Objective: Mixed (subsyndromal hypomanic) features are prevalent in patients with bipolar depression and are associated with more severe and complex illness, including increased risk for suicide attempts, higher switch to mania during antidepressant therapy, and a higher rate of recurrence. The aim of this post hoc analysis was to evaluate the efficacy and safety of lurasidone in the treatment of patients with bipolar depression presenting with mixed features.
Method: Patients with a DSM-IV-TR diagnosis of major depressive episode associated with bipolar I disorder, with or without rapid cycling, and with a Montgomery-Asberg Depression Rating Scale (MADRS) score ≥ 20 and a Young Mania Rating Scale (YMRS) score ≤ 12 were randomly assigned to 6 weeks of double-blind, once-daily treatment with lurasidone 20-60 mg, lurasidone 80-120 mg, or placebo. The presence of mixed features was defined as a YMRS score ≥ 4 at study baseline. Efficacy analyses included change in MADRS total score from baseline to week 6 (the primary outcome in the original study, conducted between April 2009 and February 2012).
Results: At baseline, mixed features were present in 56% of patients (lurasidone, n = 182/323; placebo, n = 90/162). Treatment with lurasidone (vs placebo) was associated with significantly greater reductions in MADRS scores in the mixed features group (−15.7 vs −10.9; P = .001; week 6; mixed model for repeated measures [MMRM]; effect size, 0.48) and in the group without mixed features (−15.2 vs −10.8; P = .002; week 6; MMRM; effect size, 0.48). Rates of protocol-defined treatment-emergent hypomania or mania were similar for patients with mixed features (lurasidone, 2.2%; placebo, 3.2%) and without mixed features (lurasidone, 3.4%; placebo, 0.0%).
Conclusions: Lurasidone was found in this post hoc analysis to be efficacious in the treatment of patients with bipolar depression who present with mixed features (assessed cross-sectionally at study baseline). No increased risk of treatment-emergent mania was observed in either group.
Trial Registration: ClinicalTrials.gov identifier: NCT00868699
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
ABSTRACT
Objective: Mixed (subsyndromal hypomanic) features are prevalent in patients with bipolar depression and are associated with more severe and complex illness, including increased risk for suicide attempts, higher switch to mania during antidepressant therapy, and a higher rate of recurrence. The aim of this post hoc analysis was to evaluate the efficacy and safety of lurasidone in the treatment of patients with bipolar depression presenting with mixed features.
Method: Patients with a DSM-IV-TR diagnosis of major depressive episode associated with bipolar I disorder, with or without rapid cycling, and with a Montgomery-Asberg Depression Rating Scale (MADRS) score ≥ 20 and a Young Mania Rating Scale (YMRS) score ≤ 12 were randomly assigned to 6 weeks of double-blind, once-daily treatment with lurasidone 20-60 mg, lurasidone 80-120 mg, or placebo. The presence of mixed features was defined as a YMRS score ≥ 4 at study baseline. Efficacy analyses included change in MADRS total score from baseline to week 6 (the primary outcome in the original study, conducted between April 2009 and February 2012).
Results: At baseline, mixed features were present in 56% of patients (lurasidone, n = 182/323; placebo, n = 90/162). Treatment with lurasidone (vs placebo) was associated with significantly greater reductions in MADRS scores in the mixed features group (−15.7 vs −10.9; P = .001; week 6; mixed model for repeated measures [MMRM]; effect size, 0.48) and in the group without mixed features (−15.2 vs −10.8; P = .002; week 6; MMRM; effect size, 0.48). Rates of protocol-defined treatment-emergent hypomania or mania were similar for patients with mixed features (lurasidone, 2.2%; placebo, 3.2%) and without mixed features (lurasidone, 3.4%; placebo, 0.0%).
Conclusions: Lurasidone was found in this post hoc analysis to be efficacious in the treatment of patients with bipolar depression who present with mixed features (assessed cross-sectionally at study baseline). No increased risk of treatment-emergent mania was observed in either group.
Trial Registration: ClinicalTrials.gov identifier: NCT00868699
J Clin Psychiatry 2015;76(4):398-405
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: July 25, 2014; accepted January 13, 2015.
Online ahead of print: March 3, 2015 (doi:10.4088/JCP.14m09410).
Corresponding author: Roger S. McIntyre, MD, University of Toronto, Departments of Psychiatry and Pharmacology, Mood Disorders Psychopharmacology Unit, 399 Bathurst St, MP 9-325, Toronto, ON, Canada M5T 2S8 ([email protected]).
Mixed (ie, subsyndromal hypomanic) features are now recognized as a key aspect of diagnostic nosology in adults presenting with both unipolar and bipolar depression. Multiple lines of evidence indicate that the presence of mixed features during a depressive episode identifies a bipolar disorder population characterized by more severe and/or chronic depressive episodes, with shorter interepisode remissions, a higher recurrence rate, an increased risk of switch to mania during antidepressant therapy, higher rates of comorbidity (most notably anxiety disorders and/or substance use disorders), and a higher risk of suicide attempts.1-9
Prevalence estimates for mixed features vary widely depending on the criteria used and on the setting and population studied.1,2 In a community sample, 41.4% of individuals who met DSM-IV criteria for major depression also met criteria for subthreshold hypomania (≥ 4 symptoms, but not meeting DSM-IV criteria for hypomania, based on the Munich Composite International Diagnostic Interview).3 In psychiatric practice settings, the prevalence of mixed features has been reported to range from 11%-54%, depending on the threshold number of manic symptoms required.4,5,10 In patients diagnosed with DSM-IV bipolar depression who were enrolled in 2 clinical trials of olanzapine/fluoxetine combination or olanzapine, the prevalence of mixed features was 78% if 1-2 manic symptoms were present at study baseline and 31% if ≥ 3 were present.11
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)12 defined mixed states categorically on the basis of the co-occurrence of full manic and major depressive syndromes. DSM-513 has broadened the concept of mixed states by instituting a dimensional approach that utilizes (in the context of bipolar depression) a “with mixed features” specifier, applicable to patients who present with 3 or more specific manic symptoms during the “majority of days of the current or most recent episode of depression.” However, a number of studies indicate that the presence of as few as 2 or 3 manic symptoms, occurring at a single timepoint, is sufficient to define a mixed population with characteristic clinical course and outcome.2,5,9,11
Lurasidone is approved in the United States and Canada for the treatment of adults with major depressive episodes associated with bipolar I disorder (bipolar depression), both as monotherapy and as adjunctive therapy with lithium or valproate.14-16 The aim of this post hoc analysis was to evaluate the efficacy of lurasidone in patients with bipolar depression with mixed features, based on a cross-sectional assessment of manic symptom severity and number at study baseline.
