Background: Brexpiprazole is a serotonin-dopamine activity modulator. We evaluated the effects of adjunctive treatment with brexpiprazole on sleep disturbances in patients with DSM-IV-TR major depressive disorder (MDD) and inadequate response to antidepressant treatment.
Methods: This study was conducted between September 27, 2013, and August 19, 2014. Patients with inadequate response to antidepressant treatment and sleep disturbances continued treatment with their current antidepressant for 2 weeks. Patients still having inadequate response and sleep efficiency less than 85% measured by baseline polysomnography (PSG) received 8-week open-label treatment with their current antidepressant treatment and adjunctive brexpiprazole (target dose: 3 mg/d). Assessments included PSG recordings and scales of insomnia severity, depressive symptoms, and daytime alertness and functioning. Changes from baseline to week 8 were analyzed.
Results: Forty-four patients were treated. Improvements (P < .05) measured by PSG and Consensus Sleep Diary for Morning, respectively, were observed in sleep efficiency (10.4 and 15.4 percentage points), total sleep time (49.0 and 84.5 min), sleep onset latency (−19.7 and −42.6 min), wake-time after sleep onset (−26.4 and −48.0 min), and latency to persistent sleep (−24.9 min, PSG only). Insomnia Severity Index (ISI) total score was improved (−9.2), as was daytime sleepiness (−2.1) as measured by the Epworth Sleepiness Scale (ESS) total score and morning sleepiness (−9.2) as measured by the Bond-Lader Visual Analog Scale (all P < .05). Reaction time was slightly decreased (−0.2 sec−1) by treatment (P < .05). Depressive symptoms improved (Montgomery-Asberg Depression Rating Scale [MADRS]: −16.0 and Clinical Global Impressions—Severity [CGI-S]: −1.8), as did functioning (−8.4) assessed by the Massachusetts General Hospital—Cognitive and Physical Functioning Questionnaire (all P < .05). Improvements in depressive symptoms were dependent on sleep (as assessed by ISI) (P < .0001) and improvements in daytime alertness (as assessed by ESS) were dependent on improvements in ISI (P = .009). No new safety concerns were observed compared to previous brexpiprazole studies.
Conclusions: In patients with inadequate response to antidepressant treatment and sleep disturbances treated with adjunctive brexpiprazole, physiologic measures of sleep and daytime alertness were improved.
Trial registration: ClinicalTrials.gov identifier: NCT01942733
Background: Brexpiprazole is a serotonin-dopamine activity modulator. We evaluated the effects of adjunctive treatment with brexpiprazole on sleep disturbances in patients with DSM-IV-TR major depressive disorder (MDD) and inadequate response to antidepressant treatment.
Methods: This study was conducted between September 27, 2013, and August 19, 2014. Patients with inadequate response to antidepressant treatment and sleep disturbances continued treatment with their current antidepressant for 2 weeks. Patients still having inadequate response and sleep efficiency less than 85% measured by baseline polysomnography (PSG) received 8-week open-label treatment with their current antidepressant treatment and adjunctive brexpiprazole (target dose: 3 mg/d). Assessments included PSG recordings and scales of insomnia severity, depressive symptoms, and daytime alertness and functioning. Changes from baseline to week 8 were analyzed.
Results: Forty-four patients were treated. Improvements (P < .05) measured by PSG and Consensus Sleep Diary for Morning, respectively, were observed in sleep efficiency (10.4 and 15.4 percentage points), total sleep time (49.0 and 84.5 min), sleep onset latency (−19.7 and −42.6 min), wake-time after sleep onset (−26.4 and −48.0 min), and latency to persistent sleep (−24.9 min, PSG only). Insomnia Severity Index (ISI) total score was improved (−9.2), as was daytime sleepiness (−2.1) as measured by the Epworth Sleepiness Scale (ESS) total score and morning sleepiness (−9.2) as measured by the Bond-Lader Visual Analog Scale (all P < .05). Reaction time was slightly decreased (−0.2 sec−1) by treatment (P < .05). Depressive symptoms improved (Montgomery-Asberg Depression Rating Scale [MADRS]: −16.0 and Clinical Global Impressions—Severity [CGI-S]: −1.8), as did functioning (−8.4) assessed by the Massachusetts General Hospital—Cognitive and Physical Functioning Questionnaire (all P < .05). Improvements in depressive symptoms were dependent on sleep (as assessed by ISI) (P < .0001) and improvements in daytime alertness (as assessed by ESS) were dependent on improvements in ISI (P = .009). No new safety concerns were observed compared to previous brexpiprazole studies.
Conclusions: In patients with inadequate response to antidepressant treatment and sleep disturbances treated with adjunctive brexpiprazole, physiologic measures of sleep and daytime alertness were improved.
Trial registration: ClinicalTrials.gov identifier: NCT01942733
Prim Care Companion CNS Disord 2016;18(5):doi:10.4088/PCC.15m01914
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDuke University Hospital, Durham, North Carolina
bH. Lundbeck A/S, Valby, Denmark
cOtsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey
*Corresponding author: Andrew D. Krystal, MD, Duke University Hospital, Durham, NC 27710 ([email protected]).
Sleep complaints are reported in a large percentage of patients with major depressive disorder (MDD).1 Subjective complaints of insomnia (problems falling asleep, frequent awakenings during the night, early morning awakening, or nonrestorative sleep) represent the most common form of sleep disturbances. The common occurrence of mood and sleep disorders has prompted suggestions of a shared diathesis.2 Alterations in sleep patterns are often conceptualized to represent a biological concomitant of clinical depression. However, the presence of sleep disturbance has also been proposed to represent a risk factor for the development of depression.3,4 Sleep disturbance also predicts poorer clinical outcomes3,5-7 and is an independent risk factor for suicidal ideation and completed suicide.8-11 In addition, residual insomnia following otherwise successful antidepressant therapy predicts a greater risk for relapse.12 Such findings have led to the hypothesis that sleep disturbance might be linked to inadequate response to antidepressant therapy and that improving sleep might be critical for optimizing antidepressant outcomes.13,14 However, few data exist that test this hypothesis.
