Randomized Placebo-Controlled Adjunctive Study of an Extract of Withania somnifera for Cognitive Dysfunction in Bipolar Disorder
ABSTRACT
Objective: Cognitive impairments contribute significantly to inadequate functional recovery following illness episodes in bipolar disorder, yet data on treatment interventions are sparse. We assessed the cognitive effects of a standardized extract of the medicinal herb Withania somnifera (WSE) in bipolar disorder.
Method: Sixty euthymic subjects with DSM-IV bipolar disorder were enrolled in an 8-week, double-blind, placebo-controlled, randomized study of WSE (500 mg/d) as a procognitive agent added adjunctively to the medications being used as maintenance treatment for bipolar disorder. Study enrollment and data analyses were completed between December 2008 and September 2012. Cognitive testing at baseline and 8 weeks assessed primary efficacy outcomes. Psychopathology and adverse events were
monitored at scheduled visits.
Results: Fifty-three patients completed the study (WSE, n = 24; placebo, n = 29), and the 2 groups were matched in terms of demographic, illness, and treatment characteristics. Compared to placebo, WSE provided significant benefits for 3 cognitive tasks: digit span backward (P = .035), Flanker neutral response time (P = .033), and the social cognition response rating of the Penn Emotional Acuity Test (P = .045). The size of the WSE treatment effect for digit span backward was in the medium range (Cohen d = 0.51; 95% CI, 0.25–0.77). None of the other cognitive tasks showed significant between-group differences. Mood and anxiety scale scores remained stable, and adverse events were minor.
Conclusions: Although results are preliminary, WSE appears to improve auditory-verbal working memory (digit span backward), a measure of reaction time, and a measure of social cognition in bipolar disorder. Given the paucity of data for improving cognitive capacity in bipolar disorder, WSE offers promise, appears to have a benign side-effects profile, and merits further study.
Trial Registration: ClinicalTrials.gov identifier: NCT00761761
J Clin Psychiatry 2013;74(11):1076–1083
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: February 7, 2013; accepted May 3, 2013 (doi:10.4088/JCP.13m08413).
Corresponding author: K. N. Roy Chengappa, MD, Comprehensive Recovery Services, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, 3811 O’Hara St, Pittsburgh, PA ([email protected]).
Meta-analyses from independent research groups have confirmed that individuals with bipolar disorder show cognitive impairments that persist during euthymic intervals.1–8 Medium to large effect size impairments have been reported in executive functioning, working memory, processing speed, episodic memory, fluency, and problem-solving and perceptual cognitive domains; in other words, a broad cognitive deficit although less severe than those reported in persons with schizophrenia.9
In schizophrenia, cognitive impairments are consistently associated with poor functional outcomes.10 Likewise, in 6 of 8 studies, cognitive impairments were linked to worse functioning in persons with bipolar disorder even after controlling for demographic, illness, and mood variables.11 Importantly, in previously employed individuals with bipolar disorder who had experienced a manic episode, changes in specific cognitive test scores robustly predicted occupational recovery 3 months after symptomatic recovery.12 So, it would stand to reason that improving cognitive capacity in bipolar disorder should be accorded research priority. However, controlled data on such treatment efforts are surprisingly sparse. Burdick and colleagues13 evaluated a dopamine D2/D3 agonist, pramipexole, in an 8-week placebo-controlled trial to improve cognition in subjects with bipolar disorder. The primary analyses did not show cognitive improvements in the expected direction; yet, when the sample was restricted to a strictly euthymic bipolar disorder group, 2 tasks—digits backward and Stroop color—showed clinically relevant treatment effects with pramipexole.13
The mechanisms that underlie cognitive impairment in bipolar disorder remain elusive. Pharmacologic strategies (among others) to improve cognition in bipolar disorder14 have included enhancement of dopaminergic function,13,15,16 increased cholinergic function,17,18 or decreasing hypercortisolemia using a glucocorticoid receptor antagonist.19 Cognitive rehabilitation has also been assessed in an open study.20
We assessed a standardized extract of the medicinal plant Withania somnifera (WSE, Sensoril) as a procognitive agent in bipolar disorder. W somnifera has been used for centuries in Ayurvedic medicine in India as “Rasayana” or an “adaptogen” (increasing bodily resistance to stress and disease). Modern chemistry data indicate several important bioactive constituents in WSE, including steroidal lactones termed glycowithanolides and sitoindosides, as well as Withaferin A.21 Glycowithanolides have shown brain antioxidant, neuroprotective, and memory-enhancing activity.22–25 Withanolide A from WSE reversed memory deficits and induced regeneration of dendritic spines and axons in mice.26 Furthermore, in a rat model of stress, WSEs attenuated damage to hippocampal neurons in the CA2 and CA3 region by 80%.24 In stress models, rodents pretreated with WSE showed significant diminution in hypercortisolemia and other indices of stress.23,27–29 WSEs have shown procholinergic but not glutamatergic or gabaergic effects in rat brain30; furthermore, WSEs show cholinesterase inhibiting activity.31–33 Given these diverse pharmacologic actions, we postulated that adjunctive WSE treatment would improve cognitive functions broadly in subjects with bipolar disorder, eg, executive functions, working memory, attention, processing speed, and memory.
METHOD
An 8-week clinical trial was conducted using a random-assignment, parallel-group, double-blind, placebo-controlled design and was registered at clinicaltrials.gov (identifier: NCT00761761). WSE or placebo was added adjunctively to the medications being used as maintenance treatment for bipolar disorder. The study was conducted at Western Psychiatric Institute and Clinic–University of Pittsburgh Medical Center. An investigational new drug application (IND) was submitted to the US Food and Drug Administration, and approval was obtained to use a standardized preparation of WSE (Sensoril) as described in this report. Sensoril and placebo were kindly provided by Natreon, Inc (New Brunswick, New Jersey). Coded, identical-looking hard gelatin capsules containing WSE (250 mg) or placebo (containing inert substances or excipients) of identical fill weights were utilized. Sensoril is concentrated to a minimum of 8% withanolides, 32% oligosaccharides (carrier molecules), and a maximum of 2% Withaferin A (high concentrations can be toxic) and is based on a standardized aqueous extraction process. Placebo capsules were exposed to cloth-covered sachets containing WSE. After a few days, the smell permeated the placebo gelatin capsules, which then smelled similar to the WSE capsules. The study was approved by the University of Pittsburgh Institutional Review Board. The study enrolled subjects starting December 2008, and final data analyses were completed in September 2012.
