Objective: Low-dose doxepin has produced favorable results in healthy adults and elderly persons with chronic or transient insomnia, while exhibiting an amenable adverse event profile. The aim of this article is to investigate the efficacy and safety of low-dose doxepin for insomnia in depressed patients.
Method: In this retrospective case series analysis, the files of 17 inpatients diagnosed with major depressive disorder (MDD) and comorbid insomnia between January 1, 2011, and October 1, 2012 who had received a course of off-label doxepin (< 25 mg/d) were analyzed with regard to dose, efficacy, and safety for up to 4 weeks of treatment. Hamilton Depression Rating Scale (HDRS) sleep item scores were used to estimate efficacy.
Results: Our results showed no improvement in sleep onset and sleep maintenance insomnia in patients with MDD during the 4 weeks of treatment. We found a significant improvement in insomnia between baseline and week 3 when considering all 3 HDRS sleep items (P = .058).
Conclusions: Contrasting previous results in healthy subjects, low-dose doxepin does not seem to improve sleep onset or maintenance in patients with MDD. Further research, preferably placebo-controlled, double-blind sleep laboratory trials, is necessary to determine whether low-dose doxepin may be beneficial in this important patient subgroup.
Prim Care Companion CNS Disord 2014;16(1):doi:10.4088/PCC.13m01567
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: August 5, 2013; accepted October 31, 2013.
Published online: January 16, 2014.
Corresponding author: Arnim Quante, MD, Department of Psychiatry and Psychotherapy, Charité, University Medicine Berlin, Campus Benjamin Franklin Hindenburgdamm 30, 12203 Berlin, Germany ([email protected]).
Depression and sleep disturbances are closely linked; the relationship between these 2 entities is bidirectional, with sleep disturbances leading to depression and depression inducing sleep disturbances. Insomnia or hypersomnia are among the 9 DSM-IV-TR criteria for diagnosing depression, and it has been estimated that as many as 90% of depressed patients also suffer from poor sleep quality, amounting to around 3% of the American population.1-4
Besides being common, insufficient or nonrestorative sleep must be considered more than just an inconvenience. Nonrestorative sleep has a high impact on daily functioning, and recent studies have shown that insufficiently treated insomnia may be associated with a poorer prognosis for patients with major depression.5,6
The current American Psychiatric Association guidelines on major depressive disorder (MDD) recommend various pharmacologic treatment options for comorbid insomnia.7 These options include sedating antidepressants such as tertiary amine nonselective monoamine reuptake inhibitors (NSMRIs) or specific second-generation antidepressants (ie, mirtazapine, trazodone, nefazodone, or agomelatine). In pronounced cases, the use of adjunct sedative and hypnotic medication, including benzodiazepines or other γ-aminobutyric acid (GABA) agonists, is proposed. Most selective serotonin reuptake inhibitors (SSRIs), a popular first choice of treatment, are known to cause initial restlessness and insomnia, therefore necessitating additional sedative treatment. While addressing comorbid insomnia is paramount to achieving full remission of a major depressive episode, many of these medications exhibit a notable adverse effect profile.