METHOD
The post hoc analysis summarized here are based on data from a previously reported double-blind, placebo-controlled trial in patients with bipolar depression (ClinicalTrials.gov identifier: NCT00868699) for which study methods have been summarized in detail.14 The study was approved by an institutional review board at each investigational site and was conducted in accordance with the International Conference on Harmonization Good Clinical Practices guidelines and with the ethical principles of the Declaration of Helsinki. All patients signed an informed consent document explaining study procedures and potential risks before study entry. The study was conducted between April 2009 and February 2012.
Study Patients and Study Design
This multiregional study, conducted in the United States and 7 other countries, enrolled outpatients 18 to 75 years of age who were diagnosed with bipolar I disorder and were experiencing a major depressive episode (DSM-IV-TR criteria, ≥ 4 weeks and < 12 months in duration), with or without rapid cycling, without psychotic features, and with a history of at least 1 lifetime bipolar manic or mixed manic episode. A Montgomery-Asberg Depression Rating Scale (MADRS)17 score of ≥ 20 and a Young Mania Rating Scale (YMRS)18 score of ≤ 12 were required at both screening and baseline. Eligible patients were randomly assigned to receive 6 weeks of double-blind treatment with placebo or 1 of 2 fixed-flexible dose ranges of lurasidone: 20-60 mg/d (starting dose: 20 mg/d for 7 days) or 80-120 mg/d (20 mg/d on days 1-2, 40 mg/d on days 3-4, 60 mg/d on days 5-6, and 80 mg/d on day 7). Lurasidone dose adjustments were permitted after 7 days to optimize efficacy and tolerability.

- Subsyndromal hypomanic symptoms (mixed features) are prevalent in patients with bipolar depression and are associated with more severe and complex illness, including increased risk for suicide attempts, higher rates of switch to mania during antidepressant therapy, and a more recurrent form of illness. In addition, clinical evidence suggests reduced responsiveness to antidepressants in patients with this condition.
- The results of this post hoc analysis indicate that lurasidone may be an effective treatment for patients who experience mixed (hypomanic) features in association with episodes of bipolar depression.
In the current analysis, patients were categorized as having mixed features based on the presence of a YMRS score ≥ 4 assessed cross-sectionally at baseline. The YMRS consists of a checklist of 11 items that are scored on a scale of 0-4 (7 items) or 0-8 (4 items).18 The range of scores is 0-60. Previous studies indicate that a YMRS score ≥ 4, assessed at a single timepoint, defines a mixed features population with treatment response characteristics that appear distinct from those of patients without mixed features.6,11 Patients with a YMRS score < 4 at baseline defined a group without mixed features. An additional analysis was performed that defined mixed features based on the presence at baseline of 2 or more YMRS symptoms with a severity score of ≥ 2. This analysis is consistent with alternative definitions of mixed features that require the presence of either 2 or 3 hypomanic symptoms.2-5,11
Assessments
We assessed efficacy in the current analysis utilizing the MADRS total score and the Clinical Global Impression, Bipolar—Severity of Illness depression score (CGI-BP-S).19 Additional efficacy assessments included the YMRS, the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (QIDS-SR16),20 the Hamilton Anxiety Rating Scale (HARS),21 the Sheehan Disability Scale (SDS),22 and the Quality of Life Enjoyment and Satisfaction Questionnaire—Short Form (Q-LES-Q-SF).23
Treatment-emergent hypomania or mania was defined, a priori, as (1) a YMRS score of ≥ 16 at any 2 consecutive postbaseline visits or at the final visit or (2) an adverse event of mania or hypomania. Suicidal ideation and behavior were assessed using the Columbia-Suicide Severity Rating Scale (C-SSRS).24
Safety and tolerability were assessed by the incidence and severity of adverse events during the study. Additional safety evaluations included movement disorder scales, vital signs, laboratory tests, 12-lead electrocardiogram, and physical examination.
Statistical Analysis
The modified intent-to-treat (mITT) population, which was used for all efficacy analyses, consisted of randomized patients who received at least 1 dose of study medication and had at least 1 postbaseline efficacy assessment. The MADRS, CGI-BP-S, YMRS, and QIDS-SR16 were assessed using a mixed model for repeated measures (MMRM) that included fixed-effect terms for treatment, visit, pooled center, baseline score as a covariate, and a treatment-by-visit interaction term, using an unstructured covariance matrix for within-patient correlation. Changes from baseline in additional efficacy measures were evaluated using an analysis of covariance model using last observation carried forward (LOCF), with fixed effects for treatment, pooled center, and baseline score as a covariate. Efficacy analyses were conducted on the combined dosage groups and on the 2 individual dosage groups. The presence or absence of mixed features was used to stratify efficacy models for post hoc analyses and was also added as an interaction term in selected models for the primary (MADRS) and secondary outcomes. The proportions of responders (≥ 50% reduction from baseline in MADRS total at LOCF endpoint) and remitters (MADRS total ≤ 12 at LOCF endpoint) were compared between the lurasidone and placebo treatment groups using logistic regression. Number needed to treat (NNT) was calculated as the reciprocal of the difference between the proportion of subjects in each treatment group achieving an endpoint of interest. All statistical tests were 2-sided, with statistical significance set at α = .05. Between-treatment group differences were reported using least-squares (LS) means with standard error (SE). No adjustment was made for multiple comparisons with respect to post hoc analyses. All statistical analyses were performed using SAS version 9.2.25
Cohen d effect sizes were calculated for the primary and secondary efficacy outcome variables (MADRS, CGI-BP-S) as the difference in LS mean change scores between treatment and placebo divided by the model estimate of the pooled standard deviation.