Subjects
Adult men or women aged between 18 and 65 years of any ethnicity with a Mini-International Neuropsychiatric Interview34 (MINI)–affirmed DSM-IV diagnosis of bipolar I, II, or NOS disorder (supplemented by discussions with referring clinicians, medical chart review, and a consensus reached among the investigators) who were outpatients and who provided written informed consent were enrolled. Patients with diagnosed neurologic disorders, unstable medical conditions, or known allergy or side-effects to WSE; receiving cholinesterase inhibitors; or taking over-the-counter agents—St John’s wort, Ginkgo biloba, or omega-3 fish oil—were excluded. Pregnant or breast-feeding women were excluded. Recent instability in mental status, especially suicidal and homicidal behavior or ECT treatment in the past 6 months, was also grounds for exclusion. At screening, potential subjects had to have Young Mania Rating Scale (YMRS)35 and Montgomery-Asberg Depression Rating Scale (MADRS)36 scores < 10 for at least 4 weeks, and their main mood-stabilizing medication had to have been used in stable doses for ≥ 4 weeks.
Procedures
At screening, an electrocardiogram (ECG), psychopathology ratings, and medical history were obtained from subjects. Laboratory tests among others included a screen for drugs of abuse, pregnancy, thyroid indices, and mood-stabilizer levels. A baseline cognition assessment was completed. Subjects meeting all eligibility criteria proceeded to a computer-generated 1:1 randomization allocation schedule to either WSE or placebo. Titration of WSE (or placebo) 250 mg/d orally began on day 1 for the first week, increasing to 250 mg twice daily in the second week; the 500-mg/d dosage continued throughout the study period. A minimum dosage of WSE of 250 mg/d was permissible for lack of tolerability. The daily dosage was based on a study that used the same WSE (Sensoril) for ameliorating stress in subjects attending an Ayurvedic clinic.37 Mood-stabilizer or other concomitant medications used for bipolar disorder treatment continued unchanged throughout the study. Among others, exit criteria utilized by the investigative team with oversight of a Data and Safety Monitoring Board included decompensation of mental status (clinical determination and rating scales), serious adverse events, hospitalization, requirement of alternative mood-stabilizing medications, and nonadherence to study procedures or visits.
Measures
Cognition was assessed using tests developed by The Cognition Group (TCG; London, United Kingdom, and Newark, Delaware). Testing procedures and consistency were assured by the same staff-patient dyad for each assessment, and a TCG staff person had previously trained the research staff.38 Executive functions, processing speed, attention/working memory, memory, and psychomotor speed were assessed using the Set Shifting Test (SST), Strategic Target Detection Test (STDT), Flanker Test, Auditory Digit Span, Word List Memory, and the Finger Tapping Tests, respectively. Social cognition was assessed using the Penn Emotional Acuity Test. The subjects rate the emotional valence of happy, neutral, and sad faces on a 7-point Likert scale, with 4 being neutral; scores above 4 reflect slightly, moderately, and very happy; and scores below 4 are sad ratings.
Details of these cognitive tests are available at http://www.cogtest.com, and are also described in other studies.38–40 Patients took approximately 60 minutes to complete the testing. The 2 main cognition testing time-points were the pre-randomization baseline and 8 weeks. One time-point at 4 weeks was added to assess a secondary outcome, ie, to evaluate if there was a difference in time to onset of improvements (if any) in cognitive test scores between the 2 treatments. Several tests in the cognition battery have alternate forms to reduce the potential for practice effects.
Psychopathology was assessed utilizing the YMRS, the MADRS, and the Hamilton Anxiety Rating Scale (HARS).41 Ratings were done by a trained rater blind to treatment assignment at 6 scheduled visits during which vital signs and any treatment-emergent adverse events were also recorded. Adherence to study medication was determined by reconciling pill counts. The Functioning Assessment Short Test (FAST) was administered at baseline and 8 weeks.42 The final visit also included a medical review of body systems, laboratory, and ECG results.
Statistical Analyses
Demographic, clinical, and treatment characteristics were examined using independent group Student t tests or the χ2 test. The change scores for the psychopathology rating scales between WSE and placebo were evaluated using an analysis of covariance (ANCOVA) with the baseline total score of each scale as the covariate. Similar analyses were applied to the FAST total and individual domain scores. All randomized subjects with a baseline rating were included in the safety analyses, and those with at least 1 post-randomization assessment were included in the primary and secondary outcomes analysis. Treatment-emergent adverse events were grouped by body system and assessed for between–treatment group differences using the Fisher exact test. Statistical analyses were done blind to treatment assignment.
The primary outcomes were the evaluation of changes in the scores of cognitive tests from baseline to 8 weeks following WSE treatment (relative to placebo). Based on past studies,38 the cognition variables were assessed for normal distribution. The primary outcomes, ie, cognition variables, were analyzed using ANCOVA with treatment and visit as fixed effects and baseline cognitive score as the covariate. Statistically significant differences between the 2 treatment groups for changes from baseline to end of study were evaluated using 2-sided P values and least-squares (LS) means as point estimates. Two-sided 95% confidence intervals (CIs) were also calculated for the estimated differences between treatments. Effect size (Cohen d) was computed for the significant cognitive results as the mean differences in the change in cognitive scores between treatments divided by the pooled standard deviation. Model assumptions of parallelism and normality were assessed by including treatment by visit interactions in the model and assessment of model residuals. Interaction terms were removed from the final model in the event of constancy over time, and log transformations were applied in the event of demonstration of
non-normality. An adjustment for multiple comparisons was not applied as this study was a preliminary investigation with a modest sample size. Furthermore, overcorrection of type 1 error would very likely increase the type 2 error and thereby mask real differences in these cognitive tasks.