Benzodiazepines present significant risk of tolerance and dependence and are associated with next-day residual sedation, impaired cognition, gait instability, and rebound insomnia upon discontinuation. Elderly patients are particularly at risk, as paradoxical effects may occur and increased vulnerability to particular adverse effects such as gait instability and falls applies. Special care should be taken when treating subjects with comorbid psychiatric disorders, such as mood disorders and alcohol abuse, making benzodiazepines a below-optimum choice for depressed patients.8
Nonbenzodiazepines, comprising zolpidem, zopiclone, eszopiclone, and zaleplon, display an adverse effect profile comparable to that of benzodiazepines with few differences. Whereas their effects on sleep architecture and next-day residual sedation are significantly reduced, nonbenzodiazepines are regularly associated with parasomnia, psychomotor impairment, and reduced cognitive performance. Depression, which is a rare (< 1%) adverse effect of benzodiazepines, occurs more frequently in nonbenzodiazepine users (1%-10%).8-10
The sedating properties of NSMRIs and certain novel antidepressants may also be utilized to improve sleep disturbances in the form of monotherapeutic or adjunct therapy treatment strategies. Commonly used NSMRIs comprise amitriptyline and trimipramine, while newer agents include trazodone, nefazodone, mirtazapine, and agomelatine. All of these substances are associated with a wide spectrum of adverse events. Amitriptyline, like other NSMRIs, often causes extensive peripheral and central anticholinergic effects, as well as cardiac adverse events, orthostatic hypotension, weight gain, and a reduced seizure threshold. Rare but serious clinical concerns for trazodone include priapism, heart failure, and hallucinations; mirtazapine frequently induces weight gain and may cause leucopenia, while nefazodone and agomelatine are associated with hepatotoxicity.8
Although not explicitly mentioned in the above guidelines, there are additional pharmacologic treatment options that we will briefly discuss here for completeness. Antihistamines, like diphenhydramine, may be prescribed or attained over-the-counter to relieve comorbid insomnia. Next-day residual sedation, impaired cognition, and electrocardiographic changes at high doses are known adverse effects. Alternatively, anticonvulsants or antipsychotics with sedating properties can be considered, however, only if a prior indication for this medication exists, as both pose significant risk of serious adverse events. Recent research has focused on melatonergic modulation and orexin antagonists among others, like serotonin 5-HT2 receptor modulators, including eplivanserin and pimavanserin; however, only a few of these substances have been clinically approved.8,11

- Treating comorbid insomnia in depressed patients is important.
- Low-dose doxepin failed to significantly improve sleep disturbances in depressed patients.
The above-mentioned agents exert their sedating properties either by enhancing the effects of sleep-promoting GABA or by blocking wake-promoting neurotransmitters, chiefly histamine. Considering histamine antagonists, the desired sleep-promoting effects are very likely due to H1 receptor antagonism; however, a large share of adverse events seem to be caused by modulation at various other receptors (adrenergic, cholinergic, dopaminergic, and serotonergic). Therefore, it follows that truly selective H1 antagonism would produce more satisfying results. This can be achieved either by developing dose-independent H1-specific antihistamines or by making multifunctional agents with potent H1 antagonism specific by a reduction in dosage.12
Doxepin is a well-established NSMRI with an over 100-fold magnitude potency separation between antidepressant actions and H1 antagonism; consequently, low-dose doxepin is considered a highly H1-selective antihistamine.12 Since its US Food and Drug Administration approval for sleep maintenance insomnia in 2010, low-dose doxepin in 1, 3, and 6 mg has produced a range of favorable results compared to previous hypnotics.13-18
Roth et al,15,18 Krystal et al,16 and Scharf et al17 found that, while showing significant improvements in various polysomnographic and subjective sleep measures, including sleep efficiency in the final third of the night, residual sedation, anticholinergic effects, or other adverse events were comparable to placebo. Later, long-term assessments confirmed that there was also no increased risk of memory impairment, parasomnia, weight gain, increased appetite, or rebound insomnia upon discontinuation. These results were reproduced in elderly patients with chronic insomnia, as well as healthy adults suffering from transient insomnia.
In 2011, Mansbach et al19 investigated the effects of low-dose doxepin on QT elongation and discovered that neither 6 mg nor 50 mg of doxepin administered over the course of 7 days led to significant alterations in QT values.
The aim of this article is to investigate the efficacy and safety of low-dose doxepin in depressed patients with comorbid insomnia. Low-dose doxepin treatment has provided promising results in healthy adults and elderly persons with chronic or transient insomnia, but, to our knowledge, no trials have explored its application in depressed patients until now. A series of inpatients diagnosed with MDD and comorbid insomnia will be analyzed with regard to dose, duration, efficacy, and adverse effects of doxepin treatment.