Polysomnographic (PSG) studies,3 the accepted “gold standard” for sleep assessment in a laboratory environment,15 have consistently demonstrated alterations in sleep physiology in depressed patients. The most common reported alterations in sleep parameters of depressed patients include disturbance of initiation and maintenance of sleep, diminished slow-wave sleep during the first non-rapid eye movement (non-REM) period, and alterations in REM sleep including shortened latency from sleep onset to the first REM period, increased amount and percentage of REM sleep, and greater number of eye movements per minute of REM sleep (increased REM density).16
Most antidepressants alter the physiologic patterns of sleep and are thought to lead to improvement in sleep disturbances, along with other symptoms of depression.17 However, the available data suggest that in many cases, sleep disturbances may persist after otherwise successful antidepressant therapy, and many antidepressants also have unwanted adverse effects on sleep; notably, they may cause or worsen insomnia, daytime sleepiness, or sedation.17-19 Yet, there is surprisingly little research completed on the effects of antidepressant interventions on sleep. Notably, there are no PSG studies of antidepressant regimens aimed at improving sleep outcomes in patients with depression. There are 3 studies20-22 documenting improved sleep outcomes in patients administered initial treatment with the combination of an antidepressant medication and a hypnotic agent. Zolpidem has been used for persistent insomnia in selective serotonin reuptake inhibitor (SSRI)-treated depressed patients.23 Notably, PSG studies aimed at investigating the effects of antidepressant augmentation strategies on sleep parameters have not been performed, despite the fact that augmentation therapy is widespread due to the relatively limited remission rate associated with initial antidepressant treatment.24-26 Increasingly common is the addition of certain atypical antipsychotics to treatment regimens, a practice supported by a meta-analysis.27 However, data on the effects of antipsychotics on sleep architecture are sparse, and results are variable.16 To our knowledge, the effect of antipsychotic augmentation therapy on sleep-wake patterns has not yet been assessed.
Brexpiprazole is a serotonin-dopamine activity modulator that has primary pharmacologic effects that may lead to improvements in sleep. Brexpiprazole shows potent partial agonistic activities at 5-HT1A and D2 receptors and antagonistic activity at 5-HT2A and α1B and α2C receptors. It has moderate affinity for histamine-1.28 The efficacy, tolerability, and safety of brexpiprazole as adjunctive treatment in patients with MDD were demonstrated in 2 pivotal randomized, double-blind, placebo-controlled studies29,30 (NCT01360645 and NCT01360632, respectively).
The objective of the present study was to explore the effects of brexpiprazole as an adjunctive treatment to antidepressant monotherapy on sleep-wake patterns, including PSG assessment of sleep, in depressed patients with sleep disturbances.
METHOD
Patients
This open-label, flexible-dose, exploratory study included 44 patients enrolled at 12 sites in the United States between September 27, 2013, and August 19, 2014 (ClinicalTrials.gov identifier: NCT01942733). The study was conducted in compliance with the Declaration of Helsinki and the principles of Good Clinical Practice and was approved by the appropriate institutional review boards. All patients provided written informed consent before enrollment. At screening, eligible patients were men and women aged 18-65 years with a diagnosis of MDD according to DSM-IV-TR criteria. The current major depressive episode (MDE) had to be confirmed using the Mini-International Neuropsychiatric Interview.31 Further, patients had to have displayed an inadequate response to at least 1 antidepressant treatment in the current MDE, as documented by the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire.32 Patients had to have a Montgomery-Asberg Depression Rating Scale (MADRS)33 total score > 18 and a Clinical Global Impressions−Severity of Illness (CGI-S)34 score ≥ 3.

- Sleep disturbance in major depressive disorder predicts poorer clinical outcomes and is an independent risk factor for suicidal ideation and completed suicide. In addition, residual insomnia following otherwise successful antidepressant therapy predicts a greater risk for relapse.
- In patients with inadequate response to antidepressant therapy and sleep disturbances treated with adjunctive brexpiprazole, physiologic measures of sleep and daytime alertness were improved.
The duration of the current MDE was required to be more than 10 weeks. Individuals were currently treated for the existing MDE with an SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressant treatment at the same dosage for more than 2 weeks and for a total period of more than 6 weeks. Importantly, eligible patients had to have been experiencing sleep disturbances (difficulty falling asleep, difficulty staying asleep, problem waking up too early), confirmed by an Insomnia Severity Index (ISI)35 score ≥ 8. At baseline, patients must have still fulfilled the DSM-IV-TR criteria for MDE and received the same SSRI or SNRI antidepressant treatment at an adequate dose for the entire 2-week screening period. Patients with a MADRS total score > 18 with an improvement of less than 25% compared with values at screening were considered eligible. Patients also had to have a CGI-Improvement34 score ≥ 3, had to present with sleep disturbances confirmed by an ISI score ≥ 8, and had to have a mean latency to persistent sleep ≥ 20 minutes during 2 consecutive nights of PSG36 monitoring. Patients must have had a latency to persistent sleep of no less than 15 minutes on either night, as well as an average sleep efficiency < 85% on both nights.
Patients with any current psychiatric disorder or Axis I disorder (DSM-IV-TR criteria), established as the principal diagnosis, other than MDD or a current Axis II (DSM-IV-TR) diagnosis were excluded. Patients were excluded if they had experienced or were experiencing hallucinations, delusions, or any psychotic symptomatology in the current MDE. Another key exclusion criterion was that the patient, in the opinion of the investigator or based on the Columbia-Suicide Severity Rating Scale (C-SSRS),37 was at significant risk of suicide. For the full list of selection criteria, please see Appendix 1.