RESULTS
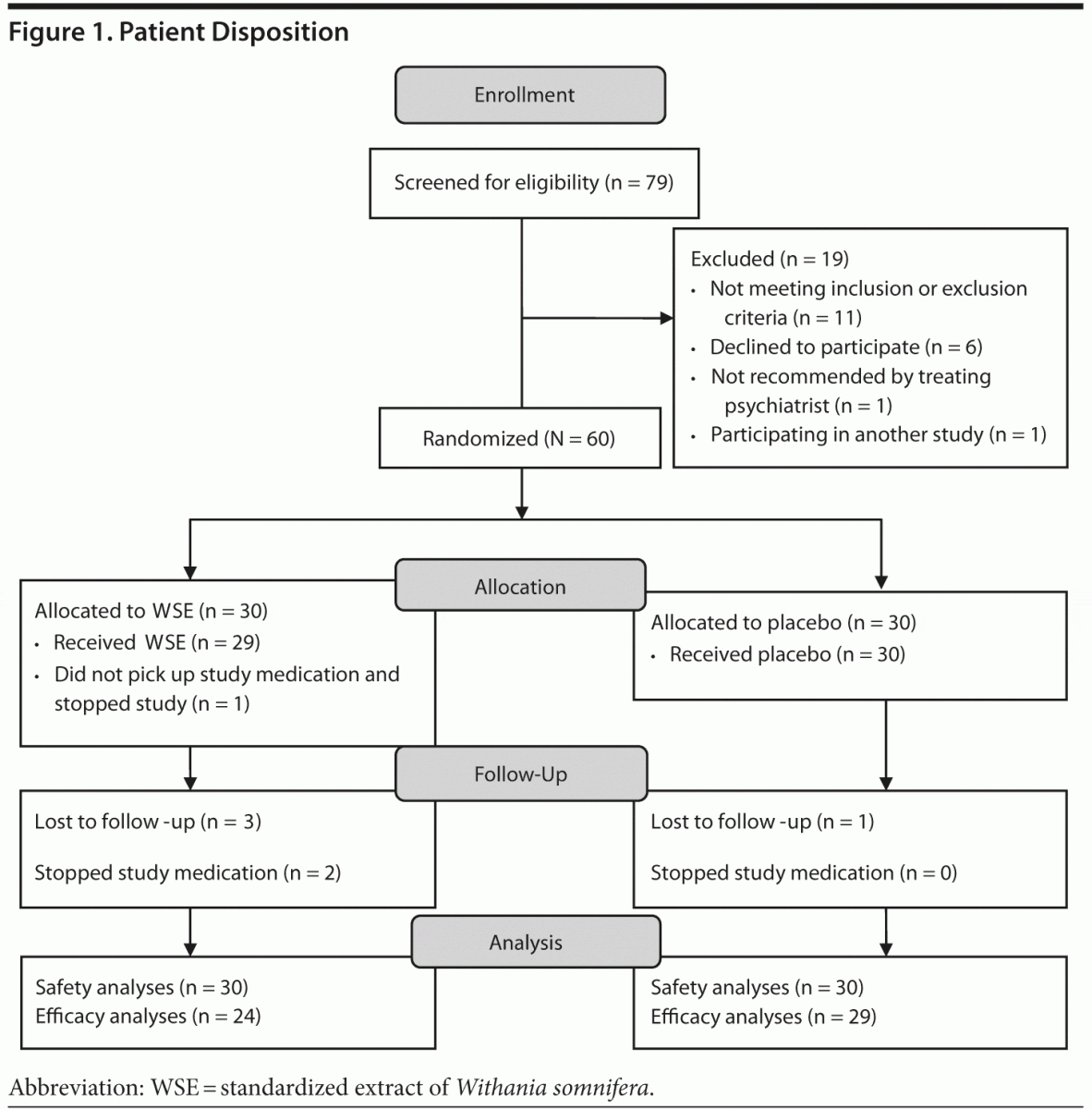
Seventy-nine subjects with DSM-IV bipolar disorder provided informed consent and 19 subjects did not meet eligibility criteria (see Figure 1). Sixty patients were randomly assigned to WSE (n = 30) or placebo (n = 30). The demographic, illness, and treatment characteristics of the randomized participants are described in Table 1, and there were no statistically significant differences between the treatment groups, even though the WSE-treated group was slightly older and reported more life-time hospitalizations. Lithium and valproate levels were in the range of 0.55 to 1.11 mEq/L and 54 to 106 mg/dL, respectively, with no significant differences between treatments (data not shown).
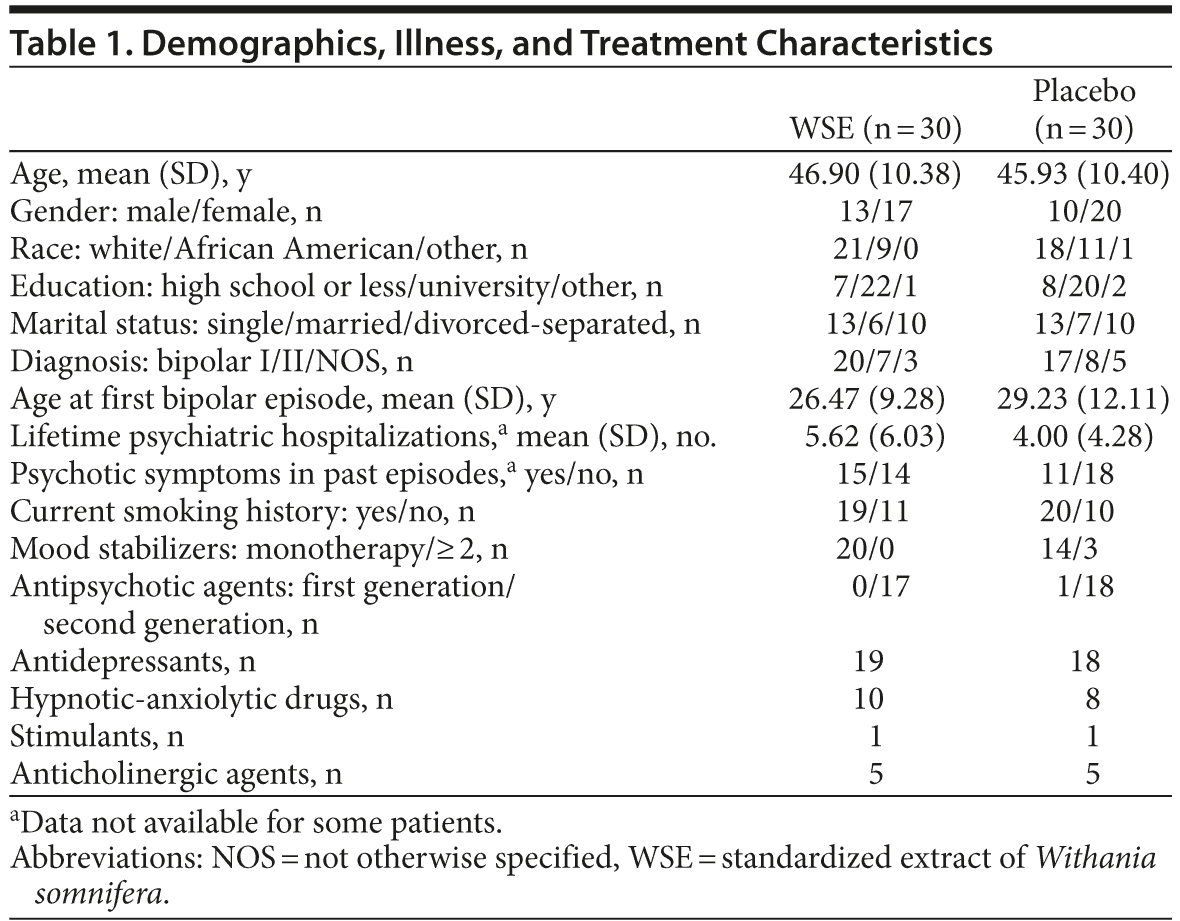
The YMRS, MADRS, and HARS scores are shown in Table 2 and reflect a patient population whose symptoms were well controlled (ie, euthymic) and remained stable through the study period. There were no statistically significant differences between treatment groups in any of the psychopathology scale scores from baseline to study endpoint. Similarly, there were no statistically significant between-group differences in either the total or individual domain scores of the FAST from baseline to endpoint (higher scores indicate worse functioning) (Table 2). Only the cognitive domain score of the FAST indicated a within-group improvement for the WSE-treated group (t107 = 2.07, P < .041), but not the placebo group (t107 = 1.12, P < .266).