RESULTS
Baseline Characteristics
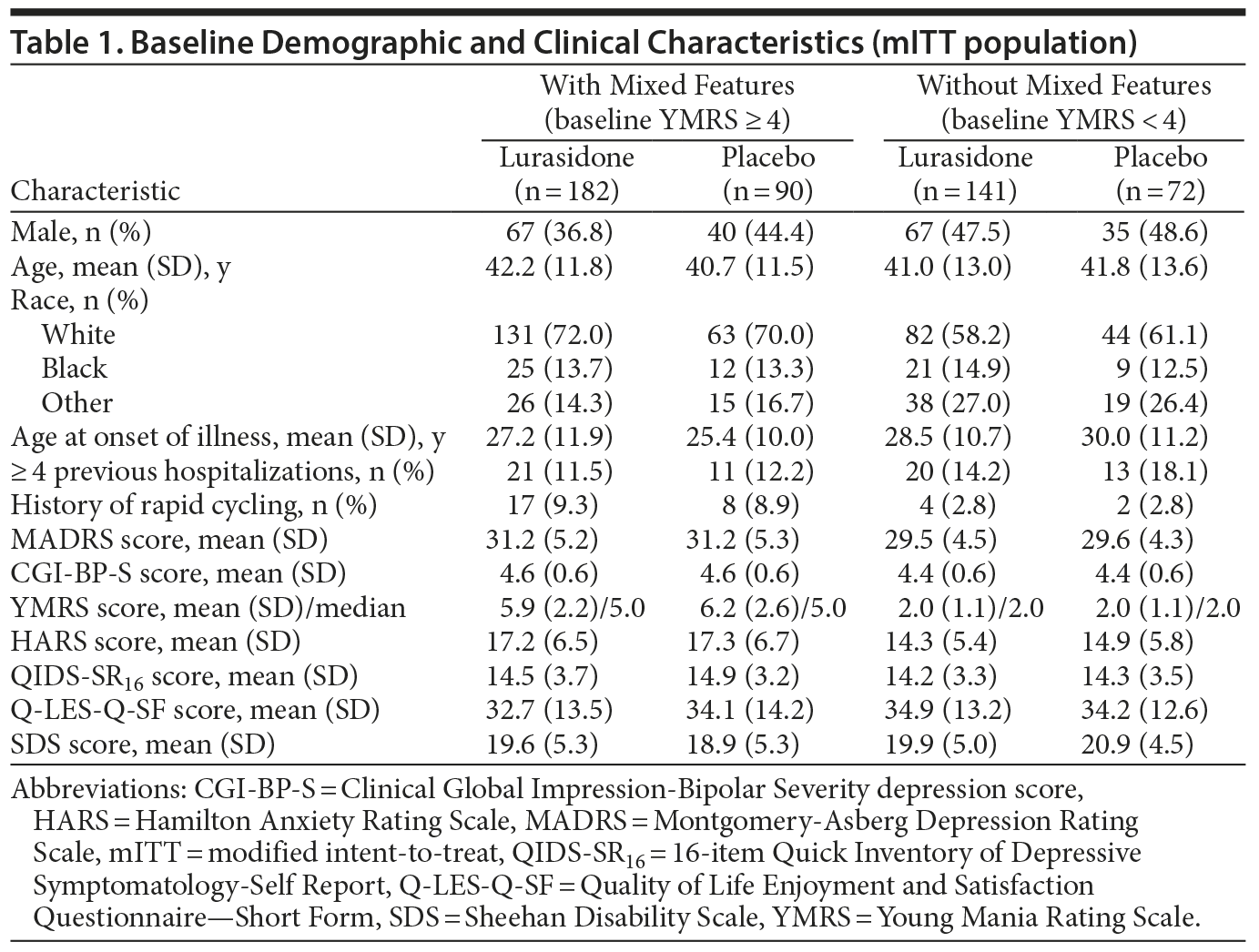
The modified intent-to-treat (mITT) population used in the current post hoc analysis consisted of 485 patients, of whom 272 (56.1%) met the baseline YMRS criterion (score ≥ 4) for mixed features. In the group with (vs without) mixed features, the mean MADRS score at baseline was 31.2 versus 29.5, and the mean YMRS score was 6.0 versus 2.0 (Table 1). At baseline, 26.0% of patients had a YMRS score of 6-12, 14.4% had a score of 8-12, and 6.2% had a score of 10-12.
At baseline, the group with mixed features (vs the group without mixed features) included a higher proportion of women (60.7% vs 52.1%; P = .0065), a higher proportion of whites (71.3% vs 59.2%; P = .002), an earlier age at onset of bipolar illness (26.6 years vs 29.0 years; P = .020), a history of rapid cycling (9.2% vs 2.8%; P = .005), and higher levels of anxiety (mean HARS score, 17.2 vs 14.5; P < .001).
Patient Disposition
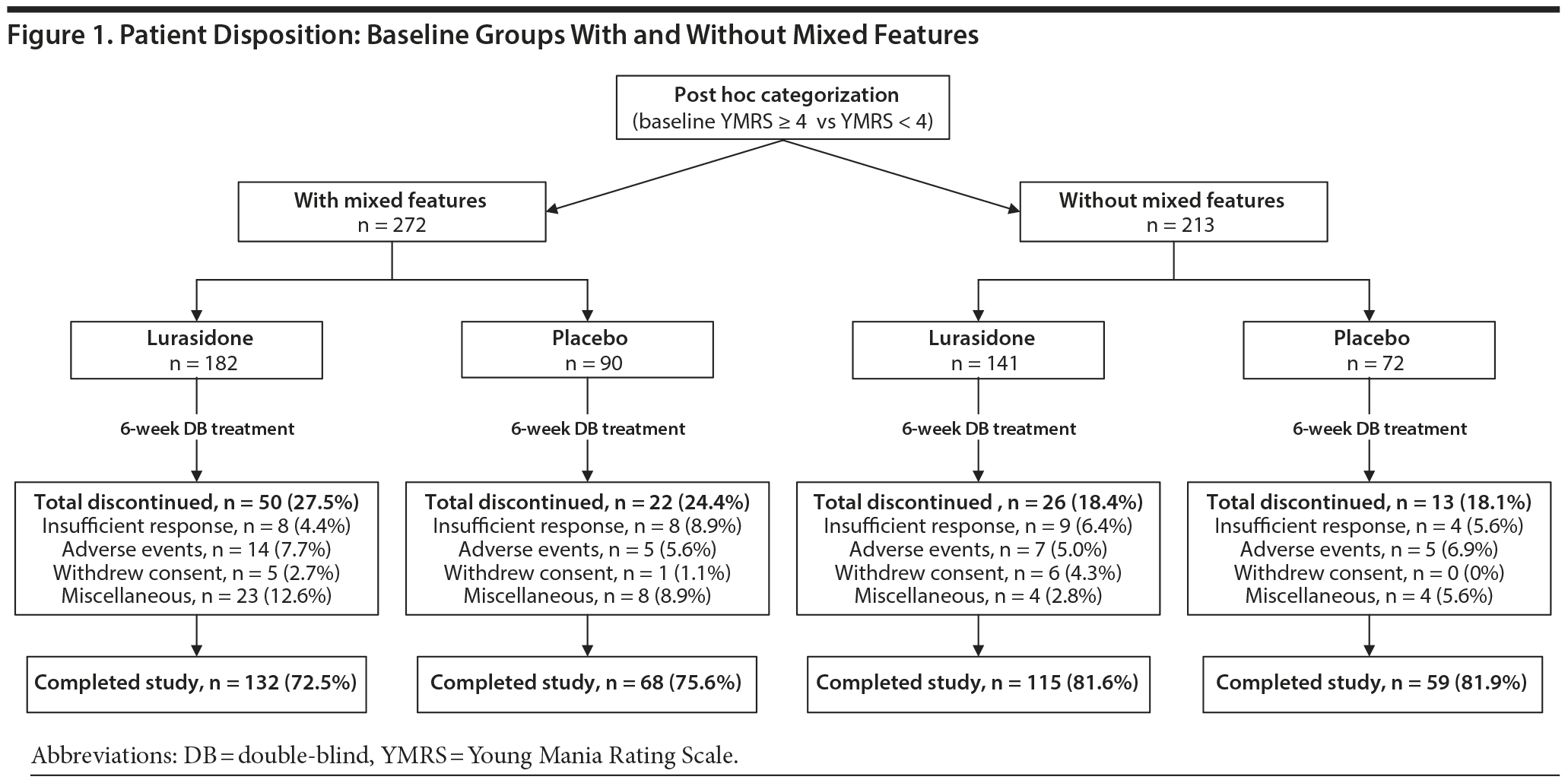
Study completion rates were somewhat lower for patients with mixed features (72.5%) compared to patients without mixed features (81.6%; Figure 1). All-cause discontinuation rates were similar for lurasidone and placebo, respectively, in the group with mixed features (27.5% vs 24.4%) and in the nonmixed features group (18.4% vs 18.1%).