As the current data suggest that low-dose doxepin produces significant improvements in various sleep parameters of otherwise healthy subjects, while adverse events remain comparable to placebo,13-18 we predict that our observations will support these findings. By revealing initial results for this particular patient subgroup, which is commonly affected by insomnia, we hope to build a basis for further research in this area.
METHOD
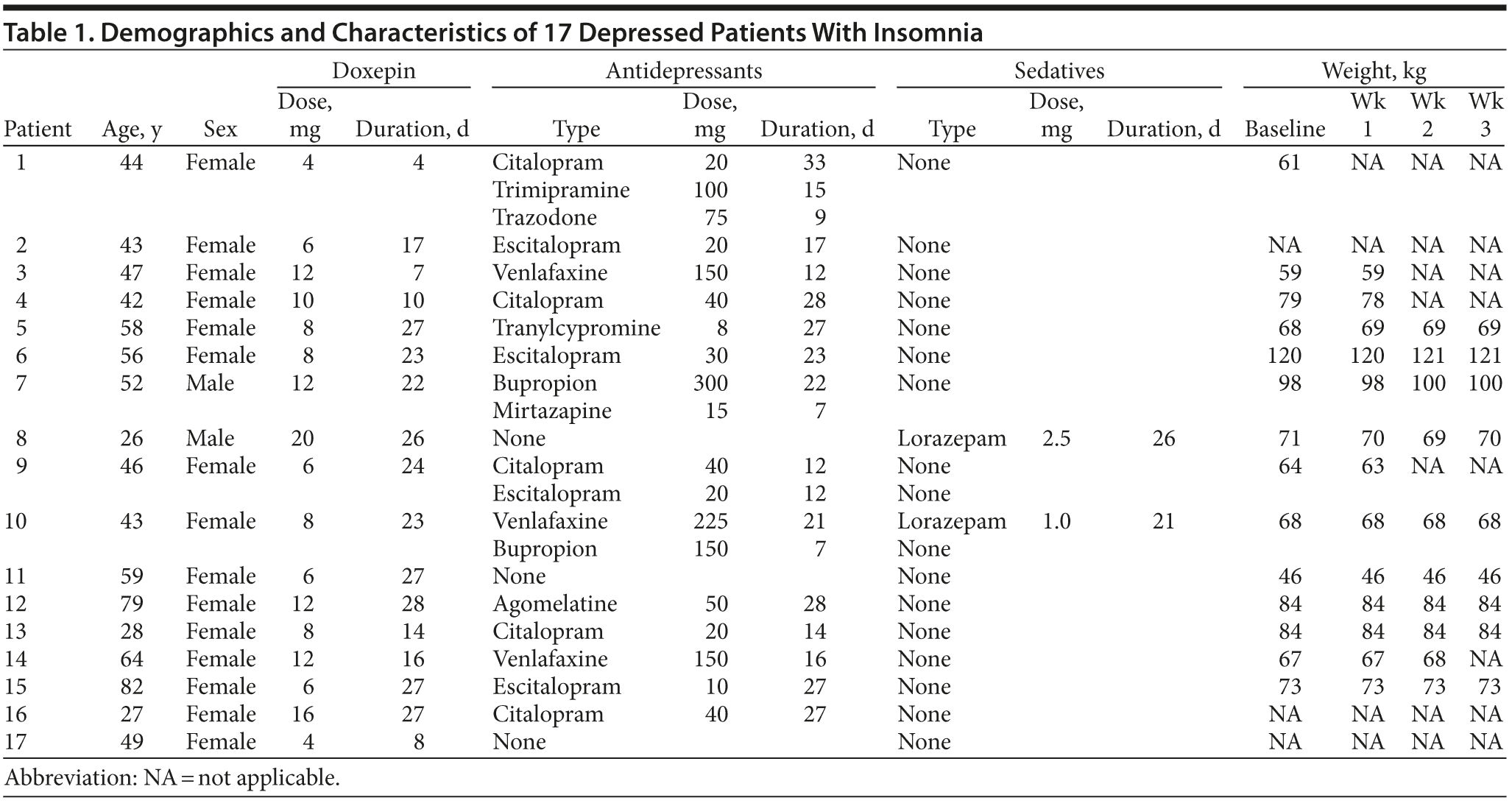
All inpatients diagnosed with MDD and comorbid insomnia at the Department of Psychiatry and Psychotherapy, Charité University Medicine, Berlin, Germany, between January 1, 2011 and October 1, 2012, who had received a course of off-label low-dose doxepin (< 25 mg/d) treatment to relieve sleep disturbances, were included in this retrospective case series analysis. Each diagnosis was made by the attending psychiatrist in accordance with DSM-IV criteria and approved by a senior psychiatric consultant.