All except 3 subjects who were titrated back down to 250 mg/d (WSE: n = 2, complaints of “vivid dreams” and “sleepiness”; placebo: n = 1, complaint of “vivid dreams”) tolerated the 500 mg/d of study medicine. Based on reconciled pill counts, adherence ranged from 82.9% to 100%, with no significant differences between those assigned to WSE versus placebo.
Primary Outcomes
WSE-treated patients achieved significantly greater improvement compared to those receiving placebo on the following cognitive tasks: mean digit span backward (Auditory Digit Span), neutral mean response time (Flanker Test), and the mean social cognition response rating (Penn Emotional Acuity Test) (Table 3). The size of the treatment effect (Cohen d) was 0.51 (95% CI, 0.246 to 0.774) for mean digit span backward, 0.62 (95% CI, −16.35 to 15.15) for Flanker neutral mean response time, and 0.26 (95% CI, 0.202 to 0.308) for mean response task of the Penn Emotional Acuity Test. Significant between–treatment group differences for these cognitive variables were noted at the end of the study (8 weeks), not at 4 weeks.
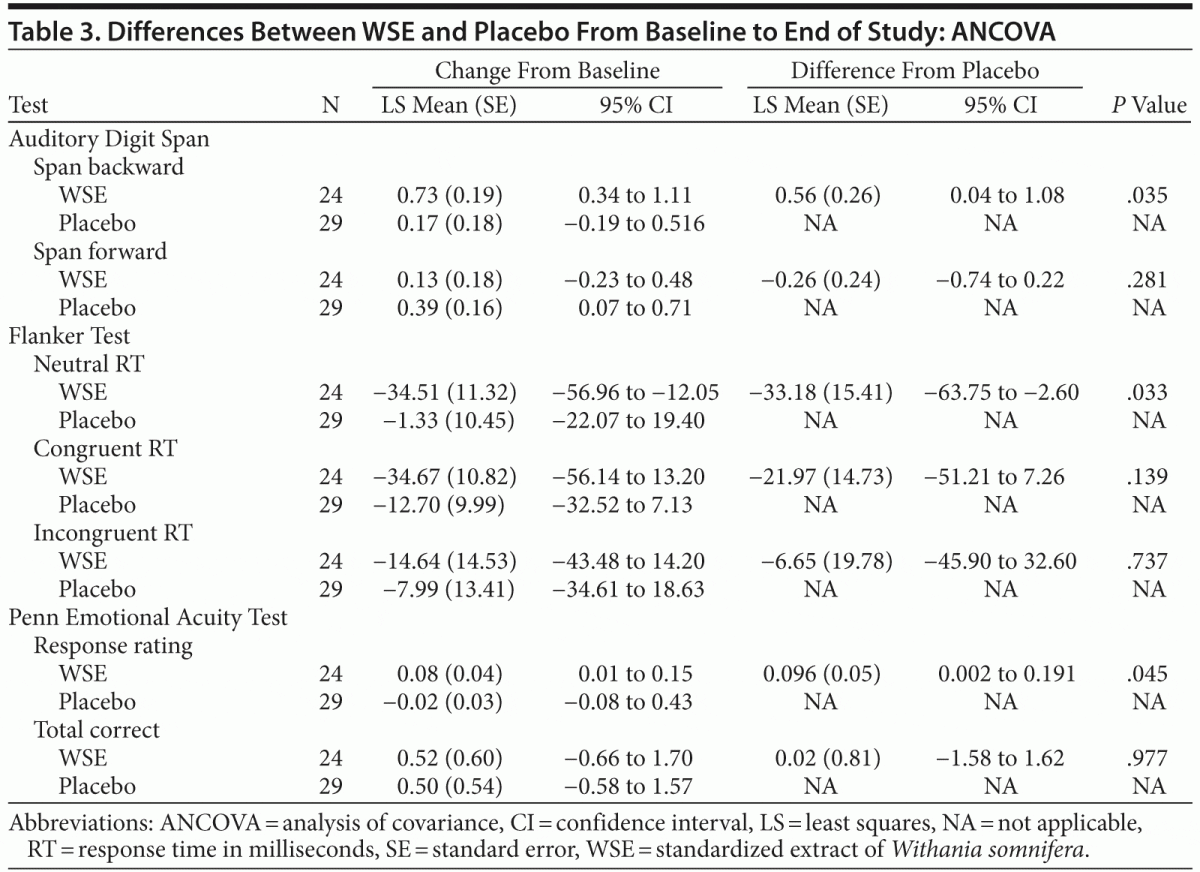
The forward digit span change scores of the Auditory Digit Span were not significantly different between the 2 treatments. Even though the WSE-treated group achieved faster congruent and incongruent mean response processing times on the 2 other Flanker Test variables, these differences were not statistically significantly different from placebo (Table 3). On the social cognition test of the Penn Emotional Acuity Test, the proportions of total mean correct responses for neutral, sad, and happy faces were not significantly different between treatments. None of the other cognitive task variables showed significant differences between treatment groups (Table 4).
