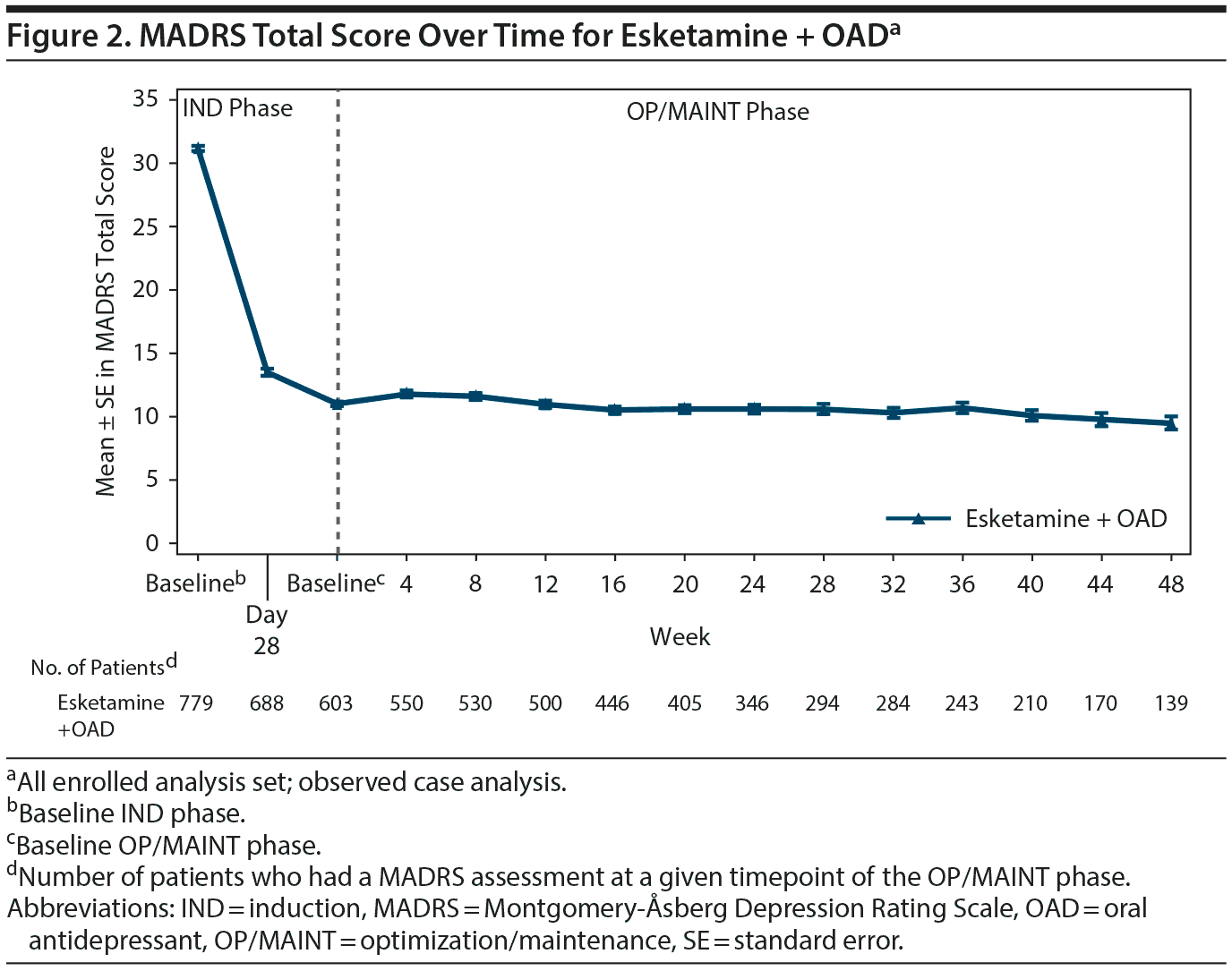
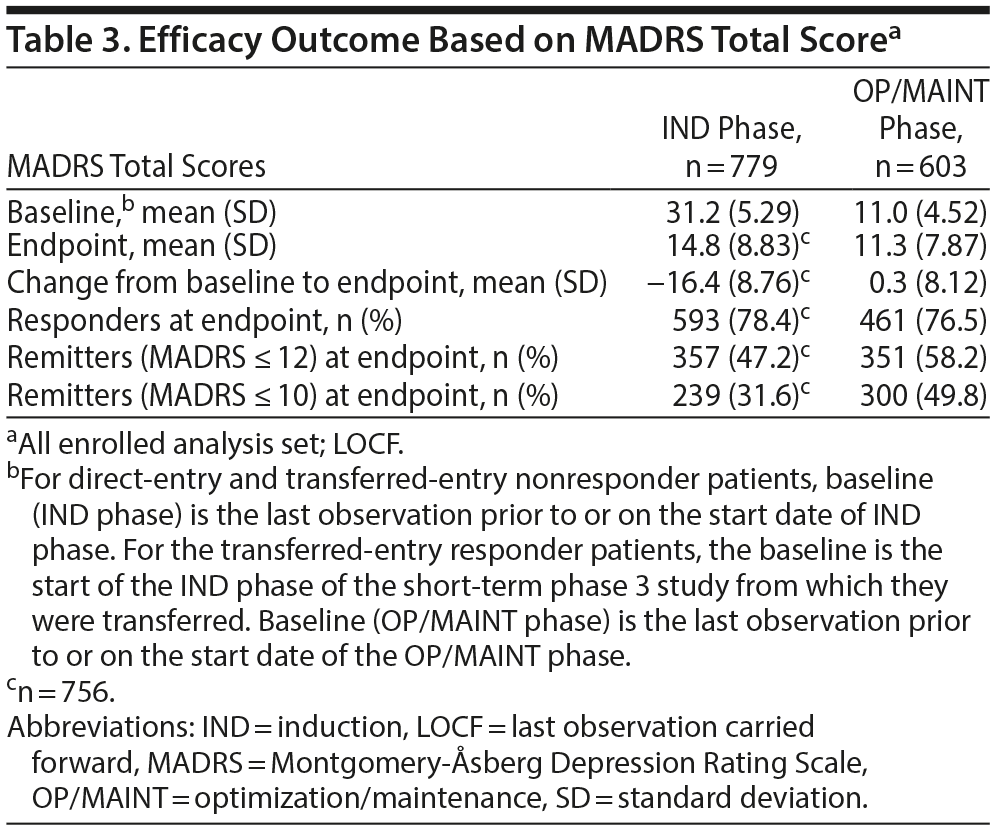
The mean MADRS total score decreased in the IND phase (mean [SD] change from IND baseline to endpoint: −16.4 [8.76], n = 756). The improvement in MADRS total score appeared to be sustained in patients who were responders (direct and transfer entry) and who continued treatment for up to 1 year of exposure (mean [SD] change from OP/MAINT baseline to endpoint: 0.3 [8.12]) (Table 3 and Figure 2; also see “Efficacy results” in Supplementary Appendix 2 and Supplementary Tables 6 and 7).
In the IND phase, the percentage of responders and remitters increased over time, with 78.4% responders and 47.2% remitters (MADRS ≤ 12) at endpoint. The percentage of responders and remitters at the OP/MAINT phase endpoint was 76.5% and 58.2%, respectively (Table 3).
DISCUSSION
The present study is one of the first to demonstrate the long-term (up to 1 year) safety and tolerability of weekly or every-other-week treatment with esketamine nasal spray plus a new OAD. Previously, only 1 study28 with a small sample size (n = 54) showed manageable tolerability and low discontinuation rate due to adverse events with long-term (up to 29 months) intravenous ketamine treatment in patients with severe, treatment-resistant mood disorders.
Overall, the nature of TEAEs reported in this study was consistent with the known safety profile of esketamine reported in placebo-controlled, short-term studies1-5,10 of patients with TRD. The majority of TEAEs were mild or moderate in severity, and the incidence of serious TEAEs was low. Most patients with serious TEAEs either recovered or were recovering at study closure. Two deaths reported during the study were not attributed to esketamine as judged by the study site investigator. Reductions in depressive symptoms were observed during the first 4 weeks of treatment (IND phase) and appeared to be sustained through the 1-year exposure period. In addition, reduction in dosing frequency from weekly to every-other-week regimens was consistently achieved in a considerable proportion (38.1%) of patients. As dosing frequency was based on treatment response together with tolerability, for most patients, the findings suggested consistent benefits and acceptable tolerability of esketamine nasal spray treatment over a period of up to 1 year.