Efficacy
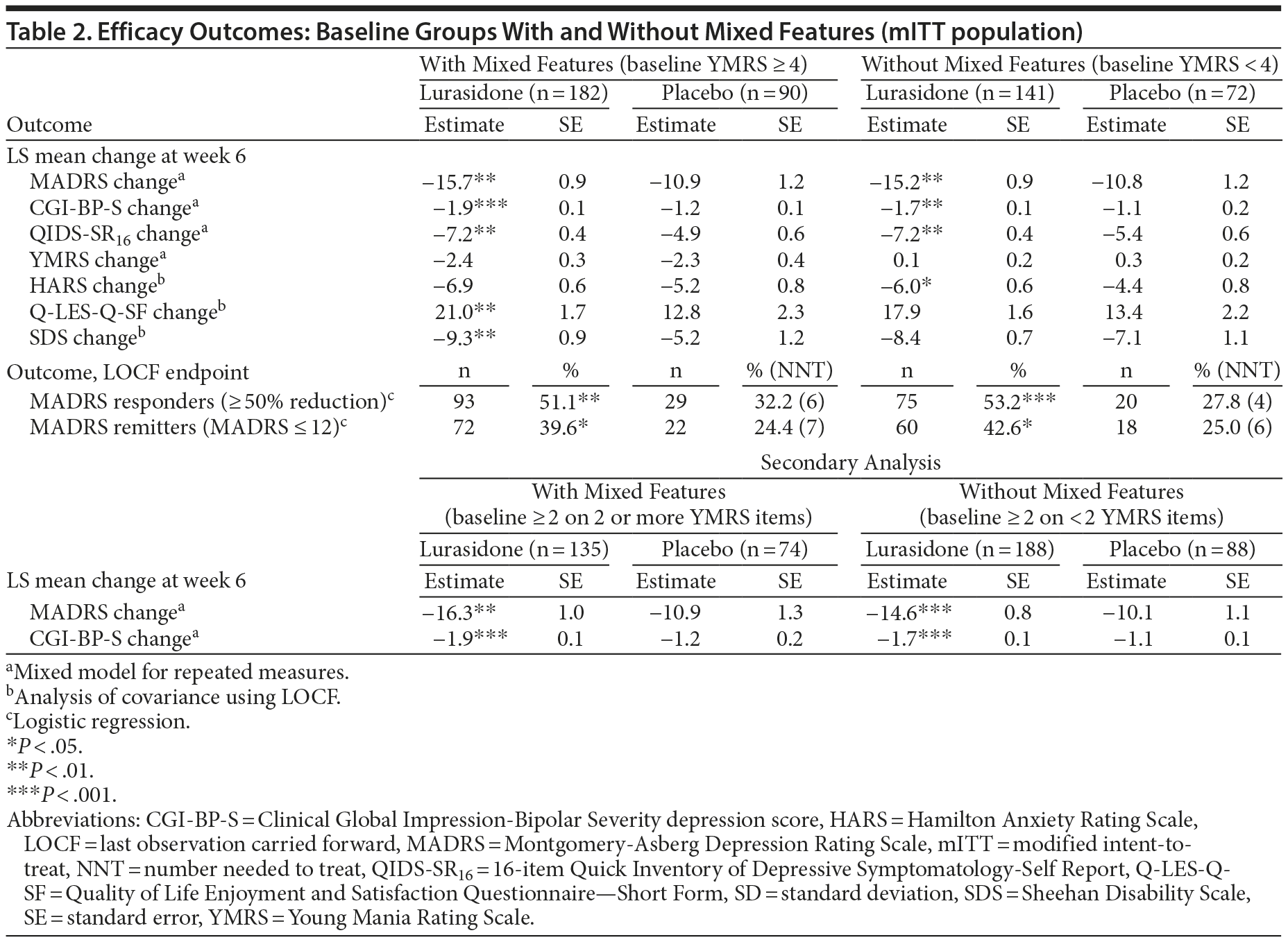
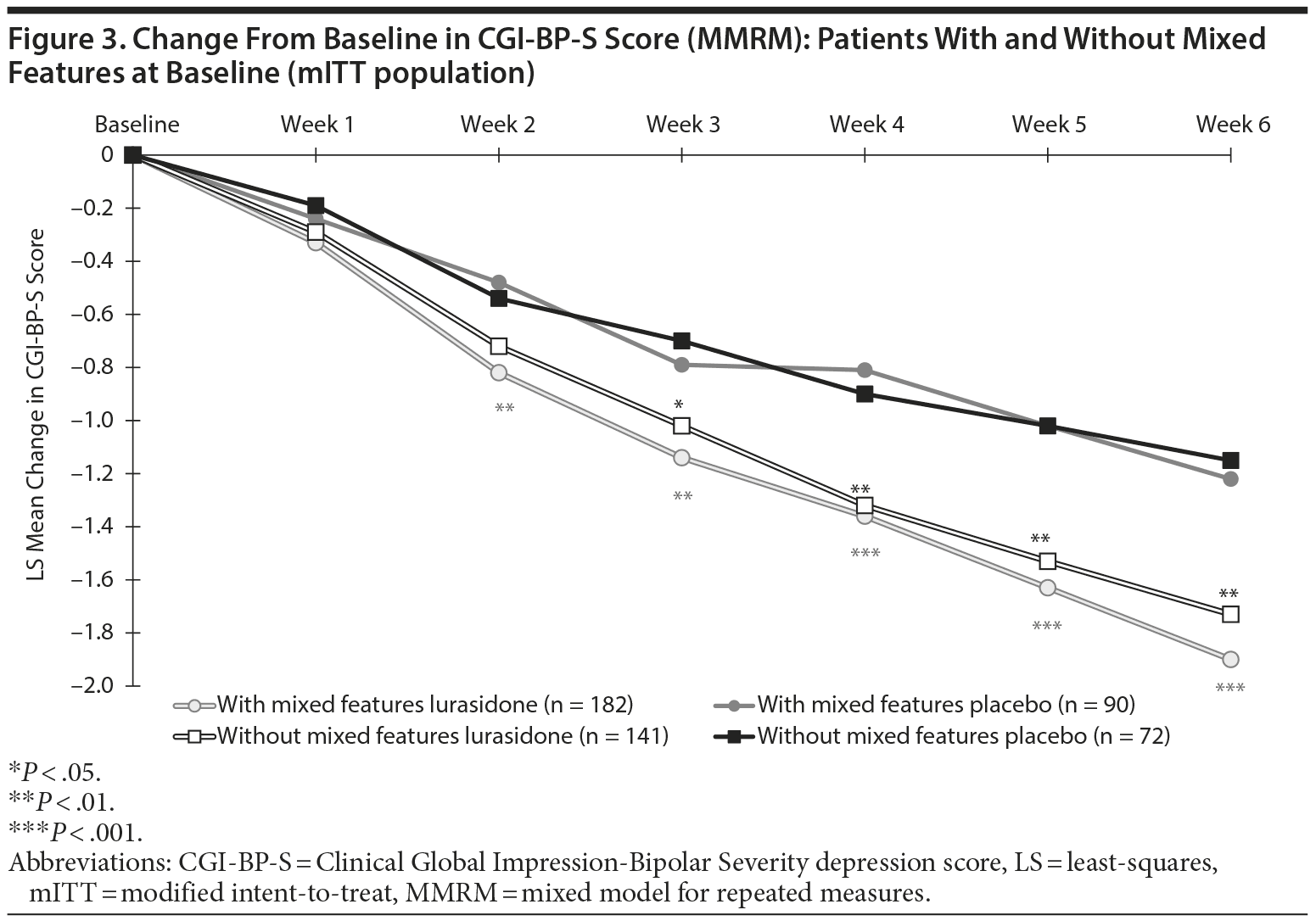
The LS mean change from baseline to week 6 in MADRS total score was significantly greater for the lurasidone group versus placebo both in patients with mixed features (−15.7 vs −10.9; P = .001; effect size, 0.48) and in patients without mixed features (−15.2 vs −10.8; P = .002 [Table 2]; effect size, 0.48). The LS mean change in the CGI-BP-S depression score was also significantly greater for the lurasidone group versus placebo both in patients with mixed features (−1.9 vs −1.2; P < .001; effect size, 0.57) and in patients without mixed features (−1.7 vs −1.1; P = .002 [Table 2]; effect size, 0.49).
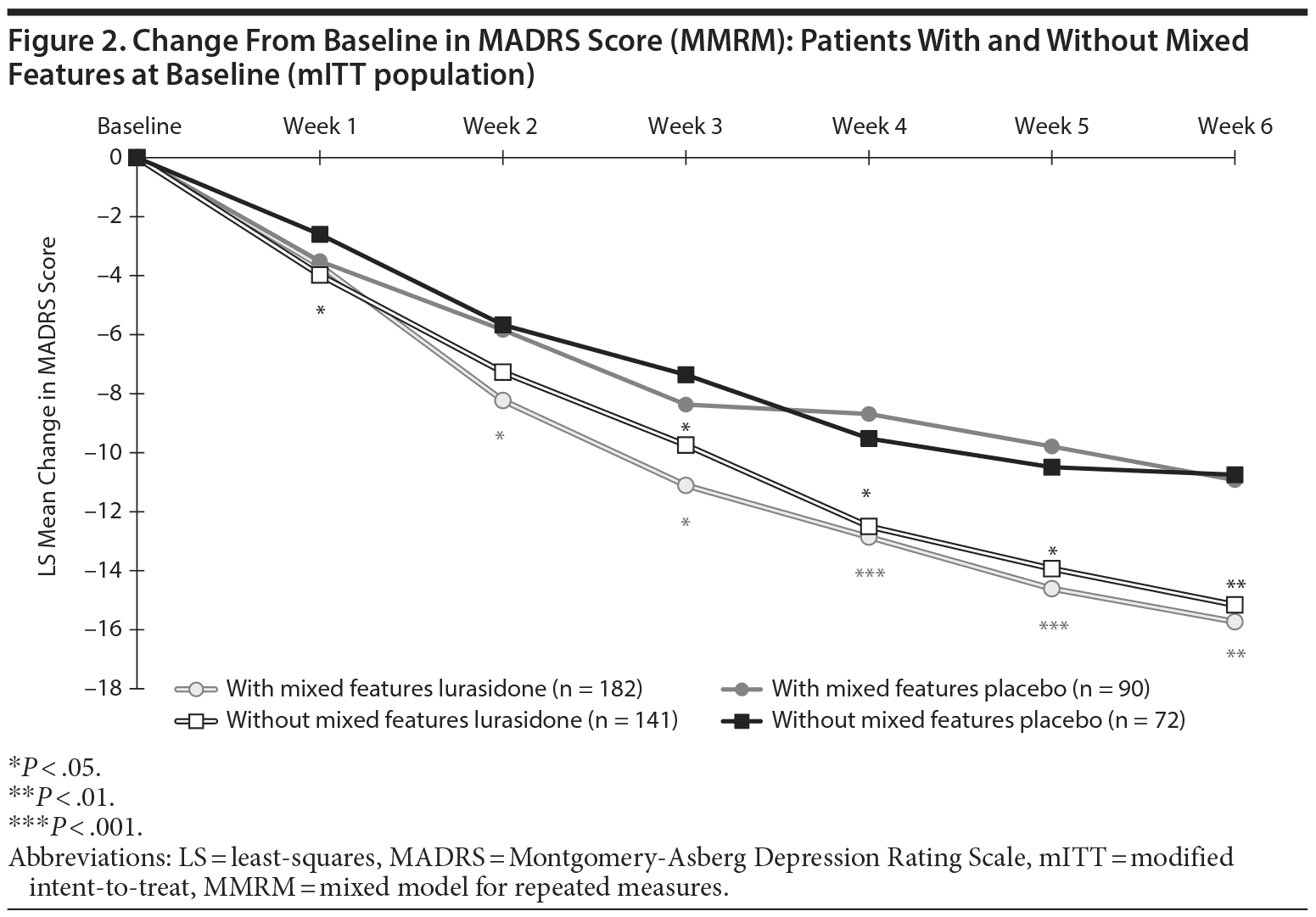
Superiority versus placebo was observed from week 2 onward for lurasidone treatment in patients with mixed features with regard to both MADRS (Figure 2) and CGI-BP-S depression scores (Figure 3). Similar superiority in favor of lurasidone was observed from week 3 onward in patients without mixed features with regard to MADRS (Figure 2) and CGI-BP-S depression scores (Figure 3).