The above patients were informed that, due to positive results in recent publications on low-dose doxepin in healthy subjects, this treatment may be beneficial and may present a more amenable adverse effect profile compared to conventional hypnotic medication. As this is a retrospective naturalistic investigation, no informed consent or ethics committee vote could be included. All antidepressive therapy was continued under a treatment-as-usual model throughout the hospitalization period, as this was a retrospective clinical observation. SSRIs or selective-norepinephrine reuptake inhibitors were the most common antidepressants administered in addition to doxepin treatment.
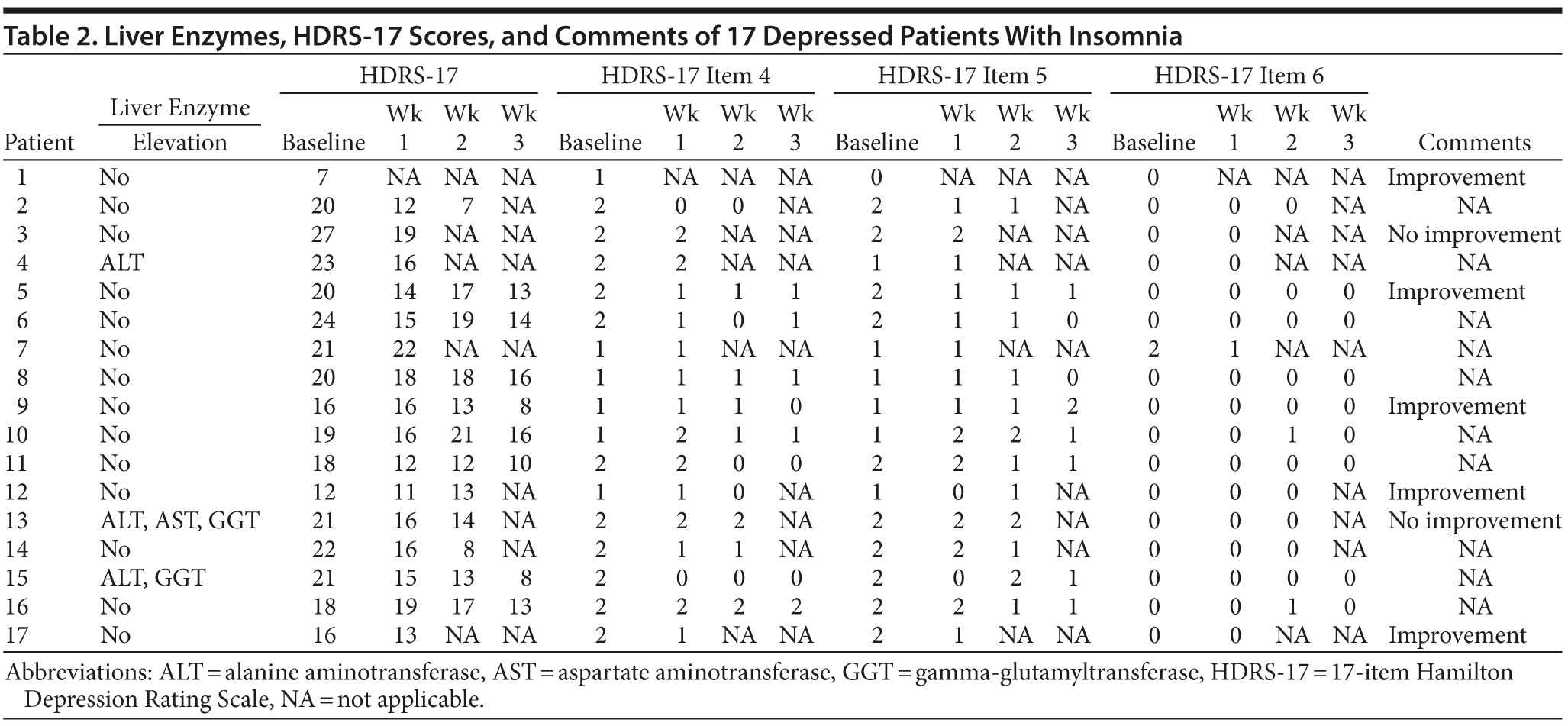
Solely the information included in the patients’ files, particularly the physician’s letter, was considered to establish dose, duration, efficacy, and acceptability of doxepin treatment over a maximum period of 4 weeks, with values collected in week 1 being equivalent to baseline. As this is a retrospective analysis, only standard rating scales could be used in order to determine outcome. The 17-item Hamilton Depression Rating Scale20 (HDRS-17) sleep item scores (items 4, 5, and 6) were analyzed to estimate efficacy, categorized as sleep onset, sleep maintenance, and early morning awakenings, as all patients at our clinic are routinely evaluated by the HDRS-17 on a weekly basis. Additionally, total HDRS-17 scores were used to estimate depression severity as a secondary outcome, because changes in depression severity may also influence sleep disturbances.