The majority of clinically-relevant esketamine-related TEAEs in this study were transient and resolved on the day of administration. In patients with low cardiovascular risk at baseline, increases in BP observed after esketamine administration were generally transient and led to discontinuation in 0.7% of patients. No case of interstitial/ulcerative cystitis was reported, and TEAEs of cystitis were mostly of mild severity, transient and self-limiting, and considered to be suggestive of an infectious etiology (ie, UTI). UTIs account for nearly a quarter of common infections and are expected events in a long-term study of a predominantly middle- to older-age study sample with a preponderance of female patients.29,30 The incidence of UTI (8.1%) in the present study was analogous to rates observed in a general adult female population (over 10%) over a 1-year period.31 The serious TEAEs related to renal and urinary tract disorders were assessed as not related to esketamine and resolved while the treatment was ongoing.
Suicidal ideation and behavior are associated with TRD with estimated incidence rates of 0.47 completed and 4.66 attempted suicides per 100 patient years. These rates are substantially higher than those in the general MDD population and similar to those found before and after treatments such as electroconvulsive therapy.32 Another study33 reported that in TRD patients, the life-time risk of suicide attempts is assessed to be close to 30%. In the present study, 14.5% of patients who did not have suicidal ideation at baseline reported new suicidal ideation, 6 patients (0.7%) attempted suicide (1.571 per 100 patient years), and 1 patient completed suicide (0.262 per 100 patient years).
Cognitive deficits, impairments in working memory, and decrements in episodic memory have been documented following acute and repeated recreational abuse of ketamine.8 Although a single subanesthetic dose (0.2 and 0.5-mg/kg) of ketamine showed no cognitive impairment (working memory and go/no-go tasks on days 3 and 14 postketamine infusion) in patients with TRD, effects on cognitive performance with repeated use of ketamine over longer periods remains elusive.34 In the present study, preservation or improvement in all clinically significant cognitive domains on the Cogstate and HLVT-R was observed, except in patients aged ≥ 65 years, who had slowing of performance only on simple (DET) and choice reaction-time (IDN) tests of the Cogstate. The interpretation of this effect was limited by the substantial intraindividual variability in reaction time among patients ≥ 65 years across successive study visits, the lack of a control group, small sample size, and the absence of impaired reaction times on day 28 of the IND phase, at the OP/MAINT phase endpoint, and at follow-up. In addition, there was no significant change in the more complex cognitive domains (verbal, visual, working memory, and executive function), suggesting that the slowing in reaction times may be an isolated observation, not implicating attentional deficit.35 Further investigation in specially designed, controlled, long-term studies in ≥ 65-year-old patients is warranted to understand and interpret the effect of esketamine on reaction time in this population. Finally, alternate forms (HVLT-R: Forms 1-6; Cogstate: playing-cards-based tests [DET, IDN, OCL, and ONB tasks] and 18 parallel maze forms for the GMLT) were used for all cognitive tests to attenuate practice effects.36 Nonetheless, it cannot be known whether practice effects occurred that might have obscured some decline in cognitive performance. Although, in a longitudinal, randomized withdrawal trial37 in which patients with TRD who had responded to 28-day induction treatment with esketamine plus OAD were randomized to esketamine plus OAD or OAD plus placebo, performance of patients aged 18-64 years on the Cogstate and HVLT-R was similar across treatment arms through week 44 of maintenance treatment, and on some tests (eg, HVLT-R), performance of patients who continued on esketamine and OAD appeared to show improvement over patients in the placebo group. Further methodological research should be considered to enable better understanding and quantification of practice effects and optimal control strategies in longitudinal trials involving repeat cognitive assessments.
In this study, there was no indication of abuse of esketamine, and the duration of TEAEs of dizziness, dissociation, and somnolence was generally limited to the day of esketamine administration. The mean (SD) PWC-20 total score 2 weeks after discontinuation of esketamine (8.0 [7.42]) was similar to that observed at end of treatment (8.0 [7.61]) and was approximately 2- to 3-times lower than the total scores reported in patients after successful (17.2 [11.6]) and unsuccessful (24.6 [12.6]) tapering-off of their benzodiazepines.19 Collectively, these findings and discontinuation-related symptoms from the PWC-20 assessment suggest that withdrawal symptoms were infrequent, mild, and in quality appeared generally indistinguishable from early return in MDD symptoms.