Procedures
The study consisted of a 2-week period with an open-label treatment with the current antidepressant treatment, an 8-week open-label adjunctive treatment period with brexpiprazole, and a 4-week safety follow-up period. The dose of the current antidepressant treatment remained the same during the duration of the study. During the 8-week adjunctive treatment period, brexpiprazole was titrated from 1 mg once daily for 1 week, to 2 mg once daily for 1 week, and then increased to 3 mg once daily as a target dosage for the remaining 6 weeks. If tolerability issues arose, the dose could have been reduced to 2 mg once daily after week 2 until week 8. The 2-mg once-daily dose was then kept stable. If tolerability issues arose at 2-mg doses, the patients could be withdrawn. A safety follow-up visit was performed 4 weeks after completion of or withdrawal from the study. The antidepressant treatment could be continued or changed at the discretion of the investigator during this latter period.
Exploratory analyses evaluated the effect on sleep quality, sleep architecture, insomnia, daytime alertness, and functioning during 8 weeks of treatment with brexpiprazole as adjunctive treatment to antidepressant treatment. Assessments included PSG recording, patient-rated Consensus Sleep Diary for Morning (CSD-M),38 ISI, Epworth Sleepiness Scale (ESS),39 and Massachusetts General Hospital—Cognitive and Physical Functioning Questionnaire (CPFQ).40 Also, a 10-minute Psychomotor Vigilance Task (PVT)41 and the Bond-Lader Visual Analog Scale (BL-VAS)42 were administered. Changes from baseline in clinician-rated CGI-S34 and MADRS total score were also measured. On days of PSG assessments, patients were awakened after 8 hours of recording. PSG recordings were performed on 2 consecutive nights, and both days of sleep data were used in the analyses. The PSG, actigraphy, and PVT data from each individual sleep laboratory participating in the study were transferred digitally to a Core Sleep Data Center for centralized scoring. A sleep assessment manual was provided to each site to standardize the collection, processing, and transfer of these data. Safety assessments included treatment-emergent adverse events (TEAEs), clinical safety laboratory tests, vital signs, and electrocardiogram (ECG) parameters. Risk of suicide was assessed using the C-SSRS.43