A significantly greater proportion of patients treated with lurasidone compared with placebo were treatment responders in the group with mixed features (51.1% vs 32.2%; P = .003; NNT = 6; LOCF endpoint) and in the group without mixed features (53.2% vs 27.8%; P < .001; NNT = 4; LOCF endpoint; Table 2). Similarly, a significantly greater proportion of patients treated with lurasidone compared with placebo met remission criteria in the group with mixed features (39.6% vs 24.4%; P = .014; NNT = 7; LOCF endpoint) and in the group without mixed features (42.6% vs 25.0%; P = .012; NNT = 6; LOCF endpoint; Table 2).
In the group with mixed features, treatment with lurasidone was associated with significantly greater endpoint improvement, compared with placebo, on patient-rated assessments of depression symptoms (QIDS-SR16), quality of life (Q-LES-Q), and functioning (SDS; Table 2) and nonsignificantly greater improvement (P = .065) in the HARS total score. In the group without mixed features, treatment with lurasidone was associated with significantly greater endpoint improvement, compared with placebo, on the QIDS-SR16, and the HARS and nonsignificantly greater improvement on the Q-LES-Q (P = .058) and the SDS total score (P = .281; Table 2).
The presence/absence of mixed features was added as an interaction term to the efficacy models and was found to be nonsignificant for the MADRS (P = .376) and the CGI-BP-S (P = .818), suggesting that treatment effect sizes were similar for patients with and without mixed features. Lurasidone dose level was also examined as an interaction term in the efficacy model and was found to be nonsignificant (P = .127); in the group with mixed features, the effect size for endpoint MADRS change was 0.58 in the lurasidone 20-60 mg group and 0.36 in the 80-120 mg group; in the group without mixed features, the effect size for endpoint MADRS change was 0.31 in the lurasidone 20-60 mg group and 0.64 in the 80-120 mg group.
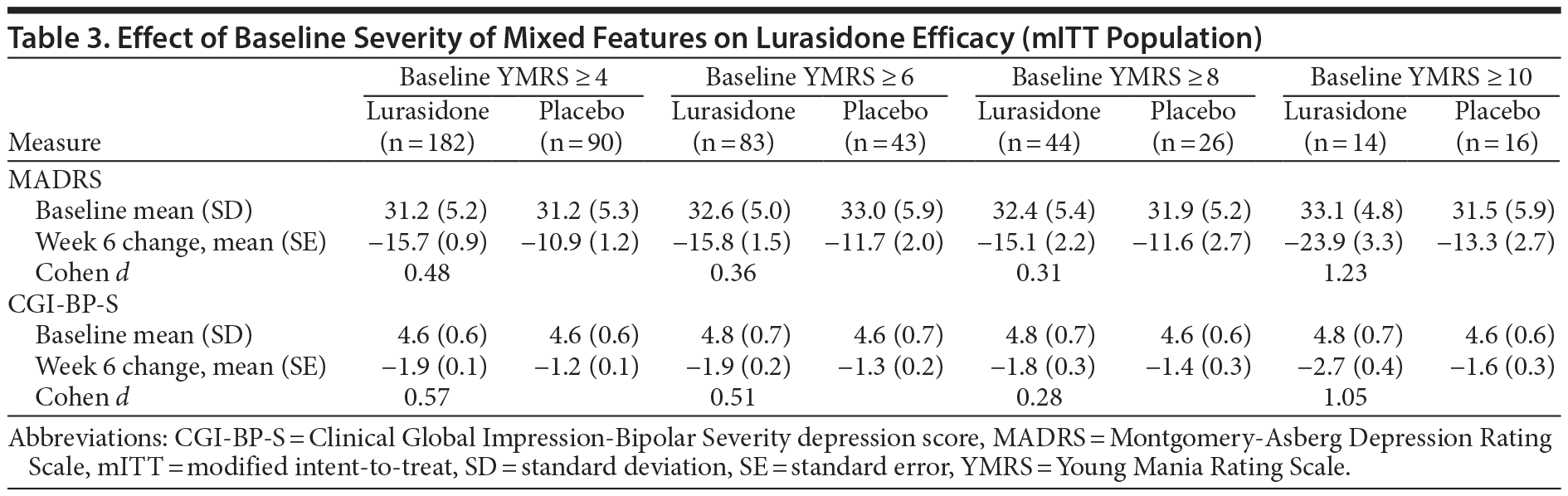
A secondary analysis was performed, utilizing a baseline score ≥ 2 on 2 or more YMRS items as the criterion for mixed features. At baseline, 209/485 (43.1%) of patients met this criterion for mixed features, with a mean MADRS score of 31.9 at baseline. In this analysis, the LS mean change from baseline to week 6 was significantly greater for the lurasidone group versus placebo on both the MADRS total score (−16.3 vs −10.9; P = .002; Table 2) and CGI-BP-S depression score (−1.9 vs −1.2; P < .001; Table 2). A significantly greater proportion of patients meeting the secondary definition of mixed features who were treated with lurasidone and placebo, respectively, were responders (51.1% vs 31.1%; P = .005; NNT = 5; LOCF endpoint) or remitters (39.3% vs 21.6%; P = .009; NNT = 6; LOCF endpoint). The relationship between the severity of mixed features at baseline and the efficacy of lurasidone (Table 3) was assessed. For MADRS change at week 6, lurasidone effect sizes were as follows for each baseline YMRS severity subgroup: YMRS score of 4-12: 0.48; 6-12: 0.36; 8-12: 0.31; and 10-12: 1.23. For CGI-BP-S change at week 6, lurasidone effect sizes were as follows for each baseline YMRS severity subgroup: YMRS score of 4-12: 0.57, 6-12: 0.51, 8-12: 0.28, and 10-12: 1.05.