Other variables that were retrieved whenever possible encompass the dose and duration of any additional psychotropic medication, weight change, elevation in liver enzymes, and patients’ comments regarding adverse events and quality of sleep.
RESULTS
Demographics
The rather small sample size most likely led to unusual demographic figures; however, some of the patients’ baseline characteristics may be consistent with previous studies. The case series included 17 patients, of whom 2 were male and 15 were female. Age ranged from 26 to 82 years (mean = 49.71, SD = 15.93). All patients were diagnosed with MDD and comorbid insomnia at the beginning of their hospitalization period. As shown by HDRS scores, all except 1 patient exhibited sleep maintenance insomnia, for which low-dose doxepin is formally approved.
A summary of the patients’ data, treatment outcomes, and tolerability is provided in Tables 1 and 2.
Treatment Outcome
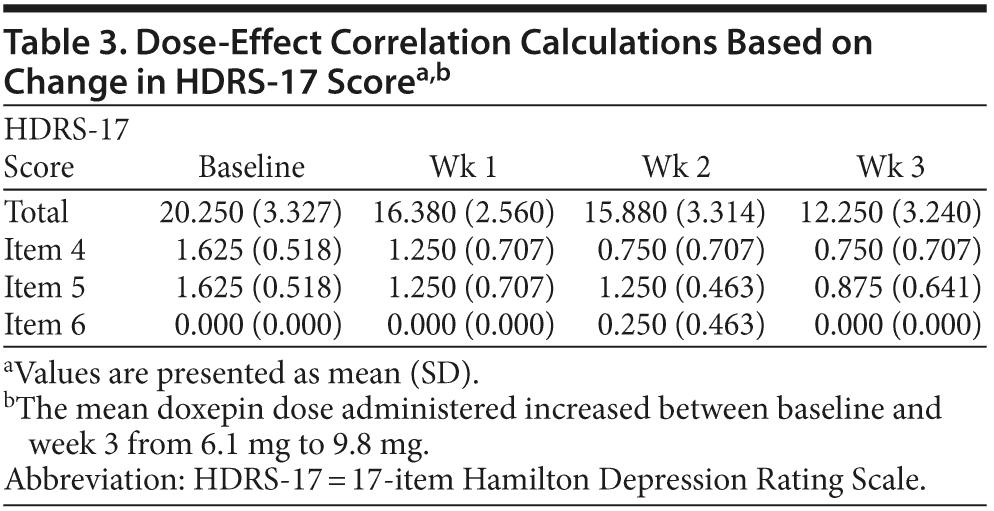
HDRS scores. A 1-way repeated-measure analysis of variance was conducted to analyze the effect of low-dose doxepin on insomnia, categorized as sleep onset, sleep maintenance and early morning awakenings. HDRS-17 values 4, 5, and 6 were used to estimate all patients’ sleep disturbances. As mentioned in the Method, all values obtained in the first week of treatment equate to baseline; hence, our calculations include these values as well as the consecutive 3 weeks of treatment.