For an overview of the timing of all study assessments, as well as details of the study visit procedures, including details of the PSG assessments, please see Appendix 1.
Statistical Analyses
Safety analyses were conducted on the all-patients-treated set, comprising all patients who took at least 1 dose of brexpiprazole. Efficacy analyses were conducted on the full analysis set (using observed cases), comprising all patients in the all-patients-treated set with a valid baseline assessment and at least 1 valid postbaseline efficacy assessment. For all endpoints, change from baseline to week 8 was summarized using descriptive statistics. In addition, for ISI, ESS, CPFQ, MADRS total score, CGI-S, and CSD-M, the change from baseline to week 8 was analyzed using a mixed-model repeated-measures approach. Relationships among the change from baseline in ISI, ESS, CPFQ, and MADRS (without item 4: sleep) were investigated performing multivariate regression analyses. The statistical software used was SAS, Version 9.4.
RESULTS
Patients
Of the 156 screened patients, 44 were included and treated. Of these, 93% (41/44) completed the 8-week treatment period and 7% (3/44) were withdrawn from the study (1 patient for noncompliance with study medication, 1 patient was lost to follow-up, and 1 patient due to administrative or other reasons). Patients had an average of 5 previous MDEs, with the mean duration of the current MDE being 13 months. The mean duration of the patients’ last period of wellness was 28 months.
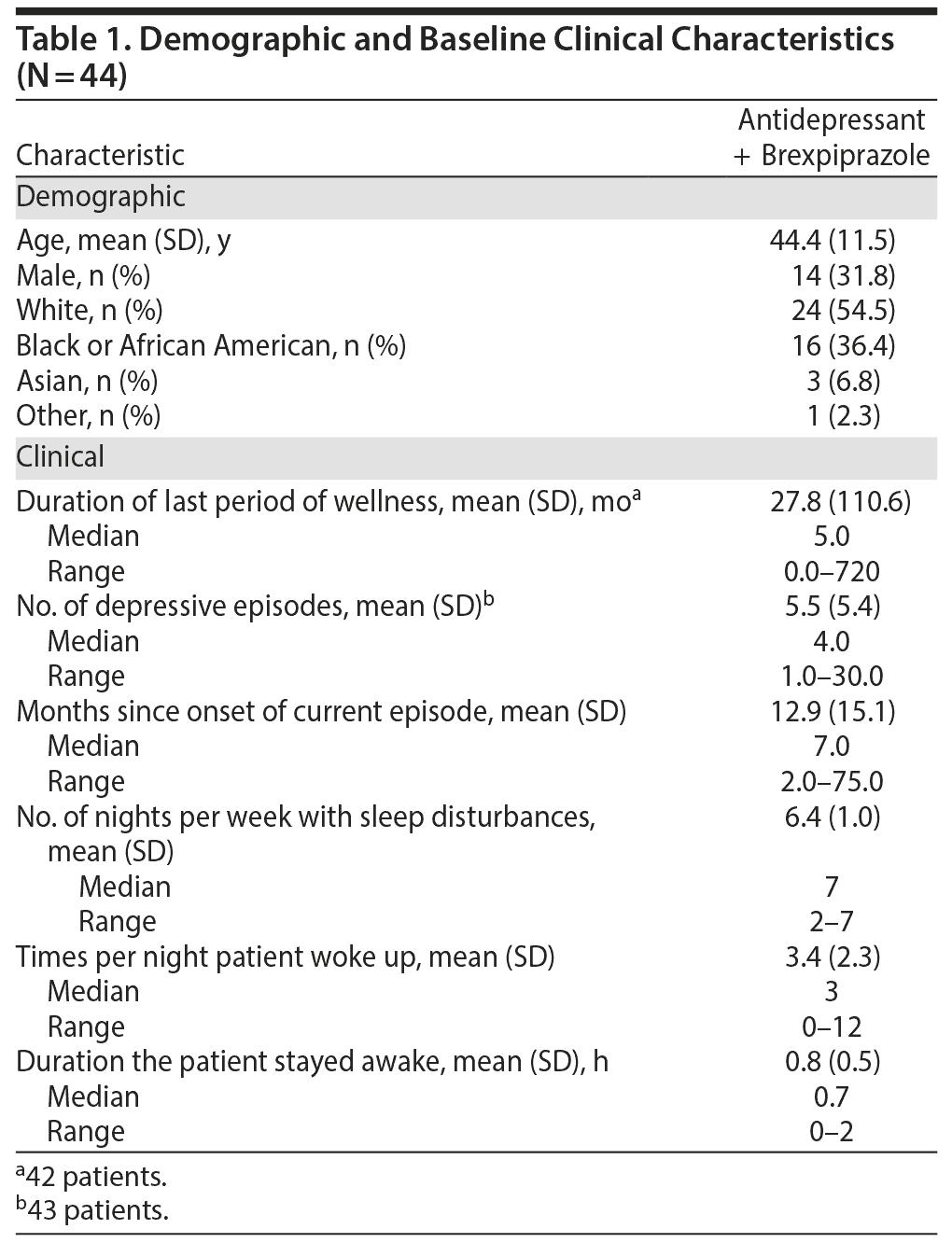
At baseline, patients had sleep disturbances for a mean of 6 nights per week during the previous month. During the nights on which sleep problems were apparent, patients woke up a mean of 3 times per night, and when the patients were awake, they stayed awake for a mean of 0.8 hours. Baseline patient demographics and clinical characteristics are summarized in Table 1.
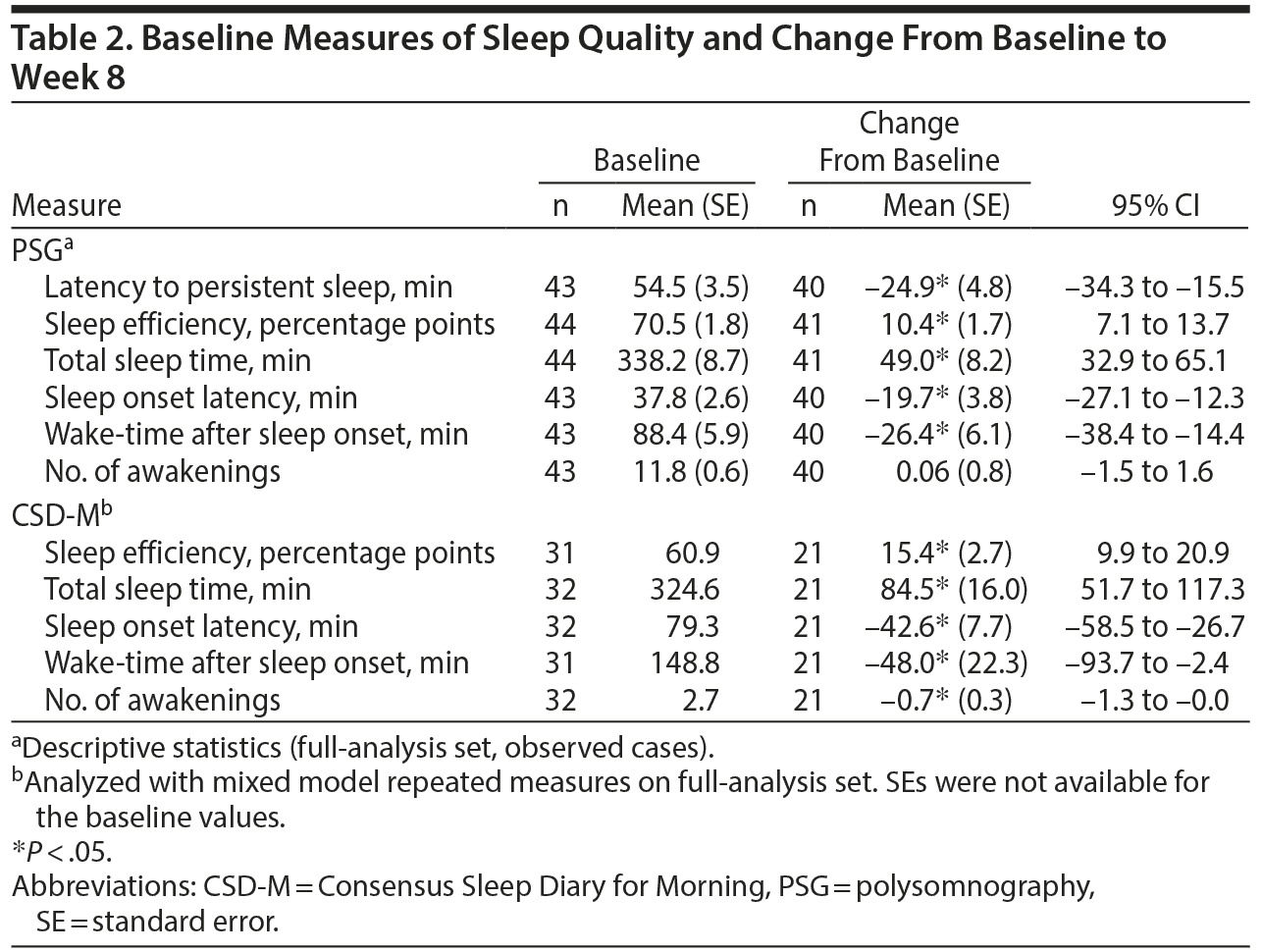
As measured by PSG at baseline, mean latency to persistent sleep was 54.5 minutes, while sleep efficiency was 70.5%; total sleep time was 338.2 minutes, sleep onset latency was 37.8 minutes, wake-time after sleep onset was 88.4 minutes, and patients had a mean of 11.8 awakenings (Table 2).