Safety and Tolerability
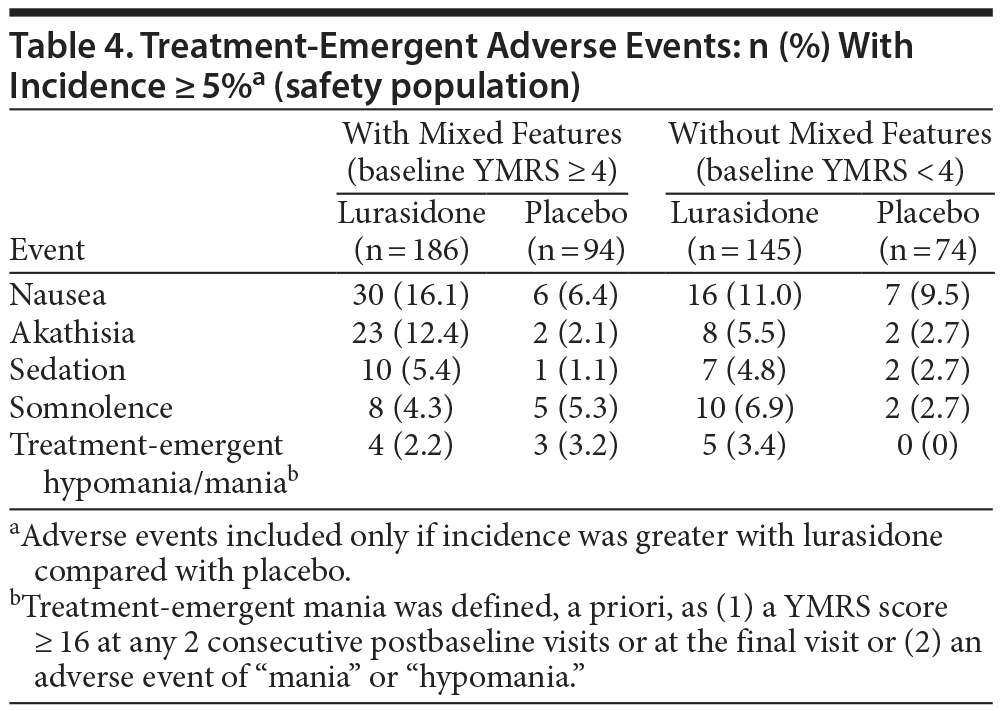
Safety and tolerability findings for the overall study population have been previously reported.10 In the current post hoc analysis, discontinuation rates due to an adverse event were similar, for lurasidone and placebo, respectively, in the group with mixed features (7.7% vs 5.6%) and in the nonmixed group (5.0% vs 6.9%; Figure 1). Table 4 summarizes the adverse events that occurred with an incidence of at least 5% (and more frequently in the lurasidone group compared with placebo). Treatment with lurasidone was associated with a higher incidence, in the mixed versus nonmixed groups, respectively, of nausea (16.1% vs 11.0%) and akathisia (12.4% vs 5.5%).
In the group with mixed features, treatment-emergent hypomania/mania, defined as a YMRS score of ≥ 16 at any 2 consecutive postbaseline visits, or at the final visit, or an adverse event of mania or hypomania, occurred at a similarly low rate in the lurasidone vs placebo groups (2.2% vs 3.2%; Table 4). In the group without mixed features, treatment-emergent hypomania/mania occurred in 3.4% of patients treated with lurasidone and in no patients treated with placebo. The mean endpoint change in YMRS scores was similar for lurasidone and placebo in the group with mixed features (−2.4 vs −2.3) and in the group without mixed features (+0.1 vs +0.3; Table 2).
The proportion of patients in the mixed features group with at least 1 instance of treatment-emergent suicidal ideation, based on the C-SSRS, was 17.0% in the lurasidone group and 21.1% in the placebo group. In the group without mixed features, treatment-emergent suicidal ideation occurred in 10.6% of the lurasidone group and 4.2% of the placebo group. Suicidal behavior or completed suicide was not observed on lurasidone or placebo in either mixed features group.
DISCUSSION
In this post hoc analysis of a placebo-controlled study that investigated the efficacy and safety of lurasidone in the treatment of patients with bipolar depression, the majority of individuals (56.1%) met the severity criterion (YMRS score ≥ 4) for mixed features, and a somewhat lower proportion (43.1%) met an alternative item-based criterion (YMRS score ≥ 2 on 2 or more items). These findings are consistent with prevalence rates, ranging from 11% to 54% (depending on setting and criteria employed), that have been previously reported.4,5,10
In this analysis, patients with mixed features were more likely to be female, be white, and have an earlier age at onset of bipolar illness, a history of rapid cycling, and higher baseline levels of anxiety compared to patients without mixed features. These patient characteristics are consistent with results from previous studies of patients with mixed features.5,11,26
The bipolar depressed population with mixed features has been reported to be characterized by more severe and chronic episodes, with higher recurrence rates, higher rates of comorbidity, and poorer overall clinical course and outcomes.1-5 In this post hoc analysis, lurasidone was found to be significantly more effective than placebo in reducing depression symptom severity in both mixed and nonmixed bipolar depressed groups. Effect sizes were similar in the groups with or without mixed features, respectively, for week 6 change in the MADRS (0.48 vs 0.48) and in the CGI-BP-S (0.57 vs 0.49). The results of a statistical interaction analysis were nonsignificant for the presence or absence of mixed features, further supporting the finding that the presence of mixed features did not influence lurasidone treatment response in our sample. Improvement in depressive symptoms in the subgroup with mixed features did not appear to be influenced by the baseline severity of hypomanic symptoms.
DSM-5 specifier criteria require that mixed features be present for the “majority” of the depressive episode, whereas in the current analysis the presence of mixed features was defined based on cross-sectional assessment at study baseline. Cross-sectional assessment has been utilized extensively to define the presence of mixed features and appears to yield a mixed feature population whose course and outcome is similar to populations defined using a longitudinal approach.4,6,11,27 However, additional empirical data are needed to evaluate the relative merits of these respective approaches.
Few studies are available that have examined the effect of mixed features on treatment response in patients with bipolar depression. Frye and colleagues6 reported that patients with bipolar depression and mildly elevated baseline YMRS scores were more likely to be nonresponders during combined antidepressant/mood stabilizer treatment and to experience treatment-emergent mania. Among patients with bipolar depression enrolled in 2 short-term clinical trials,11 Tohen and colleagues reported that low levels of mixed features were associated with a progressive reduction in responder rates to olanzapine monotherapy as the number of hypomanic symptoms increased from 0 (responders, 44.8%) to 4 (responders, 19.7%) to 7-8 (responders, 7%-9%). The olanzapine/fluoxetine combination was associated with a similar, albeit smaller, reduction in responder rates as the number of hypomanic symptoms increased from 0 (responders, 48.0%) to 4 (responders, 44.3%) to 7-8 (responders, 41.5%).11
Treatment with lurasidone was well tolerated, with relatively low all-cause discontinuation rates in both mixed features groups. The incidence of akathisia was higher among patients treated with lurasidone in the group with (vs without) mixed features (12.4% vs 5.5%; number needed to harm [NNH] = 15). Rates of treatment-emergent hypomania/mania were comparable among patients treated with lurasidone (vs placebo) in both the group with mixed features (2.2% vs 3.2%) and in the group without mixed features (3.4% vs 0%; NNH = 30). Mean YMRS scores at study endpoint showed a small (2-3 point) reduction on both lurasidone and placebo in the mixed group and minimal change in the group without mixed features (mean, 0.1-0.2).
Limitations of the current analysis include that the findings are not based on a prospective trial intended to evaluate the efficacy of lurasidone in bipolar depressed patients with mixed features. The criteria for mixed features utilized in the current analysis were based on the YMRS, which includes several items (irritability, disruptive/aggressive, appearance, insight) that are not included in the DSM-5 mixed features criteria. As previously discussed, DSM-5 specifier criteria also require that mixed features be present for the “majority” of the depressive episode, while the current analysis relied on a cross-sectional assessment to determine the presence of mixed features.
