Bright Light Treatment as Add-On Therapy for Depression in 28 Adolescents: A Randomized Trial [Duplicate Publication]

ABSTRACT
Background: In the last decade, a significant incidence of depression in the younger population has been observed. Bright light therapy, an effective therapeutic option for depressed adults, could also provide safe, economical, and effective rapid recovery in adolescents.
Method: The randomized trial included 28 inpatients (18 females and 10 males) between 14 and 17 years old with depressive complaints. The study was conducted between February and December of 2010 in Rodewisch, Germany. Half of the patients (n = 14) first received placebo (50 lux) 1 hour a day in the morning from 9:00 am to 10:00 am for 1 week and then received bright light therapy (2,500 lux) for 1 week in the morning from 9:00 am to 10:00 am. The other half (n = 14) first received bright light therapy and then received placebo. Patients were encouraged to continue ongoing treatment (fluoxetine 20 mg/day and 2 sessions of psychotherapy/week) because there were no changes in medication/dosage and psychotherapy since 1 month before the 4-week study period. For assessment of depressive symptoms, the Beck Depression Inventory (BDI) was administered 1 week before and 1 day before placebo treatment, on the day between placebo and bright light treatment, and on the day after and 1 week after bright light treatment. Saliva samples of melatonin and cortisol were collected at 8:00 am and 8:00 pm 1 week before and 1 day before placebo treatment, on the day between placebo and bright light treatment, on the day after bright light treatment, and 1 week after bright light treatment and were assayed for melatonin and cortisol to observe any change in circadian timing.
Results: The BDI scores improved significantly (P = .015). The assays of saliva showed significant differences between treatment and placebo for evening melatonin (P = .040). No significant adverse reactions were observed.
Conclusions: Antidepressant response to bright light treatment in this age group was statistically superior to placebo.
Trial Registration: World Health Organization International Clinical Trials Registry Platform identifier: DRKS00003309
Prim Care Companion CNS Disord 2011;13(6):doi:10.4088/PCC.11m01194
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: April 7, 2011; accepted June 22, 2011.
Published online: December 22, 2011.
Corresponding author: Helmut Niederhofer, MD, PhD, Saechsisches Krankenhaus, Rodewisch, Child and Adolescent Psychiatry, 08228 Rodewisch, Germany ([email protected]).
Depression in adolescents is often treated, and although antidepressants are effective treatments for psychiatric disorders in adolescents, there is a greater risk of medication side effects. These difficulties suggest that additional biological treatment options for depressed adolescents either to supplement or replace pharmacotherapy should be established. Bright light treatment could benefit adolescents with depression. Bright light treatment for seasonal depression has been accepted in the Clinical Practice Guidelines issued by the US Department of Health and Human Services1 and in Treatment of Psychiatric Disorders by the American Psychiatric Association2 because it is effective, rapid, safe, and inexpensive.
A bright light treatment study was conducted to investigate nonseasonal major depression and demonstrated that bright light treatment is effective for these disorders.3 In nonseasonal depression, light treatment may produce more benefit when coadministered with antidepressant drugs.4 The mechanisms by which light produces antidepressant effects are of basic scientific interest in order to understand the underlying etiologies of depressive illnesses and to develop treatment strategies.
There is some evidence that treatment with morning light is superior to evening light for seasonal depression,5-8 but some studies have found little difference between timings.9,10 The advantage of morning light could be partly explained by an anomalous order effect in crossover designs.11 Another explanation for the special effect of morning light could be that it might work by suppressing or phase advancing an overly late melatonin offset.12,13 Furthermore, conditions in which mood complaints are most prominent might have a common etiology in the circadian phase of malsynchronization, which is characterized by abnormal entrainment of circadian rhythms to the solar day and/or abnormal relationships among rhythms in the body. The work of Neumeister et al,14 Loving et al,15 and Bloching et al16 suggested that bright light in combination with partial sleep deprivation produces remarkable antidepressant responses, as confirmed by dramatic contrasts between bright light and placebo.
This article reports a randomized clinical trial of bright light treatment as add-on therapy in 28 adolescents. It is based on a case series wherein preliminary results showed that bright light treatment is superior to placebo with regard to depression scores and saliva melatonin levels.17 This randomized trial was performed to demonstrate greater improvements in mood and sleep among patients receiving 1 week of bright light treatment compared to placebo.