Latency to persistent sleep decreased (improved) (P < .05) by 24.9 minutes from baseline to week 8 (Table 2). Further, improvements (P < .05) at week 8 as measured by PSG and the CSD-M were observed in sleep efficiency (10.4 percentage points and 15.4 percentage points, respectively), total sleep time (49.0 min and 84.5 min, respectively), sleep onset latency (−19.7 min and −42.6 min, respectively), and wake-time after sleep onset (−26.4 and −48.0, respectively). In contrast, no meaningful changes (although significant for CSD-M) from baseline in the number of awakenings were observed when using PSG or CSD-M (0.06 and −0.7, respectively); it should be noted that the number of awakenings at baseline for CSD-M was very low (mean of 2.7).
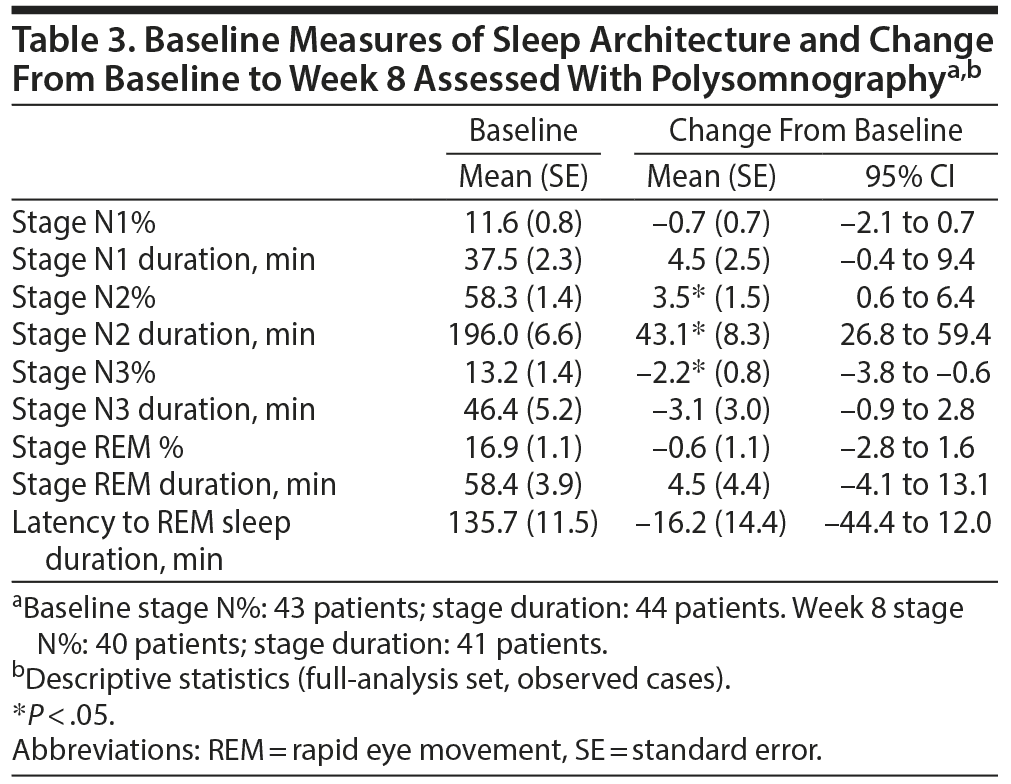
An increase (P < .05) in the fraction of stage N2 sleep by 3.5% and duration by 43.1 minutes and a reduction (P < .05) in the fraction of stage N3 sleep by 2.2% were observed at week 8. No other significant changes in sleep architecture were detected (Table 3).
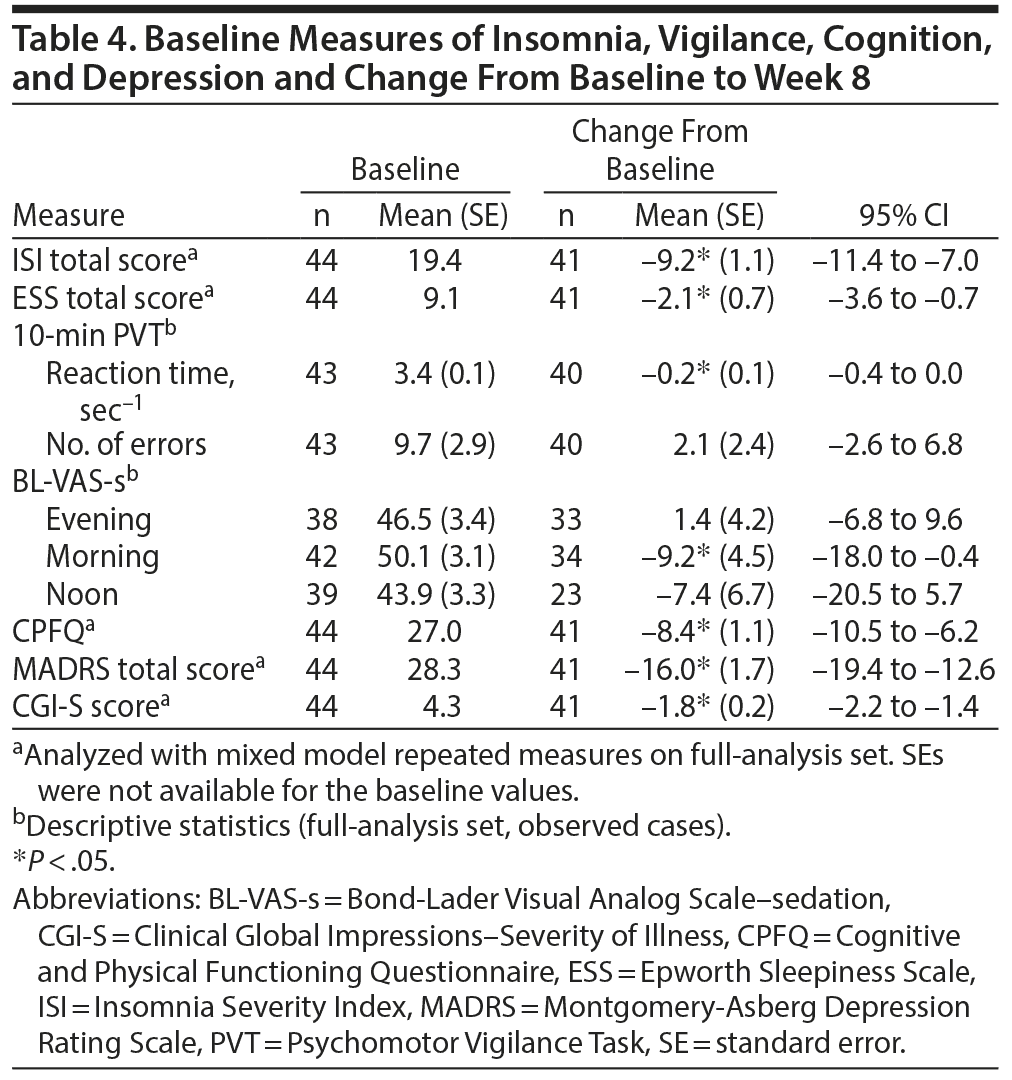
Syndromal severity of insomnia, as measured by the patient-rated ISI total score, was improved by −9.2 at week 8 (P < .05) from a baseline score of 19.4 (Table 4). In addition, the degree of sedation, as measured by the patient-rated ESS, was reduced (−2.1) when assessed at week 8 (P < .05). Morning, but not daytime or evening, BL-VAS scores improved following 8-week adjunctive treatment with brexpiprazole (P < .05). Reaction time, as measured by the PVT, improved by 0.2 sec−1 from baseline scores of 3.4 sec−1 (P < .05); the number of errors was not affected (Table 4).