Notably, the rapid clearance from plasma, short half-life, and low frequency of dosing (weekly or every-other-week) did not allow esketamine to reach steady-state in patients dosed in this study. Consistent with the rapid plasma clearance, dissociative symptoms and dizziness were transient and mostly resolved within 1.5 hours after dosing. Similar to that in earlier reports of esketamine,1-5,10 severity of dissociative symptoms in this study attenuated over time in most cases, although in rare instances transient reemergence of higher intensity dissociative symptoms at a later dosing session was observed.
This study had several limitations including its open-label study design, the simultaneous initiation of a new OAD, and the absence of a control group; however, the high response and remission rates suggest that long-term (up to 1 year) esketamine treatment appears to provide sustained symptom improvement. Remission rate observed in this treatment-resistant population at end of the OP/MAINT phase was 58.2% using MADRS ≤ 12 criteria and 49.8% using MADRS ≤ 10 cutoff. The definition of remission as a MADRS score ≤ 12 was selected taking into account that the patients manifested TRD prior to entry and thus had higher MADRS scores at baseline than those typically included for studies assessing the efficacy of new treatments in the general MDD population. Nevertheless, the same definition has also been used previously in clinical studies20,21 of patients with MDD who were selected irrespective of response to previous treatment. In the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study,38 remission rates assessed by a different instrument and more strict criteria (Quick Inventory of Depressive Symptomatology-Self-Report [QIDS-SR] ≤ 5) were 30.6% after 2, 13.7% after 3, and 13.0% after 4 failed AD trials. In this study, improvements in patient-reported measures of depressive symptoms (PHQ-9; Supplementary Table 6), functionality, and associated disability (SDS; Supplementary Table 7) appear to further support the long-term efficacy of esketamine.
This study involved difficult-to-treat patients with a long-term history of TRD, moderate to severe depression at baseline, and a mean duration of the current depressive episode of about 3 years, and approximately a quarter of the sample had a prior history of suicidal ideation.39 Furthermore, the high retention rates (75% of patients from the IND phase entered the OP/MAINT phase) and attainment of adequate exposure requirements (as outlined in the International Council for Harmonisation [ICH] E1 guideline)40 adds to the comprehensiveness of the data collected during this longitudinal study.41,42 Exclusion of patients with clinically relevant psychiatric or medical comorbidities or substance dependence may potentially limit the generalizability of study findings. To standardize treatment conditions and reduce variability, the 4 OADs used in this study were among the commonly available standard-of-care treatments in the participating countries.
In summary, esketamine nasal spray plus a new OAD demonstrated favorable long-term safety and manageable tolerability in long-term (up to 1 year) intermittent treatment in patients with TRD. The adverse event profile of weekly or every-other-week dosing of esketamine was consistent with findings from earlier short-term studies.
Submitted: April 26, 2019; accepted October 28, 2019.
Published online: April 28, 2020.
Author contributions: G.S., S.K., A.H.Y., A.H.S., C.-T.L., J.-W.P., H.J.J., and J.G. were principal investigators and S.T.W. was a sub investigator for this study. E.W., L.A., R.L.M., E.J.D., R.H., G.S., S.K., A.H.Y., A.H.S., C-.T.L., J-.W.P., H.M., D.H., W.C.D., J.B.S., J.G., H.J.J., and S.T.W. were involved in the study design and/or data collection, analysis and interpretation. P.L., R.L. and J.E.G. oversaw data analysis and interpretation. All authors met the ICMJE criteria and those who fulfilled the criteria are listed as authors. All authors had access to the study data, provided direction and formal review of the manuscript, and made the final decision about where to publish these data. All authors contributed toward drafting and revising the manuscript and agreed to be accountable for all aspects of the work.