Considering sleep onset, the results show significant changes in HDRS-17 item 4 values during the 3 weeks of treatment (F3,21 = 4.4, P = .02), but post hoc tests did not demonstrate any significant improvement in sleep onset insomnia. With regard to sleep maintenance, the results show no significant changes in HDRS-17 item 5 values during the 3 weeks of treatment (F3,21 = 2.03, P = .14).
Considering early morning awakening, the results show no significant changes in HDRS-17 item 6 values during the 3 weeks of treatment (F3,21 = 2.333, P = .103). With regard to sleep in general, when adding all 3 sleep items, the results show significant changes in HDRS-17 values (F3,21 = 3.546, P = .032), and post hoc tests revealed a significant improvement in sleep between baseline and week 3 (P = .058).
Additionally, the severity of depression was estimated using total HDRS-17 scores. With regard to depression, the results show that depression severity (HDRS-17 values) changed significantly during the 3 weeks of treatment (F3,21 = 14.3, P = .00). Post hoc tests revealed a significant improvement in depression severity between the first and the fourth (P = .00), the second and the fourth (P = .05), and the third and the fourth (P = .00) point of measure.
Doxepin dose and effect. The mean doxepin dose administered increased between baseline and week 3 from 6.1 mg to 9.8 mg. Dose-effect correlation calculations were made and revealed no significant correlation between these 2 entities (Table 3).
Patients’ comments. Five of the 17 patients stated during ward rounds that sleep had improved consequent to doxepin administration, 2 patients believed that doxepin treatment was not leading to any improvement, and the remaining 10 patients made no comments related to quality or quantity of sleep.
Other medication. All except 3 patients received at least 1 antidepressant in addition to doxepin treatment. The most common antidepressants administered were SSRIs, namely citalopram and escitalopram, which were received by 5 and 4 of the 17 patients, respectively. One patient received supplementary lorazepam, and 1 patient received lorazepam only, which was phased out during hospitalization. No other psychotropic medication, including antipsychotics, was administered. It must be pointed out that patient 1 received trimipramine and trazodone and patient 7 received mirtazapine, which have significant sedating properties. It is therefore difficult to pinpoint the cause of sleep promotion in these cases.
Acceptability and Adverse Events
Following thorough scanning of the patients’ files, we failed to find any patient comments or physician notes related to adverse events that were experienced during or after doxepin treatment, and these adverse events were usually well documented regarding other medications; however, 3 patients exhibited elevated liver enzymes in their blood test results. Patient 4 showed an elevation in alanine aminotransferase (ALT) level, which remained stable for several weeks after doxepin discontinuation. Patient 13 showed an elevation in ALT, aspartate aminotransferase, and gamma-glutamyltransferase (GGT) levels and patient 15 showed an elevation in ALT and GGT levels. Both patients’ follow-up blood tests showed that previously elevated liver enzyme parameters were regressing during the course of treatment. Furthermore, none of the patients, whose weight was recorded on a regular basis, showed a significant increase in body weight during the course of doxepin treatment.
DISCUSSION
Our results show that there was no improvement in sleep onset and sleep maintenance insomnia in patients with MDD, as estimated by HDRS scores, during 4 weeks of low-dose doxepin treatment. We found significant improvement in insomnia between baseline and week 3 only when considering all 3 HDRS sleep items.