Clinically relevant improvements were observed in the CPFQ, with −8.4 at week 8 (P < .05) from a baseline score of 27.0, and in depressive symptoms, with an improvement at week 8 in mean MADRS total score (−16.0, P < .05) and CGI-S score (−1.8, P < .05) from baseline scores of 28.3 and 4.3, respectively (Table 4).
The multivariate regression analyses revealed dependence of the change from baseline in MADRS without item 4 (sleep) and change from baseline in ISI (P < .0001). Further, there was a significant dependence of the change from baseline in ESS and change from baseline in ISI (P = .009), but not between change from baseline in ESS and change from baseline in MADRS without item 4 (sleep) (P = .21 and P = .87) or change from baseline in CPFQ (P = .56 and P = .25). Change from baseline in CPFQ was significantly dependent on change from baseline in MADRS without item 4 (sleep) (P = .006), but not change from baseline in ISI (P = .09) or ESS (P = .56) (Supplementary Table 1).
Safety Assessments
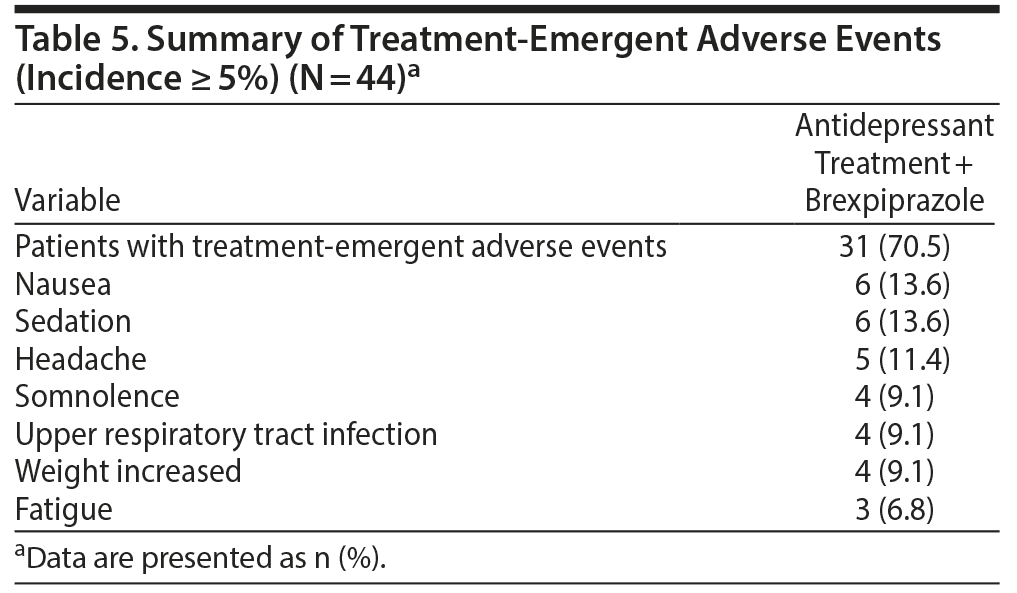
A total of 31 patients experienced 1 or more treatment-emergent adverse events (TEAEs). There were no serious adverse events or adverse events leading to withdrawal. A summary of the TEAEs is in Table 5. Of the total number of TEAEs, the highest incidences were nausea and sedation. Other TEAEs with an incidence ≥ 5% were headache, somnolence, upper respiratory tract infection, weight increased, and fatigue. A total of 3 patients presented extrapyramidal side effects−related TEAEs: 2 with restlessness (4.5%) and 1 with akathisia (2.3%). One patient (2.3%) reported insomnia.