METHOD
The randomized trial included 28 inpatients, 18 females and 10 males, aged 14-17 years, with IQs of 96 to 105 and significant depressive complaints. Informed consent was obtained from each participant in accordance with the guidelines set forth by the Declaration of Helsinki. The local ethics committee approved the study. The study was conducted between February and December of 2010 in Rodewisch, Germany (World Health Organization International Clinical Trials Registry Platform identifier: DRKS00003309).
Patients were not required to meet 5 out of the 9 DSM-IV criteria for current major depressive disorder. Three points were considered to be sufficient, because many adolescents are significantly troubled by minor depressive disorders without meeting criteria for major depressive disorder. Additional psychiatric disorders were defined as exclusion criteria. Any lifetime history of mania required exclusion, as a history of mania appears to predict a greatly increased risk of a manic switch during bright light treatment.18 Patients were encouraged to continue ongoing treatment (fluoxetine 20 mg/day and 2 sessions of psychotherapy/week) during the study, with the assumption that psychotherapy and medication effects over an interval of 5 weeks were likely to be small because there were no changes in medication/dosage and psychotherapy (2 sessions/week) since 1 month before the 4-week study period.
Depressive symptoms were assessed weekly by the Beck Depression Inventory (BDI),19 a questionnaire that consists of 21 items asking for symptoms such as sadness, suicidality, agitation, and sleeping disorders, with a score of 0 = not existent to 3 = always existing. The BDI has an excellent variation sensitivity of 81% and a specificity of 61% for DSM-IV depression. A cutoff of 9 indicates that scores > 9 are typical for depressive disorders. Saliva samples were collected at 8:00 am and 8:00 pm 1 week before and 1 day before placebo treatment, on the day between placebo and bright light treatment, on the day after bright light treatment, and 1 week after bright light treatment and assayed for melatonin and cortisol to characterize the circadian phase of the subject’s melatonin rhythms.
The patients completed a weekly Assessment for Treatment Emergent Events (SAFTEE) symptom scale.20 This physical symptom inventory consists of 20 items (range of severe, moderate, mild, minimal, not existing) and examines adverse reactions. In our trial, there were weekly symptom assessments. The investigators visited subjects weekly to assure their safety and compliance with the study and to administer and collect rating forms.
This was a randomized trial. After the baseline week, one-half of the participants (group 1; steps 1, 2, 3, and 4 below) began placebo treatment (dim white light) for 1 week followed by 1 week of bright light treatment. The second half of patients (group 2; steps: 1, 3, 2, and 4 below) received, after the initial baseline week, bright light treatment for 1 week and then placebo (dim white light) for another week. We summarized both groups so as to provide better contrasts between bright light treatment and placebo.
Step 1: Patients began with the initial baseline week of the study. The purpose of the baseline week of the study was to differentiate changes in the BDI score related to depression between placebo and bright light treatment.
Step 2: In the second week of the study, patients were asked to sit in front of the placebo light box (dim white light, 50 lux) for 60 minutes in the morning (from 9:00 am to 10:00 am). Patients were allowed to play or listen to a story. If a BDI score dropped 50% or more from the first day to the last day of the baseline week, the patient was dropped from the study. This aspect of the design followed the general principle of clinical trial design that it is easier to obtain contrasts between active and placebo treatments if early placebo responders are eliminated. According to Eastman,18 the issue of placebo responses has been a serious problem in clinical bright light studies.
Step 3: In the third week of the study, we asked patients to sit in front of the bright light box (2,500 lux) for 60 minutes in the morning (from 9:00 am to 10:00 am). The 1-hour restriction was requested for 1 hour only because drug dosages and bright light treatment time should be reduced in children, adolescents, and older adults. During this time, patients played or listened to a story.
Step 4: In the fourth week of the study, patients received neither placebo nor bright light treatment. The purpose of this week of the study was again to differentiate changes in the BDI score related to depression between placebo and bright light treatment.
Statistics were computed by SPSS V 17.0 (SPSS, Inc, Chicago, Illinois). Significance was assessed with the Wilks Lambda test, and for comparisons of bright light treatment/placebo with the pretreatment/posttreatment periods, 1-factor analysis of variance for repeated measures with Bonferoni correction was used.