We were unable to detect any immediate improvement within the first week of treatment, which we would have expected under H1 antagonism; instead, a delayed effect after 4 weeks was measured. This finding may be accountable to the positive effect of additional psychotropic medication, leading to an improvement in depressive symptoms and a consequent improvement in sleep. Doxepin is unlikely to have resulted in any antidepressant action in such low doses unless the respective patients were poor metabolizers. Hence, it follows that low-dose doxepin treatment did not result in a significant improvement in insomnia in depressed patients, as shown by HDRS sleep item scores. In short, low-dose doxepin failed to improve sleep disturbances in depressed patients.
When considering patients’ comments during ward rounds, almost one-third of patients reported a subjective improvement in sleep, which may be considered a moderate outcome; however, more than 50% of patients made no comment regarding sleep, making interpretation of these results extremely difficult.
As expected, most patients received SSRIs as concomitant antidepressant medication, which are known to cause initial restlessness and sleep disturbances. This condition may have played an additional role in producing the above results, although we would have expected the sleep-promoting effects of doxepin to outweigh the initial wake-promoting effects of SSRIs.
Regarding acceptability and safety, we found that none of the 17 patients receiving low-dose doxepin treatment reported any adverse events during and after their course of treatment. Furthermore, objective measurements, such as weight gain, were negative. The same applies for the changes in laboratory parameters exhibited by 3 of the 17 patients, as these are unlikely to have been caused by doxepin treatment. These results are in line with previous studies, wherein doxepin showed adverse event rates comparable to placebo.13-18
Further Research
Although this case series failed to show a statistically significant positive effect of low-dose doxepin for insomnia in depressed patients, these results are unreliable and should not be used as a basis for any treatment guidelines. Instead, further research, preferably placebo-controlled, double-blind sleep laboratory trials, is necessary to establish whether doxepin may be an efficacious treatment option. As many as 90% of patients with MDD also suffer from insomnia, and sleep maintenance insomnia is common in depression, making this an important patient group that could possibly benefit from low-dose doxepin treatment.4
Further exploration of these alternative treatment options is worthwhile, as traditional hypnotic medication poses a serious risk of various adverse events, including an increased risk of dementia,21 mortality, and cancer. The higher risk was shown by Kripke et al22 in a recent longitudinal 1-to-1 matched cohort analysis of 10,529 patients. They found that “patients receiving prescriptions for zolpidem, temazepam, and other hypnotics suffered over 4 times the mortality as matched, hypnotic-free control patients”22(p1) and “among patients prescribed hypnotics, cancer incidence was increased for several specific types of cancer, with an overall increase of 35% among those prescribed high doses.”22(p1) Admittedly, no such studies have been conducted for low-dose doxepin or other novel hypnotics; however, current results have displayed very low adverse event rates, making them a promising alternative at this point in time.
In the future, it may also be of interest to investigate whether other new treatment approaches with novel mechanisms, such as orexin or serotonin antagonists, may show positive results in this patient group.
Limitations
The main limitations of this case series are caused by its naturalistic, retrospective design and the small sample size. As previously mentioned, this analysis was conducted in line with a retrospective, treatment-as-usual design by consulting patients’ files for information on treatment efficacy and safety only. In some instances, relevant information, such as patients’ comments, HDRS scores, or laboratory parameters, was missing or poorly documented, leading to less reliable results.
HDRS scores were used to estimate efficacy, as this was the only recorded reference to sleep besides irregular patient comments. HDRS forms were filled in by varying physicians with different approaches to evaluating the patient. This is an imprecise representation of the patient’s sleep parameters, and detailed interviews or polysomnographic measurements would have produced more accurate results.