One patient (2%) had prior nonsuicidal self-injurious behavior, and 13 (30%) of the patients had a prior history of suicidal ideation. During the entire study period, none of the patients displayed suicidal behavior. Two patients had suicidal ideation (5%).
The majority of the mean laboratory values were within the reference ranges at all scheduled visits. However, for fasting low-density lipoprotein cholesterol, the mean values were above the upper reference range at all scheduled visits. The mean changes from baseline in fasting metabolic parameters were minimal and not clinically relevant (Supplementary Table 2). With respect to weight, at week 8, patients had a mean (SD) weight gain of 1.4 (2.7) kg compared to baseline (baseline: 81.4 [19.5] kg). None of the patients presented potentially clinically significant vital signs. With respect to ECG measurements, 1 patient had a potentially clinically significant high QRS complex duration at weeks 4 and 8. At screening, this patient’s QRS duration value of 94 ms was close to the upper limit of the reference range of 100 ms but was not reported as a TEAE.
DISCUSSION
In this exploratory open-label study, depressive symptoms improved in patients with MDD and insomnia treated with brexpiprazole flexible dose (target dose 3 mg/d) given adjunctive to an antidepressant. These improvements were consistent with findings from previous clinical studies.29,30 Further, improvement in a number of key sleep parameters (latency to persistent sleep, total sleep time, wake-time after sleep onset, sleep onset latency, and sleep efficiency) as assessed by PSG or CSD-M were observed. Changes in some sleep architecture parameters (eg, increase in the duration of stage N2 sleep) as assessed by PSG were also observed. The effects on sleep parameters in this study are of similar magnitude as those described for sleep medications such as zolpidem,44,45 eszopiclone,45,46 or ramelteon47 in placebo-controlled trials of the treatment of patients with primary insomnia. In contrast, no relevant changes were observed with number of awakenings, which may be explained by the relatively low number at baseline.
Changes to sleep pattern were also reflected in the mean reduction in the overall ISI (9.2-point decrease [47%]) following adjunctive treatment with brexpiprazole. Furthermore, only 1 patient reported insomnia as an adverse event during the study, and this finding taken together with the improvements in sleep parameters observed, suggests that adjunctive treatment with brexpiprazole tends to improve sleep and does not exacerbate sleep disturbances, which can occur with some commonly administered antidepressant medications as monotherapy.17 As revealed by the multivariate regression analyses, the improvements in depressive symptoms were dependent on sleep (as assessed by ISI).
Besides complaints of insomnia, patients with MDD also suffer from symptoms of impaired functioning, including diminished alertness.48 The present findings, as shown by the multivariate regression analyses, suggest that improved insomnia symptoms (as assessed by ISI) were associated with improvements in daytime alertness (as assessed by ESS). Improvements in self-reported cognitive and physical functioning (as assessed by CPFQ, which includes items related to daytime alertness, energy, and ability to concentrate) were dependent on improvements in depressive symptoms and occurred independently of improvements in insomnia symptoms and daytime alertness after adjunctive treatment with brexpiprazole.
PVT was not negatively affected, indicating the absence of impairment on sustained attention following treatment with brexpiprazole. This result is in line with the literature on antidepressants such as escitalopram or agomelatine.49,50
The consistent improvements on sleep-wake patterns after adjunctive treatment with brexpiprazole may be partly explained by its specific receptor binding profile, in particular its partial agonism at dopamine D2, D3, and 5-HT1A receptors, and its antagonism at 5-HT2A receptors.28 Evidence shows that 5-HT2A/2C receptor antagonists potently promote slow-wave sleep and are implicated in cognitive performance and may even improve daytime functioning.51 However, studies investigating the effects of antipsychotics that bind with high affinity to 5-HT2A/2C on sleep architecture are sparse and results are variable.16 For example, aripiprazole and olanzapine reversed MK-801-induced increase in sleep latency in rats, but quetiapine and risperidone were ineffective.52 Thus, the effects on sleep may not be mediated by 5-HT2A antagonism alone, as both quetiapine and risperidone have 5-HT2A antagonist effects. As mentioned, 5-HT2A antagonism is reliably associated with an increase in slow waves and slow-wave sleep time. We did not observe changes in slow-wave sleep in the current study, which further suggests the potential contribution of pharmacologic effects other than 5-HT2A antagonism on our results.
The study showed good tolerability for brexpiprazole in patients with MDD and sleep disturbances, which is in line with the good tolerability profile seen in other studies with brexpiprazole in MDD.29,30 Nausea and sedation were the TEAEs with higher incidence (both 14%) in this study than in previous studies with brexpiprazole; however, none of the events were considered to be severe by the investigator, and no patients withdrew due to any TEAEs.
Limitations of the Study
Limitations of this study must be taken into account when interpreting its results. First, because of the small sample size, our results should be considered as preliminary, and they need to be replicated in a large sample. Second, the single-arm open-label design does not allow us to exclude a placebo response in sleep measures and depression ratings. Third, as this is an open-label study without a control group where there is a threshold severity for inclusion, the results could be influenced by regression to the mean. Fourth, data on reasons for screen failure were not systematically collected other than it being noted that inclusion criteria were not met.
CONCLUSION
In summary, clinical improvement of sleep disturbances and depressive symptoms were observed in patients with MDD and with sleep disturbances with an inadequate response to antidepressant treatment who were treated with adjunctive brexpiprazole. Furthermore, there was a global improvement of daily alertness and functioning. Adjunctive treatment with brexpiprazole was safe and well tolerated.
Submitted: November 27, 2015; accepted June 21, 2016.
Published online: September 8, 2016.
Drug names: aripiprazole (Abilify), brexpiprazole (Rexulti), escitalopram (Lexapro and others), eszopiclone (Lunesta), olanzapine (Zyprexa and others), quetiapine (Seroquel and others), ramelteon (Rozerem), risperidone (Risperdal and others), zolpidem (Ambien, Edluar, and others).