RESULTS
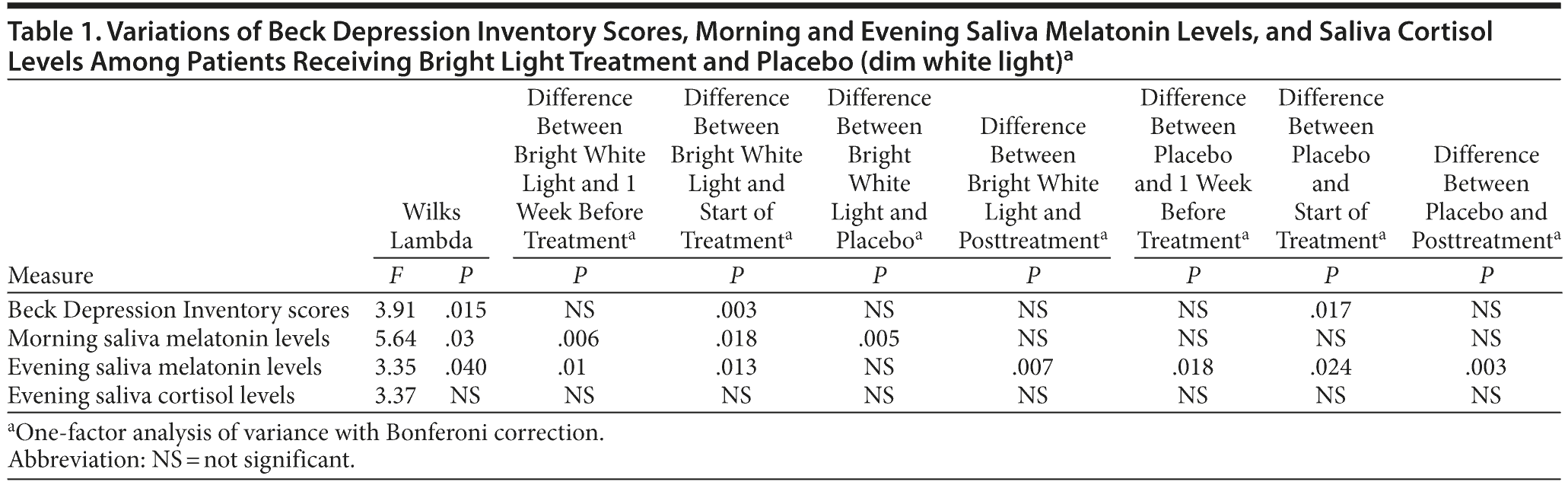
The results of comparisons between bright light treatment/placebo and the pretreatment/posttreatment periods are depicted in Table 1. The Wilks Lambda test suggested the existence of significant differences for all scores and levels within the treatment period. One-factor analysis of variance for repeated measures (Bonferoni corrected) showed that most significant differences were mainly between bright light treatment and the pretreatment/posttreatment periods. Interestingly, some significant differences between placebo and the pretreatment period could be observed (also with the posttreatment period with respect to melatonin evening levels) (Table 1).
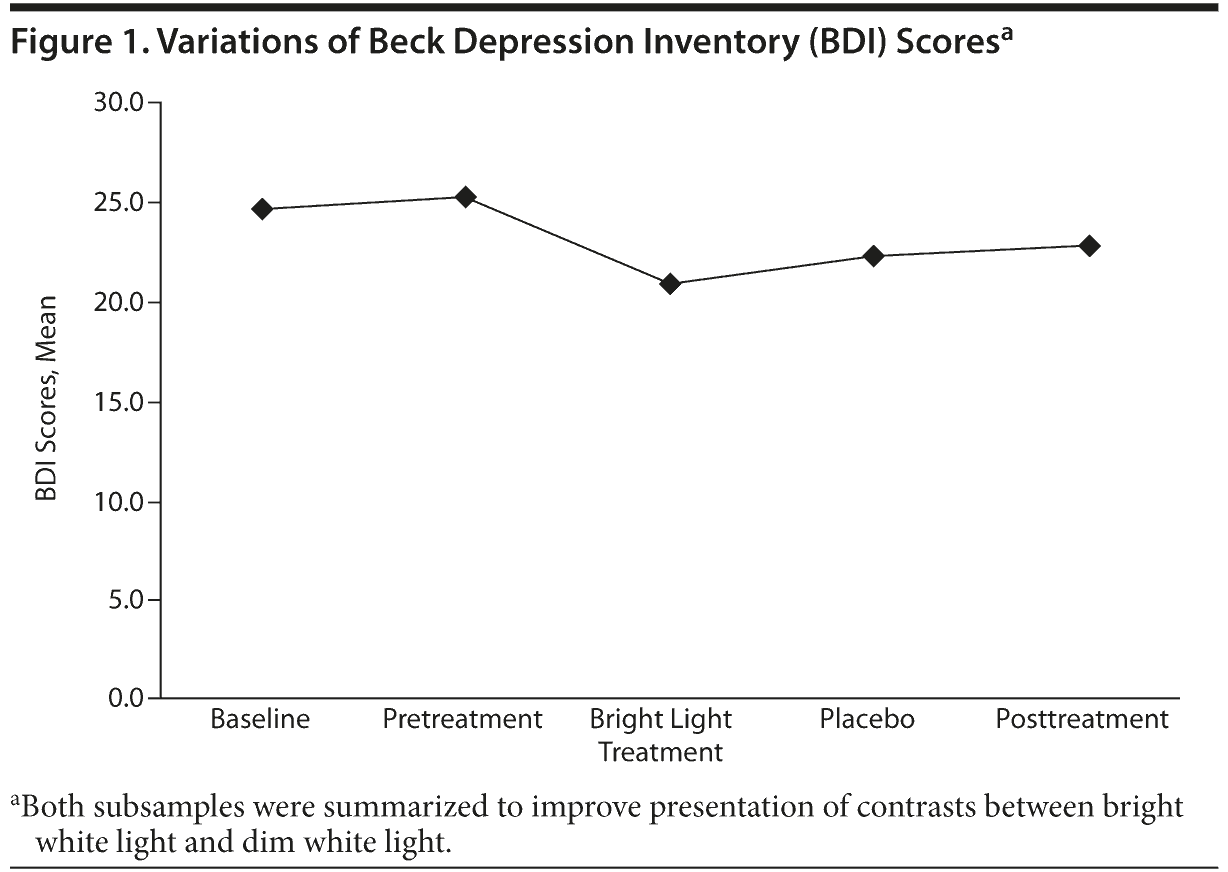
The BDI scores were stable in the pretreatment period, improved significantly (P = .017) in group 1 during treatment with placebo and then during treatment with bright light, and rose again after the following week. Group 2 dropped from an initial score equal to that of group 1 significantly (P = .003) during bright light treatment and rose again during placebo treatment. In the posttreatment period, the score rose in both groups again up to the initial values (Figure 1).
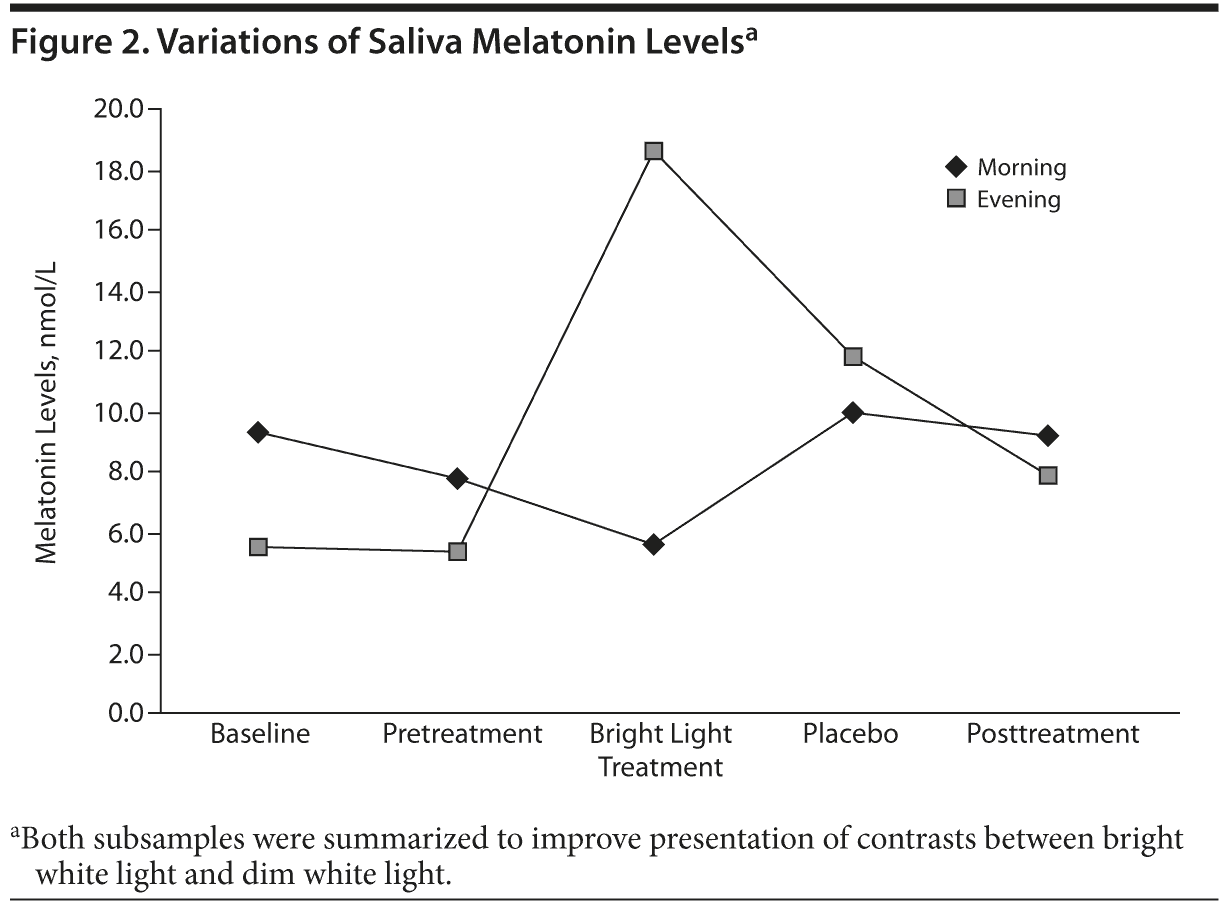
In both groups, morning salivary melatonin levels were higher than the reference values (< 5 nmol/L). Salivary melatonin levels measured in the morning did not show significant variations in the pretreatment or in the placebo period, but there was a significant (P = .019) decrease in both groups during the bright