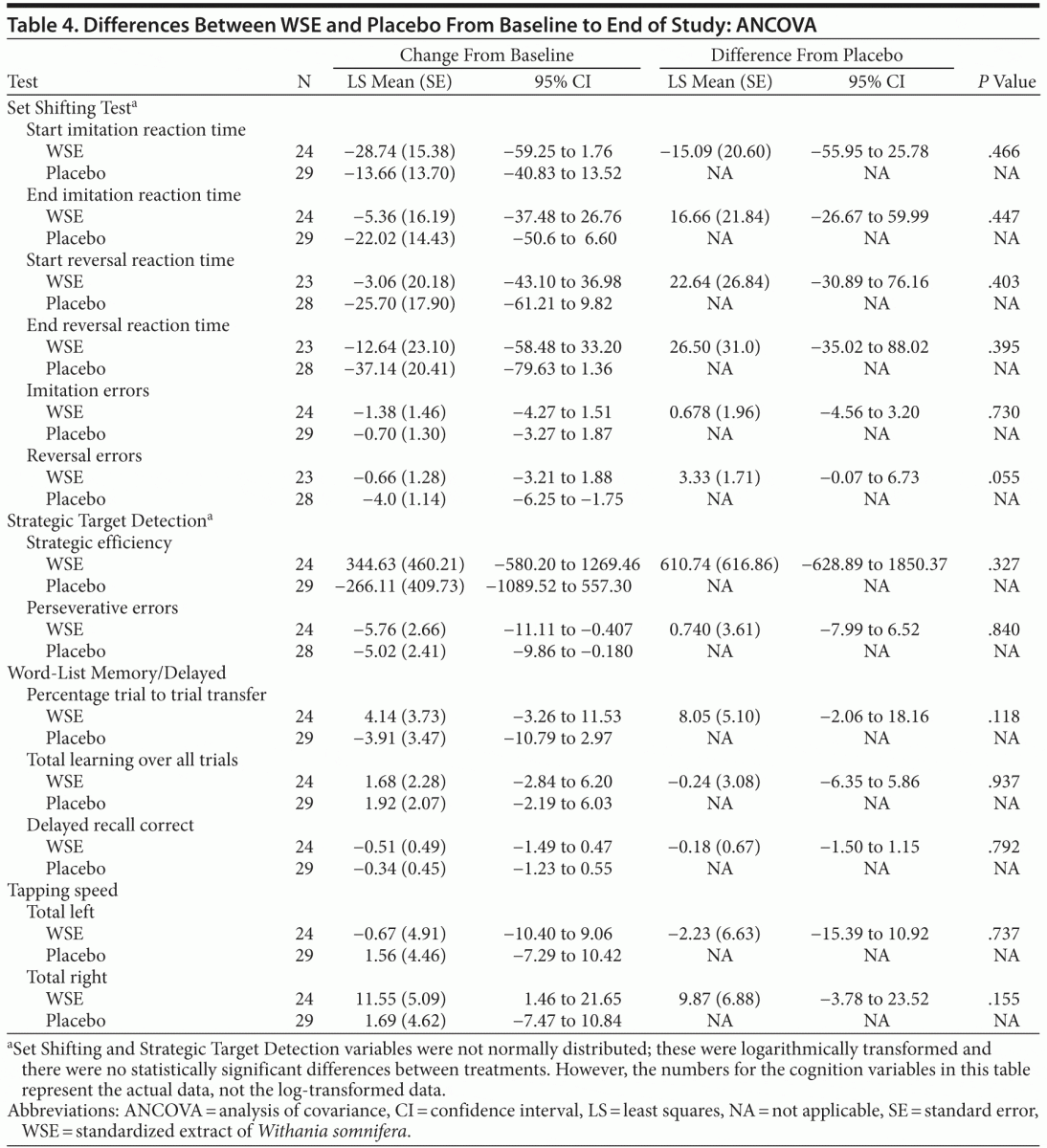
Safety
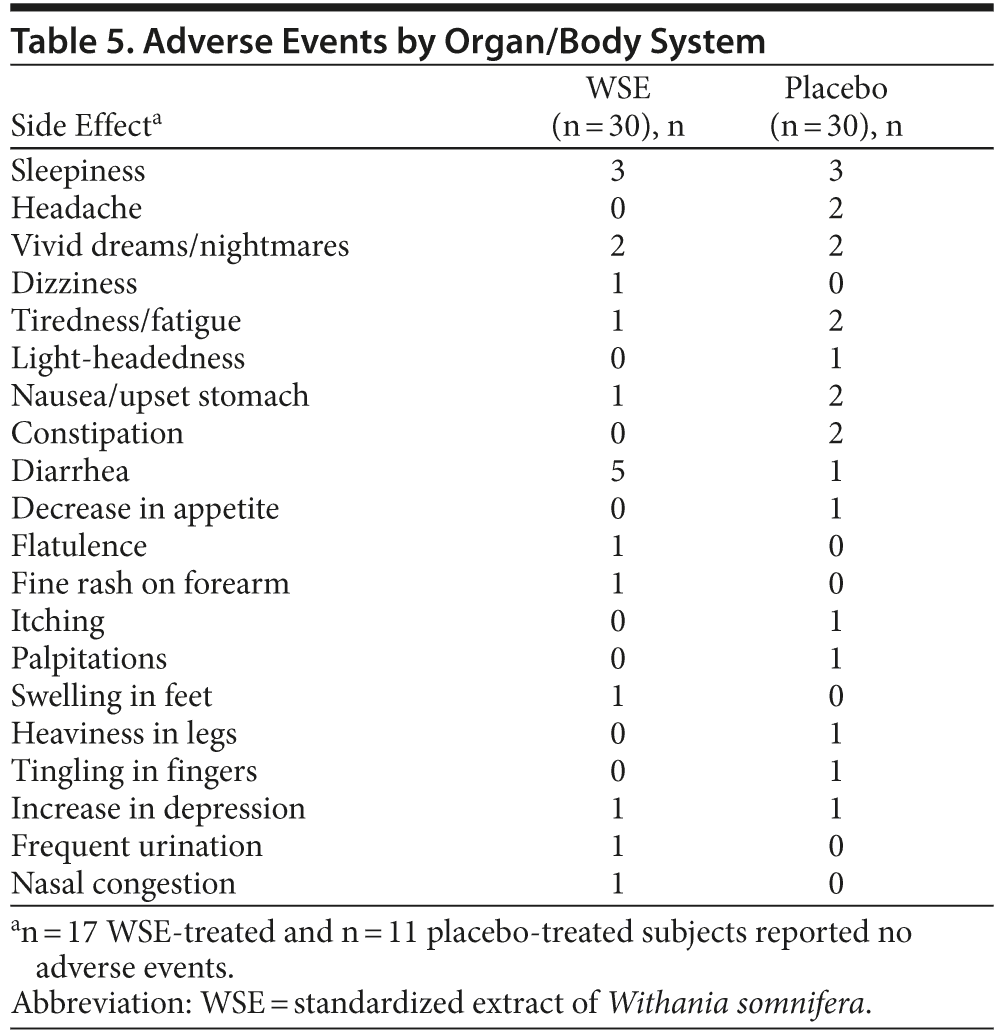
There were 26 treatment-emergent adverse events reported by 19 placebo-assigned subjects compared with 19 events reported by 13 subjects in the WSE-treated group; this difference was not statistically significant (Table 5). Some individuals in each treatment group reported more than 1 event, but 28 subjects (47%) reported no adverse events. Adverse events were mild and transient; no one discontinued the study due to adverse events, and there were no serious adverse events. Respiratory rate, pulse, and blood pressure remained stable in both groups throughout the study period; laboratory parameters and ECG readouts did not raise clinical concerns. The WSE-treated group gained 2.2 (± 4.7) lb body weight compared to 0.6 (± 9.7) lb for the placebo group, a difference that was not statistically significant.
DISCUSSION
These early data indicate that verbal working memory is significantly impacted by WSE in euthymic bipolar disorder patients. The size of the WSE treatment effect for digit span backward (0.51, medium effect) is comparable to that reported by Burdick and colleagues13 in a subset of their euthymic bipolar subjects treated with a dopamine agonist, pramipexole. A recent meta-analysis9 of cognitive impairments in euthymic bipolar disorder individuals indicated that working memory deficits were in the large
(0.8) effect size range. Furthermore, in recently manic patients who were previously employed, Bearden and colleagues12 showed that gains in working memory, episodic memory, and executive functioning robustly predicted (odds ratio > 10) occupational recovery by 3 months. So, it is possible that WSE alone or in combination with other treatments (eg, cognitive rehabilitation or remediation, pharmacotherapy) may offer an approach to enhance cognitive capacity and improve functional outcomes in persons with bipolar disorder. However, these preliminary results with WSE in persons with bipolar disorder require independent replication.
A significant improvement favoring WSE on the Flanker arrow task was obtained only for the neutral reaction time. The implications of this are unclear, since this test condition is the easiest, and significant results were not noted on the more difficult test conditions that involve executive processing. Whether this finding reflects a simple reaction time improvement or processing speed improvement must await replication and possibly the use of other cognitive tests. WSE-treated subjects rated facial expressions as “happier” (Penn Emotional Acuity Test mean response) compared to placebo-assigned subjects, but the implications of this result are unclear as this measure was computed as a group average and does not necessarily reflect more accurate ratings.