Potential conflicts of interest: Drs Wajs, Morrison, Daly, Lim, Manji, and Drevets; Mss Aluisio, Lane, and George; and Mr Holder are employees of Janssen Research & Development and hold company stock. Drs Hough and Singh were employees of Janssen Research & Development at the time of study. Dr Manji is an inventor on patents directed to this technology that are assigned to Icahn School of Medicine at Mount Sinai, Yale University, and National Institutes of Health and are exclusively licensed to Janssen; however, he will not receive any direct financial benefit therefrom. Dr Sanacora has served as a consultant for Allergan, Alkermes, AstraZeneca, Avanier Pharmaceuticals, Axsome Therapeutics Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Hoffman La-Roche, Intra-Cellular Therapies, Janssen, Merck, Naurex, Navitor Pharmaceuticals, Novartis, Noven Pharmaceuticals, Otsuka, Praxis Therapeutics, Sage Pharmaceuticals, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valeant, and Vistagen Therapeutics over the past 36 months; and received research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, and Servier over the past 36 months. Dr Sanacora holds equity in BioHaven Pharmaceuticals and is a co-inventor on a US patent (#8,778,979) held by Yale University and a co-inventor on US Provisional Patent Application No. 047162-7177P1 (00754) filed on August 20, 2018, by Yale University Office of Cooperative Research. Yale University has a financial relationship with Janssen Pharmaceuticals, and may in the future receive financial benefits from this relationship. The University has put multiple measures in place to mitigate this institutional conflict of interest. Questions about the details of these measures should be directed to Yale University’s Conflict of Interest office. Dr Young has received grants/research support for investigator-initiated studies from AstraZeneca, Eli Lilly, and Lundbeck; received honoraria or consultation fees for paid lectures and advisory boards from all major pharmaceutical companies with drugs used in affective and related disorders. Dr Kasper received grants/research support, consulting fees and/or honoraria within the past 3 years from Angelini, AOP Orphan Pharmaceuticals, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr Sulaiman has received grants, participated in advisory boards and/or spoken for Astra Zeneca, Danipone Sumitomo, Eli Lilly, Janssen Cilag, Lundbeck, Otsuka, Pfizer, Sanofi Aventis, Servier, Takeda, United Biosource, and Wyeth. Dr Wilkinson has received support from the National Institutes of Health (T32MH062994-15 and 5R25MH071584-09), the Agency for Healthcare Research and Quality (K12HS023000), Thomas Detre Fellowship, the Brain & Behavior Research Foundation, the American Psychiatric Institute for Research and Education/Janssen Resident Psychiatric Research Scholars Program, the Robert E. Leet and Clara Guthrie Patterson Trust, and the American Foundation for Suicide Prevention; and has received consulting/honoraria from Janssen. Yale University has a financial relationship with Janssen Pharmaceuticals, and may in the future receive financial benefits from this relationship. The University has put multiple measures in place to mitigate this institutional conflict of interest. Questions about the details of these measures should be directed to Yale University’s Conflict of Interest office. Ms George has received honoraria or consultation fees for advisory boards from a number of major pharmaceutical companies, including Johnson and Johnson.
Funding/support: Janssen Research & Development, USA. This study represents independent research funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Role of the sponsor: Employees of the sponsor, as noted in author contributions, were involved in study design; patient recruitment; data collection, analysis, or interpretation; and/or other aspects pertinent to the study. Authors had full access to the study data, were involved in writing and/or revising the manuscript and had final responsibility for the decision to submit for publication. Medical writing assistance for this manuscript was also supported by the sponsor.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Previous presentation: Posters presented at: American Society for Clinical Pathology (ASCP) Annual Meeting; May 29-June 1, 2018; Miami, Florida ▪ American College of Neuropsychopharmacology (ACNP) 57th Annual Meeting; Dec 9-13, 2018; Hollywood, Florida ▪ International College of Neuropsychopharmacology (CINP) World Congress; June 16-19, 2018; Vienna, Austria ▪ Canadian Psychiatric Association (CPA) Annual Conference; September 27-29, 2018; Toronto, Canada ▪ European College of Neuropsychopharmacology (ECNP) Congress; October 6-9, 2018; Barcelona, Spain ▪ ECNP 32nd Annual Meeting; Sep 7-10, 2019; Copenhagen, Denmark.
Acknowledgments: Medical writing support was provided by Priya Ganpathy, MPharm, CMPP, a full-time employee of SIRO Clinpharm, Thane, India; this assistance was funded by Janssen Research & Development. Additional editorial assistance was provided by Harry Ma, PhD, CMPP, a full-time employee of Janssen Global Services. The authors thank the study participants and the investigators for their participation in this study.
Supplementary material: See accompanying pages.