Moreover, all other forms of treatment were continued as usual during doxepin administration, so it is difficult to pinpoint which medication caused the obtained results; however, as most patients received SSRIs as concomitant medication, it is unlikely that these drugs would have resulted in an improvement in sleep. On the other hand, 2 patients received benzodiazepines for anxiety parallel to doxepin treatment, which very likely influenced our results.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), diphenhydramine (Benadryl and others), doxepin (Silenor, and others), escitalopram (Lexapro and others), eszopiclone (Lunesta), lorazepam (Ativan and others), mirtazapine (Remeron and others), temazepam (Restoril and others), tranylcypromine (Parnate and others), trazodone (Oleptro and others), trimipramine (Surmontil and others), venlafaxine (Effexor and others), zaleplon (Sonata and others), zolpidem (Ambien, Edluar, and others).
Author affiliations: Department of Psychiatry and Psychotherapy, Charité University Medicine Berlin, Benjamin Franklin Campus, Germany.
Potential conflicts of interest: None reported.
Funding/support: None reported.
REFERENCES
1. Sivertsen B, Salo P, Mykletun A, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758-765. PubMed doi:10.1097/PSY.0b013e3182648619
2. Gupta R, Lahan V. Insomnia associated with depressive disorder: primary, secondary, or mixed? Indian J Psychol Med. 2011;33(2):123-128. PubMed doi:10.4103/0253-7176.92056
3. van Mill JG, Hoogendijk WJ, Vogelzangs N, et al. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry. 2010;71(3):239-246. PubMed doi:10.4088/JCP.09m05218gry
4. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254-1269. PubMed
5. Mendlewicz J. Sleep disturbances: core symptoms of major depressive disorder rather than associated or comorbid disorders. World J Biol Psychiatry. 2009;10(4):269-275. PubMed doi:10.3109/15622970802503086
6. Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473-481. PubMed
7. Gelenberg AJ, Freeman MP, Markowitz JC. American Psychiatric Association Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. Arlington, Virginia: American Psychiatric Association; 2010.
8. Proctor A, Bianchi MT. Clinical pharmacology in sleep medicine. ISRN Pharmacol. 2012;2012:914168. PubMed
9. Gunja N. In the Zzz zone: the effects of z-drugs on human performance and driving. J Med Toxicol. 2013;9(2):163-171. PubMed doi:10.1007/s13181-013-0294-y
10. Siriwardena AN, Qureshi MZ, Dyas JV, et al. Magic bullets for insomnia? patients’ use and experiences of newer (Z drugs) versus older (benzodiazepine) hypnotics for sleep problems in primary care. Br J Gen Pract. 2008;58(551):417-422. PubMed doi:10.3399/bjgp08X299290
11. Sullivan S. Update on emerging drugs for insomnia. Expert Opin Emerg Drugs. 2012;17(3):295-298. PubMed doi:10.1517/14728214.2012.693158
12. Stahl SM. Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008;13(12):1027-1038. PubMed
13. Lankford A, Rogowski R, Essink B, et al. Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia. Sleep Med. 2012;13(2):133-138. PubMed doi:10.1016/j.sleep.2011.09.006
14. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442. PubMed
15. Roth T, Heith Durrence H, Jochelson P, et al. Efficacy and safety of doxepin 6 mg in a model of transient insomnia. Sleep Med. 2010;11(9):843-847. PubMed doi:10.1016/j.sleep.2010.07.006
16. Krystal AD, Durrence HH, Scharf M, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33(11):1553-1561. PubMed
17. Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69(10):1557-1564. PubMed doi:10.4088/JCP.v69n1005
18. Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30(11):1555-1561. PubMed
19. Mansbach RS, Ludington E, Rogowski R, et al. A placebo- and active-controlled assessment of 6- and 50-mg oral doxepin on cardiac repolarization in healthy volunteers: a thorough QT evaluation. Clin Ther. 2011;33(7):851-862. PubMed doi:10.1016/j.clinthera.2011.05.092
20. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
21. Chen PL, Lee WJ, Sun WZ, et al. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLoS ONE. 2012;7(11):e49113. PubMed doi:10.1371/journal.pone.0049113
22. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. PubMed doi:10.1136/bmjopen-2012-000850