We had expected a broader profile of cognitive benefits with WSE due to its diverse pharmacologic actions22–26,30–33; however, narrower results were obtained in this study. Recent work has indicated that WSE reversed deficits in spatial learning and working memory tasks in mouse models of Alzheimer’s disease43 probably by promoting hepatic clearance of β-amyloid. Scopolamine-induced anticholinergic actions and down-regulation of BDNF (brain-derived neurotrophic factor) or GFAP (glial fibrillary acidic protein) were reversed by WSE.44 Mechanisms that explain improvements by WSE in working memory and processing speed in bipolar disorder are worth exploring further. Moreover, questions regarding the upper and lower limits of the dosage of WSE, the ideal duration of a clinical trial to assess improvements in functioning, the use of WSE in symptomatic patients with bipolar disorder, or identifying a subgroup that may especially benefit remain unanswered and require further study. Furthermore, we used a standardized aqueous extract of Withania somnifera (Sensoril), so it is not known if extracts containing different concentrations of the various bioactive constituents or extracted utilizing other processes will provide similar benefits and/or risks.
Limitations of the present study include the relatively small number of patients, the lack of more WSE dosage treatment arms, the short duration of the clinical trial, and the fact that the results would not survive corrections for multiple comparisons, which were not applied due to the preliminary nature of the study and modest sample size. Further, even though the illness and treatment variables were not significantly different between the 2 groups, the numbers of subjects were inadequate to test whether patients with specific characteristics would benefit more or less with WSE (eg, those receiving lithium vs anticonvulsants, bipolar I vs II). This study was implemented prior to the guidance issued on the use of a cognition battery for bipolar disorder,45 an assessment that is worth considering in future studies with WSE.
Mood symptoms remained stable; no worsening was noted with WSE treatment. The low rating scale scores most likely did not allow room for improvement. Recent studies have indicated that WSE has benefits for anxiety and stress reduction.37,46,47 WSE was generally well tolerated, with low frequency and low intensity treatment-emergent adverse events; no concern with significant body weight gain was evident. No significant improvements in functioning were noted between treatments on the FAST total or subscale scores; the 8-week trial duration may have been a limiting factor, and changes in functioning would very likely take longer. Finally, the use of performance-based measures might be more optimal.48 The within-group comparison indicated that the WSE-treated group did improve significantly on the cognitive subscale of the FAST42; however, this is not a performance-based measure. In conclusion, given the lack of positive studies13,49,50 and paucity of data to improve cognition in bipolar disorder, and the importance of cognition to functioning, this standardized medicinal extract of Withania somnifera with a procognitive profile merits further investigation.