CONCLUSIONS
Lurasidone was found in this post hoc analysis to be effective in the treatment of patients with bipolar depression associated with mixed features, assessed using a manic severity criterion or number of manic symptoms as a criterion. Similar levels of improvement were observed in the mixed and nonmixed features groups in depressive symptoms and across measures of anxiety, quality of life, and functioning in patients. No increased risk of treatment-emergent hypomania/mania was observed in either group.
Drug names: fluoxetine (Prozac and others), lithium (Lithobid and others), lurasidone (Latuda), olanzapine (Zyprexa), olanzapine/fluoxetine combination (Symbyax).
Author affiliations: Departments of Psychiatry and Pharmacology, University of Toronto; Mood Disorders Psychopharmacology Unit, University Health Network, Toronto; and Institute of Medical Science, University of Toronto, Toronto, Ontario, Canada (Dr McIntyre); and Sunovion Pharmaceuticals Inc, Fort Lee, New Jersey, and Marlborough, Massachusetts (Drs Cucchiaro, Pikalov, and Loebel and Mr Kroger).
Potential conflicts of interest: Dr McIntyre has received research grants from Eli Lilly, Janssen-Ortho, Shire, AstraZeneca, Pfizer, Lundbeck, NARSAD, National Institute of Mental Health, and the Stanley Medical Research Institute; has received honoraria and/or consulting fees for work on advisory boards and speakers bureaus from AstraZeneca, Bristol-Myers Squibb, France Foundation, GlaxoSmithKline, Janssen-Ortho, Eli Lilly, Organon, Lundbeck, Pfizer, Shire, Sunovion, Merck, I3CME, Physicians Postgraduate Press, CME Outfitters, and OptumHealth; and has received fees for CME activities from AstraZeneca, Bristol-Myers Squibb, France Foundation, I3CME, Physicians Postgraduate Press, CME Outfitters, OptumHealth, Merck, Eli Lilly, and Pfizer. Drs Cucchiaro, Pikalov, and Loebel and Mr Kroger are employees of Sunovion Pharmaceuticals Inc.
Funding/support: Financial support for technical editorial and medical writing assistance was provided by Sunovion Pharmaceuticals Inc, Marlborough, Massachusetts. The original clinical research was sponsored by Sunovion Pharmaceuticals Inc.
Role of the sponsor: The sponsor was involved in the design, collection, and analysis of the data. Interpretation of the results was by the authors, and the decision to submit this manuscript for publication in The Journal of Clinical Psychiatry, was made by the authors.
Acknowledgment: Edward Schweizer, MD, of Paladin Consulting Group, provided technical editorial and medical writing assistance for this manuscript under the direction of the authors.
REFERENCES
1. Vieta E, Valent× M. Mixed states in DSM-5: implications for clinical care, education, and research. J Affect Disord. 2013;148(1):28-36. PubMed doi:10.1016/j.jad.2013.03.007
2. Swann AC, Lafer B, Perugi G, et al. Bipolar mixed states: an International Society for Bipolar Disorders task force report of symptom structure, course of illness, and diagnosis. Am J Psychiatry. 2013;170(1):31-42. PubMed doi:10.1176/appi.ajp.2012.12030301
3. Zimmermann P, Brückl T, Nocon A, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry. 2009;66(12):1341-1352. PubMed doi:10.1001/archgenpsychiatry.2009.158
4. Azorin JM, Kaladjian A, Adida M, et al. Self-assessment and characteristics of mixed depression in the French national EPIDEP study. J Affect Disord. 2012;143(1-3):109-117. PubMed doi:10.1016/j.jad.2012.05.036
5. Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166(2):173-181. PubMed doi:10.1176/appi.ajp.2008.08050746
6. Frye MA, Helleman G, McElroy SL, et al. Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry. 2009;166(2):164-172. PubMed doi:10.1176/appi.ajp.2008.08030322
7. Benazzi F. Reviewing the diagnostic validity and utility of mixed depression (depressive mixed states). Eur Psychiatry. 2008;23(1):40-48. PubMed doi:10.1016/j.eurpsy.2007.07.003
8. Benazzi F, Akiskal HS. Delineating bipolar II mixed states in the Ravenna-San Diego collaborative study: the relative prevalence and diagnostic significance of hypomanic features during major depressive episodes. J Affect Disord. 2001;67(1-3):115-122. PubMed doi:10.1016/S0165-0327(01)00444-X
9. Nusslock R, Frank E. Subthreshold bipolarity: diagnostic issues and challenges. Bipolar Disord. 2011;13(7-8):587-603. PubMed doi:10.1111/j.1399-5618.2011.00957.x
10. McIntyre RS, Soczynska JK, Cha DS, et al. The prevalence and illness characteristics of DSM-5-defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: Results from the International Mood Disorders Collaborative Project. J Affect Disord. 2014;172C:259-264. PubMed
11. Tohen M, Kanba S, McIntyre RS, et al. Efficacy of olanzapine monotherapy in the treatment of bipolar depression with mixed features. J Affect Disord. 2014;164:57-62. PubMed doi:10.1016/j.jad.2014.04.003
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Publishing, 2000.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Publishing; 2013.
14. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168. PubMed doi:10.1176/appi.ajp.2013.13070984
15. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-177. PubMed doi:10.1176/appi.ajp.2013.13070985
16. Citrome L, Ketter TA, Cucchiaro J, et al. Clinical assessment of lurasidone benefit and risk in the treatment of bipolar I depression using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2014;155:20-27. PubMed doi:10.1016/j.jad.2013.10.040
17. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
18. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429-435. PubMed doi:10.1192/bjp.133.5.429
19. Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159-171. PubMed doi:10.1016/S0165-1781(97)00123-6
20. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
21. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
22. Sheehan DV. The Anxiety Disease. New York, NY: Bantam Books; 1983.
23. Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321-326. PubMed
24. Chappell P, Feltner DE, Makumi C, et al. Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale. Am J Psychiatry. 2012;169(6):662-663, author reply 663. PubMed doi:10.1176/appi.ajp.2012.12010123
25. Version SAS. 9.2 [computer program]. Cary, NC: SAS Institute Inc; 2008.
26. Cassidy F, Yatham LN, Berk M, et al. Pure and mixed manic subtypes: a review of diagnostic classification and validation. Bipolar Disord. 2008;10(1, pt 2):131-143. PubMed doi:10.1111/j.1399-5618.2007.00558.x
27. Benazzi F, Berk M, Frye MA, et al. Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: a post hoc analysis. J Clin Psychiatry. 2009;70(10):1424-1431. PubMed doi:10.4088/JCP.08m04772gre
This PDF is free for all visitors!