light treatment period. During the posttreatment period, we observed levels that did not differ significantly from those of the pretreatment period. Also, in both groups, salivary melatonin measured in the evening was stable but much lower than the reference values (> 10 nmol/L) in the pretreatment period, increased significantly (P = .023) after placebo and again after bright light treatment, decreased again during the posttreatment week, and finally did not differ significantly from the values of the pretreatment period (Figure 2).
We could not detect significant changes with regard to salivary cortisol. There were no incidents of mania or hypomania during the bright light treatment. Study participants experienced no suicide attempts.
With respect to the SAFTEE, the depression subscale was scored exclusively by means of the BDI. Improvements in symptoms were as follows: headache improved with bright light treatment in 4 patients, change in appetite improved in 2 patients, insomnia improved in 9 patients, fatigue improved in 5 patients, and somnolence improved in 1 patient from mild to minimal. This improvement lasted until the end of the study. With respect to the other symptoms, patients did not report any change, ie, they were always scored as “minimal” or “not existing,” without any changes during the 4 weeks.
DISCUSSION
Bright light treatment was superior to dim placebo light, especially with respect to morning saliva melatonin levels. Also, placebo showed significant improvements (BDI score, evening saliva melatonin levels), but less than bright light treatment.
Rhythms of melatonin, sleep, and activity all peak later in depressed patients compared with the reference values. However, since increased light exposure is generally associated with more advanced rhythms (as was observed comparing the bright light and placebo period in this study), the results are consistent with the possibility that these depressed patients were subsensitive to the circadian effects of light.
The antidepressant response to placebo light treatment in this study is comparable to that reported for placebo in drug studies.21 However, placebo response is significant and should be considered as a confound also in antidepressant light treatment studies. Exclusion of bipolar participants was necessary to prevent hypomanic adverse events, but it is possible that depressed patients with bipolar 1 disorder are more light responsive.
The improvements found in the study might be attributed to several factors that were common to the treatment and control groups. Positive expectations, positive staff contacts, spontaneous remission, and the “placebo” effect may have contributed to positive responses. The social structure and regularized sleep might also have been beneficial. An hour a day engaging in a treatment could have induced a reduction in depressive symptoms. A longer period may be needed for this population with depressive symptoms to show more significant contrasts.
Altogether, bright light treatment seems to be a useful adjunct to antidepressants and psychotherapy and can be applied easily in treatment for adolescent inpatients.