Drug names: ashwagandha extract (Sensoril and others), lithium
(Lithobid and others), pramipexole (Mirapex and others).
Author affiliations: Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania (Drs Chengappa and Brar and Ms Schlicht); Queen’s University, Kingston, Ontario, Canada (Dr Bowie); Datamagik, London, England (Mr Fleet); and Veteran Administration Health Care System, Pittsburgh, Pennsylvania (Dr Jindal).
Potential conflicts of interest: None of the authors have any financial disclosures to report specifically with this study. Separately, Dr Bowie served as a consultant to Abbott Laboratories and has also served on the speaker’s bureau of Janssen-Cilag in the 12 months prior to this manuscript submission.
Funding/support: This study was funded by an Independent Investigator Award to Dr Chengappa from the National Alliance for Research on Schizophrenia and Depression (NARSAD), now known as The Brain and Behavioral Research Foundation, Great Neck, New York; the monies from that award were used to support study procedures and expenditures, and in part, salaries of research staff. Study drug/placebo was supplied by Natreon, Inc, New Brunswick, New Jersey.
Role of the sponsor: The sponsor NARSAD funded the study but played no role in design, clinical trial execution, data analyses, or interpretation of the results or writing the manuscript.
Acknowledgments: We are grateful to NARSAD, now known as The Brain and Behavior Research Foundation, Great Neck, New York, for much of the financial support for this study. We thank Dr Samuel Gershon, MD (Emeritus Professor of Psychiatry, University of Pittsburgh), for chairing the Data and Safety Monitoring Board (DSMB), Dr Scott Turkin, MD (Dubois Regional Medical Center, Dubois, Pa), and Ms Joan Buttenfield, RN (Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center), for being members of the DSMB and volunteering their time and effort. None of them have any conflicts of interest to disclose with this study. We are grateful to Dr Sanni Raju, PhD, of Natreon, Inc (New Jersey), who provided materials to file an IND application to the FDA and provided gratis the standardized extract of Withania somnifera (Sensoril) and placebo for the study. Dr Sanni Raju is CEO, Natreon, Inc, owner of the Sensoril brand of ashwagandha, which has been issued the following patents: US 7,318,938, US 6,713,092, US 6,153,198, EP1569669 A2, Canadian Patent No. 2,508,478, US 8,206,757. We also recognize Ms Eve Bender for data entry and Ms Joan Spinogatti (both from Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center [WPIC-UPMC]) for coordinating the manuscript, collaboration among authors, and assisting in submission of the manuscript. Neither of them has any conflicts of interest to disclose in connection with this study. We acknowledge the WPIC-UPMC Forbes Pharmacy staff for providing the Investigational Drug Service for the study, and the IND office of the University of Pittsburgh for assistance with the IND application. We thank past and present staff and scientists of The Cognition Group (TCG, London, UK, Newark, Delaware, and Gurgaon, India) for their assistance with staff training, data extraction, clarifications, and preparation for analyses, as well as providing the cognition hardware and software at significantly reduced cost for academic sites.
REFERENCES
1. Martínez-Arán A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224–232. doi:10.1111/j.1399-5618.2004.00111.x PubMed
2. Altshuler LL, Ventura J, van Gorp WG, et al. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56(8):560–569. doi:10.1016/j.biopsych.2004.08.002 PubMed
3. Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;
93(1–3):105–115. doi:10.1016/j.jad.2006.02.016 PubMed
4. Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand suppl. 2007;116(434):17–26. doi:10.1111/j.1600-0447.2007.01055.x PubMed
5. Arts B, Jabben N, Krabbendam L, et al. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38(6):771–785. doi:10.1017/S0033291707001675 PubMed
6. Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1–2):1–20. doi:10.1016/j.jad.2008.06.009 PubMed
7. Martino DJ, Marengo E, Igoa A, et al. Neurocognitive and symptomatic predictors of functional outcome in bipolar disorders: a prospective 1 year follow-up study. J Affect Disord. 2009;116(1–2):37–42. doi:10.1016/j.jad.2008.10.023 PubMed
8. Wingo AP, Baldessarini RJ, Holtzheimer PE, et al. Factors associated with functional recovery in bipolar disorder patients. Bipolar Disord. 2010;
12(3):319–326. doi:10.1111/j.1399-5618.2010.00808.x PubMed
9. Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord. 2011;13(4):
334–342. doi:10.1111/j.1399-5618.2011.00935.x PubMed
10. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. PubMed
11. Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009;11(2):113–125. doi:10.1111/j.1399-5618.2009.00665.x PubMed
12. Bearden CE, Shih VH, Green MF, et al. The impact of neurocognitive impairment on occupational recovery of clinically stable patients with bipolar disorder: a prospective study. Bipolar Disord. 2011;13(4):323–333. doi:10.1111/j.1399-5618.2011.00928.x PubMed
13. Burdick KE, Braga RJ, Nnadi CU, et al. Placebo-controlled adjunctive trial of pramipexole in patients with bipolar disorder: targeting cognitive dysfunction. J Clin Psychiatry. 2012;73(1):103–112. doi:10.4088/JCP.11m07299 PubMed
14. Goldberg JF, Chengappa KNR. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11(suppl 2):123–137. doi:10.1111/j.1399-5618.2009.00716.x PubMed
15. Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161(3):
564–566. doi:10.1176/appi.ajp.161.3.564 PubMed
16. Burdick KE, Braga RJ, Goldberg JF, et al. Cognitive dysfunction in bipolar disorder: future place of pharmacotherapy. CNS Drugs. 2007;21(12):971–981. doi:10.2165/00023210-200721120-00002 PubMed
17. Schrauwen E, Ghaemi SN. Galantamine treatment of cognitive impairment in bipolar disorder: four cases. Bipolar Disord. 2006;8(2):196–199. doi:10.1111/j.1399-5618.2006.00311.x PubMed
18. Iosifescu DV, Moore CM, Deckersbach T, et al. Galantamine-ER for cognitive dysfunction in bipolar disorder and correlation with hippocampal neuronal viability: a proof-of-concept study. CNS Neurosci Ther. 2009;15(4):309–319. doi:10.1111/j.1755-5949.2009.00090.x PubMed
19. Young AH, Gallagher P, Watson S, et al. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29(8):1538–1545. doi:10.1038/sj.npp.1300471 PubMed
20. Deckersbach T, Nierenberg AA, Kessler R, et al. Cognitive rehabilitation for bipolar disorder: an open trial for employed patients with residual depressive symptoms. CNS Neurosci Ther. 2010;16(5):298–307. doi:10.1111/j.1755-5949.2009.00110.x PubMed
21. Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1093–1105. doi:10.1016/j.pnpbp.2007.09.011 PubMed
22. Ghosal S, Kaur R, Srivastava RS. Sitoindosides IX and X, two new withanolide glycosides from Withania somnifera. Indian J Nat Prod. 1988;4:12–13.