REFERENCES
1. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148. PubMed CrossRef
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903. PubMed CrossRef
3. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616-630. PubMed CrossRef
4. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428-438. PubMed CrossRef
5. Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424-431. PubMed CrossRef
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78. PubMed CrossRef
7. Morgan CJ, Curran HV; Independent Scientific Committee on Drugs. Ketamine use: a review. Addiction. 2012;107(1):27-38. PubMed CrossRef
8. Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105(1):121-133. PubMed CrossRef
9. Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405. PubMed CrossRef
10.Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121-141. PubMed CrossRef
11.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
12.Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154. PubMed CrossRef
13.Benedict RH, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test-Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43-55. CrossRef
14.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed CrossRef
15.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11(1):125-136. PubMed CrossRef
16.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799-812. CrossRef
17.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244-251. PubMed
18.Humphrey L, Arbuckle R, Moldwin R, et al. The bladder pain/interstitial cystitis symptom score: development, validation, and identification of a cut score. Eur Urol. 2012;61(2):271-279. PubMed CrossRef
19.Rickels K, Garcia-Espana F, Mandos LA, et al. Physician Withdrawal Checklist (PWC-20). J Clin Psychopharmacol. 2008;28(4):447-451. PubMed CrossRef
20.Guelfi JD, Ansseau M, Timmerman L, et al; Mirtazapine-Venlafaxine Study Group. Mirtazapine versus venlafaxine in hospitalized severely depressed patients with melancholic features. J Clin Psychopharmacol. 2001;21(4):425-431. PubMed CrossRef
21.Schweitzer I, Burrows G, Tuckwell V, et al. Sustained response to open-label venlafaxine in drug-resistant major depression. J Clin Psychopharmacol. 2001;21(2):185-189. PubMed CrossRef
22.Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Åsberg Depression Rating Scale corresponding to the definition of remission on the Hamilton rating scale for depression. J Psychiatr Res. 2004;38(6):577-582. PubMed CrossRef
23.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744. PubMed CrossRef
24.Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93-105. PubMed CrossRef
25.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed CrossRef
26.Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:217-222.
27.Aluisio L, Wajs E, DiBernardo A, et al. Withdrawal Symptom Assessment—Esketamine Nasal Spray: Open-Label Safety Study in Treatment-Resistant Depression. 32nd European College of Neuropsychopharmacology (ECNP) congress; 2019; Copenhagen, Denmark.
28.Wilkinson ST, Katz RB, Toprak M, et al. Acute and longer-term outcomes using ketamine as a clinical treatment at the Yale Psychiatric Hospital. J Clin Psychiatry. 2018;79(4):17m11731. PubMed CrossRef
29.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49(2):53-70. PubMed CrossRef
30.Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging Health. 2013;9(5):ahe.13.38. PubMed CrossRef
31.Foxman B, Barlow R, D’ Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515. PubMed CrossRef
32.Bergfeld IO, Mantione M, Figee M, et al. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362-367. PubMed CrossRef
33.Dunner DL, Rush AJ, Russell JM, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688-695. PubMed CrossRef
34.Chen MH, Li CT, Lin WC, et al. Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord. 2018;241:1-7. PubMed CrossRef
35.Bruce KM, Robinson SR, Smith JA, et al. Validity of a screening tool for detecting subtle cognitive impairment in the middle-aged and elderly. Clin Interv Aging. 2014;9:2165-2176. PubMed
36.Goldberg TE, Harvey PD, Wesnes KA, et al. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst). 2015;1(1):103-111. PubMed
37.Morrison RL, Singh JB, Daly EJ, et al. Effect of Esketamine Nasal Spray on Cognition in Patients With Treatment-Resistant Depression: Results From Five Phase 3 Studies. American College of Neuropsychopharmacology (ACNP) Annual Meeting; 2018; Hollywood, Florida.
38.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed CrossRef
39.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388. PubMed CrossRef
40. ICH Harmonised tripartite guideline—The Extent Of Population Exposure To Assess Clinical Safety For Drugs Intended For Long-Term Treatment Of Non-Life-Threatening Conditions E1. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; 1994.
41.Abshire M, Dinglas VD, Cajita MI, et al. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol. 2017;17(1):30. PubMed CrossRef
42.Simmonds B, Turner N, Thomas L, et al. Patients’ experiences of participating in a large-scale trial of cognitive behavioural therapy for depression: a mixed methods study. Fam Pract. 2013;30(6):705-711. PubMed CrossRef
This PDF is free for all visitors!