Author affiliations: Child and Adolescent Psychiatry, Saechsisches Krankenhaus, Rodewisch (Dr Niederhofer); and Department of Child and Adolescent Psychiatry, University of Leipzig, Leipzig (Dr von Klitzing), Germany.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Acknowledgments: The authors thank the volunteers for their participation and Joerg Domurath, DiplPsych (Seachsisches Krankenhaus, Rodewisch, Germany), for his statistical support. Mr Domurath reports no conflicts of interest related to the subject of this article.
REFERENCES
1. Depression Guideline Panel. Depression in Primary Care: Treatment of Major Depression. Vol 2. Washington, DC: Agency for Health Care Policy and Research, HHS, AHCPR US Government Printing Office; 1993. Publication 93-0551.
2. Rosenthal NE. Light therapy. In: Gabbard GO, ed. Treatment of Psychiatric Disorders. Vol 1. Washington, DC: American Psychiatric Press; 1995:1263-1273.
3. Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004;(2):CD004050. PubMed
4. Lewy AJ, Sack RL, Singer CM, et al. Winter depression and the phase-shift hypothesis for bright light’s therapeutic effects: history, theory, and experimental evidence. J Biol Rhythms. 1988;3(2):121-134. PubMed doi:10.1177/074873048800300203
5. Sack RL, Lewy AJ, White DM, et al. Morning vs evening light treatment for winter depression: evidence that the therapeutic effects of light are mediated by circadian phase shifts. Arch Gen Psychiatry. 1990;47(4):343-351. PubMed
6. Avery DH, Khan A, Dager SR, et al. Morning or evening bright light treatment of winter depression? the significance of hypersomnia. Biol Psychiatry. 1991;29(2):117-126. PubMed doi:10.1016/0006-3223(91)90040-S
7. Terman JS, Terman M, Lo ES, et al. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58(1):69-75. PubMed doi:10.1001/archpsyc.58.1.69
8. Terman M, Terman JS, Quitkin FM, et al. Light therapy for seasonal affective disorder: a review of efficacy. Neuropsychopharmacology. 1989;2(1):1-22. PubMed doi:10.1016/0893-133X(89)90002-X
9. Terman M, Terman JS. Randomized, controlled trial of bright light, dawn simulation and negative air ionization for winter depression [abstract]. Soc Light Treatment Bio Rhythms Abst. 2002;14:25.
10. Terman M. Problems and prospects for use of bright light as a therapeutic intervention. In: Wetterberg L, ed. Light and Biological Rhythms in Man. 1. Stockholm, Sweden: Pergamon Press; 1993:421-436.
11. Tuunainen A, Kripke DF, Elliott JA, et al. Depression and endogenous melatonin in postmenopausal women. J Affect Disord. 2002;69(1-3):149-158. PubMed doi:10.1016/S0165-0327(01)00303-2
12. Wehr TA, Duncan WC Jr, Sher L, et al. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry. 2001;58(12):1108-1114. PubMed doi:10.1001/archpsyc.58.12.1108
13. Sekula LK, Lucke JF, Heist EK, et al. Neuroendocrine aspects of primary endogenous depression. XV: mathematical modeling of nocturnal melatonin secretion in major depressives and normal controls. Psychiatry Res. 1997;69(2-3):143-153. PubMed doi:10.1016/S0165-1781(96)02937-X
14. Neumeister A, Goessler R, Lucht M, et al. Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biol Psychiatry. 1996;39(1):16-21. PubMed doi:10.1016/0006-3223(95)00086-0
15. Loving RT, Kripke DF, Eliott JA, et al. Bright light treatment of depression for older adults. BMC Psychiatry. 2005;5(1):41. doi:10.1186/1471-244X-5-41
16. Bloching B, Dechene C, Taschner KL. Outlasting antidepressant effect of late partial sleep deprivation by bright light therapy. J Sleep Res. 2000;9:21.
17. Niederhofer H, Klitzing K. Therapy augmentation by bright light treatment for non-seasonal depression of adolescents. Clin Neuropsychiatry. 2011;8(3):225-227
18. Eastman CI. What the placebo literature can tell us about light therapy for SAD. Psychopharmacol Bull. 1990;26(4):495-504. PubMed
19. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. PubMed
20. Moynihan C. Instruction Manual for Systematic Assessment for Treatment Emergent Events (SAFTEE). Rockville, MD: ADAMHA; 1983.
21. Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch Gen Psychiatry. 2000;57(4):311-317. PubMed doi:10.1001/archpsyc.57.4.311