23. Panda S, Kar A. Evidence for free radical scavenging activity of Ashwagandha root powder in mice. Indian J Physiol Pharmacol. 1997;41(4):424–426. PubMed
24. Jain S, Shukla SD, Sharma K, et al. Neuroprotective effects of Withania somnifera Dunn in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15(6):544–548. doi:10.1002/ptr.802 PubMed
25. Naidu PS, Singh A, Kulkarni SK. Effect of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunction. Phytother Res. 2006;20(2):140–146. doi:10.1002/ptr.1823 PubMed
26. Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144(7):
961–971. doi:10.1038/sj.bjp.0706122 PubMed
27. Kaur P, Sharma M, Mathur S, et al. Effect of 1-oxo-5β, 6β-epoxy-with
a-2-ene-27-ethoxy-olide isolated from the roots of Withania somnifera on stress indices in Wistar rats. J Altern Complement Med. 2003;9(6):897–907. doi:10.1089/107555303771952244 PubMed
28. Bhattacharya SK, Bhattacharya A, Sairam K, et al. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7(6):463–469. doi:10.1016/S0944-7113(00)80030-6 PubMed
29. Bhattacharya SK, Muruganandam AV. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol Biochem Behav. 2003;75(3):547–555. doi:10.1016/S0091-3057(03)00110-2 PubMed
30. Schliebs R, Liebmann A, Bhattacharya SK, et al. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int. 1997;30(2):181–190. doi:10.1016/S0197-0186(96)00025-3 PubMed
31. Choudhary MI, Yousuf S, Nawaz SA, et al. Cholinesterase inhibiting withanolides from Withania somnifera. Chem Pharm Bull (Tokyo). 2004;
52(11):1358–1361. doi:10.1248/cpb.52.1358 PubMed
32. Choudhary MI, Nawaz SA, ul-Haq Z, et al. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun. 2005;334(1):276–287. doi:10.1016/j.bbrc.2005.06.086 PubMed
33. Grover A, Shandilya A, Agrawal V, et al. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J Biomol Struct Dyn. 2012;29(4):651–662. doi:10.1080/07391102.2012.10507408 PubMed
34. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. PubMed
35. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. doi:10.1192/bjp.133.5.429 PubMed
36. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi:10.1192/bjp.134.4.382 PubMed
37. Auddy B, Hazra J, Mitra A, et al. A standardized Withania Somnifera Extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. JANA. 2008;
11(1):50–56.
38. Chengappa KNR, Turkin SR, DeSanti S, et al. A preliminary, randomized, double-blind, placebo-controlled trial of L-carnosine to improve cognition
in schizophrenia. Schizophr Res. 2012;142(1–3):145–152. doi:10.1016/j.schres.2012.10.001 PubMed
39. Harvey PD, Hassman H, Mao L, et al. Cognitive functioning and acute sedative effects of risperidone and quetiapine in patients with stable bipolar I disorder: a randomized, double-blind, crossover study. J Clin Psychiatry. 2007;68(8):1186–1194. doi:10.4088/JCP.v68n0804 PubMed
40. Lindenmayer JP, Khan A. Galantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophrenia. Schizophr Res. 2011;125(2–3):267–277. doi:10.1016/j.schres.2010.08.021 PubMed
41. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi:10.1111/j.2044-8341.1959.tb00467.x PubMed
42. Rosa AR, Sánchez-Moreno J, Martínez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemol Ment Health. 2007;3(1):5. doi:10.1186/1745-0179-3-5 PubMed
43. Sehgal N, Gupta A, Valli RK, et al. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012;109(9):3510–3515. doi:10.1073/pnas.1112209109 PubMed
44. Konar A, Shah N, Singh R, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS ONE. 2011;6(11):e27265. doi:10.1371/journal.pone.0027265 PubMed
45. Yatham LN, Torres IJ, Malhi GS, et al. The International Society for Bipolar Disorders-Battery for Assessment of Neurocognition (ISBD-BANC). Bipolar Disord. 2010;12(4):351–363. doi:10.1111/j.1399-5618.2010.00830.x PubMed
46. Andrade CA, Aswath A, Chaturvedi SK, et al. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy ff an ethanolic extract of withania somnifera. Indian J Psychiatry. 2000;42(3):295–301. PubMed
47. Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS ONE. 2009;4(8):e6628. doi:10.1371/journal.pone.0006628 PubMed
48. Burdick KE, Endick CJ, Goldberg JF. Assessing cognitive deficits in bipolar disorder: are self-reports valid? Psychiatry Res. 2005;136(1):43–50. doi:10.1016/j.psychres.2004.12.009 PubMed
49. McIntyre RS, Soczynska JK, Woldeyohannes HO, et al. A randomized,
double-blind, controlled trial evaluating the effect of intranasal insulin on neurocognitive function in euthymic patients with bipolar disorder. Bipolar Disord. 2012;14(7):697–706. doi:10.1111/bdi.12006 PubMed
50. Dean OM, Bush AI, Copolov DL, et al. Effects of N-acetyl cysteine on cognitive function in bipolar disorder. Psychiatry Clin Neurosci. 2012;
66(6):514–517. doi:10.1111/j.1440-1819.2012.02392.x PubMed