No trade: agomelatine, brexpiprazole.
Potential conflicts of interest: Dr Krystal receives grant support from National Institutes of Health (NIH), Teva, Sunovion, Astellas, Abbott, Neosync, Brainsway, Janssen, ANS St Jude, and Novartis and is a consultant to Abbott, Astellas, AstraZeneca, Attentiv, Bristol-Meyers Squibb, Teva, Eisai, Eli Lilly, GlaxoSmithKline, Jazz, Janssen, Merck, Neurocrine, Otsuka, Lundbeck, Roche, Sanofi-Aventis, Somnus, Sunovion, Takeda, Transcept, and Vantia. Dr Mittoux and Mr Meisels are full-time employees of H. Lundbeck A/S, Valby, Denmark. Dr Baker is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey.
Funding/support: This work was supported by H. Lundbeck A/S, Valby, Denmark, and Otsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey.
Role of the sponsor: H. Lundbeck A/S and Otsuka Pharmaceutical Development & Commercialization, Inc, were involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the paper; and in the decision to submit the paper for publication.
Previous presentation: This research was presented at the Society of Biological Psychiatry (SOBP) 70th Annual Scientific Convention (via poster), May 14-16, 2015, Toronto, Ontario, Canada, ‘ ¢ American Society of Clinical Psychopharmacology (ASCP) Annual Meeting (via poster), June 22-25, 2015, Miami, Florida.
Acknowledgments: The authors thank Paul Redrobe, PhD, and Johan Hellsten, PhD, full-time employees of H. Lundbeck A/S, Valby, Denmark, for providing medical writing assistance in the manuscript preparation, revision, and editing. The authors are entirely responsible for the scientific content of the paper.
Supplementary material: See accompanying pages.
REFERENCES
1. Nowell PD, Buysse DJ. Treatment of insomnia in patients with mood disorders. Depress Anxiety. 2001;14(1):7-18. PubMed doi:10.1002/da.1042
2. McCarley RW. REM sleep and depression: common neurobiological control mechanisms. Am J Psychiatry. 1982;139(5):565-570. PubMed doi:10.1176/ajp.139.5.565
3. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Prim Psychiatry. 1994;1:22-27.
4. Winokur A, DeMartinis NA 3rd, McNally DP, et al. Comparative effects of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64(10):1224-1229. PubMed doi:10.4088/JCP.v64n1013
5. Buysse DJ, Reynolds CF 3rd, Houck PR, et al. Does lorazepam impair the antidepressant response to nortriptyline and psychotherapy? J Clin Psychiatry. 1997;58(10):426-432. PubMed doi:10.4088/JCP.v58n1003
6. Dew MA, Reynolds CF 3rd, Houck PR, et al. Temporal profiles of the course of depression during treatment: predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54(11):1016-1024. PubMed doi:10.1001/archpsyc.1997.01830230050007
7. Thase ME, Buysse DJ, Frank E, et al. Which depressed patients will respond to interpersonal psychotherapy? the role of abnormal EEG sleep profiles. Am J Psychiatry. 1997;154(4):502-509. PubMed doi:10.1176/ajp.154.4.502
8. Coryell W, Young EA. Clinical predictors of suicide in primary major depressive disorder. J Clin Psychiatry. 2005;66(4):412-417. PubMed doi:10.4088/JCP.v66n0401
9. Bernert RA, Joiner TE Jr, Cukrowicz KC, et al. Suicidality and sleep disturbances. Sleep. 2005;28(9):1135-1141. PubMed
10. McCall WV, Blocker JN, D’ Agostino R Jr, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010a;11(9):822-827. PubMed doi:10.1016/j.sleep.2010.04.004
11. Cukrowicz KC, Otamendi A, Pinto JV, et al. The impact of insomnia and sleep disturbances on depression and suicidality. Dreaming. 2006;16(1):1-10. doi:10.1037/1053-0797.16.1.1
12. Gulec M, Selvi Y, Boysan M, et al. Ongoing or re-emerging subjective insomnia symptoms after full/partial remission or recovery of major depressive disorder mainly with the selective serotonin reuptake inhibitors and risk of relapse or recurrence: a 52-week follow-up study. J Affect Disord. 2011;134(1-3):257-265. PubMed doi:10.1016/j.jad.2011.05.056
13. McCall WV, Blocker JN, D’ Agostino R Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010b;6(4):322-329. PubMed
14. Wichniak A, Wierzbicka A, Jernajczyk W. Sleep and antidepressant treatment. Curr Pharm Des. 2012;18(36):5802-5817. PubMed doi:10.2174/138161212803523608
15. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527. PubMed doi:10.1378/chest.10-1872
16. Sharpley AL, Cowen PJ. Effect of pharmacologic treatments on the sleep of depressed patients. Biol Psychiatry. 1995;37(2):85-98. PubMed doi:10.1016/0006-3223(94)00135-P
17. Krystal AD, Thase ME, Tucker VL, et al. Bupropion HCL and sleep in patients with depression. Curr Psychiatry Rev. 2007;3(2):123-128. doi:10.2174/157340007780599096
18. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50. PubMed doi:10.1017/S0033291709006011
19. Iovieno N, van Nieuwenhuizen A, Clain A, et al. Residual symptoms after remission of major depressive disorder with fluoxetine and risk of relapse. Depress Anxiety. 2011;28(2):137-144. PubMed doi:10.1002/da.20768
20. Nolen WA, Haffmans PM, Bouvy PF, et al. Hypnotics as concurrent medication in depression: a placebo-controlled, double-blind comparison of flunitrazepam and lormetazepam in patients with major depression, treated with a (tri)cyclic antidepressant. J Affect Disord. 1993;28(3):179-188. PubMed doi:10.1016/0165-0327(93)90103-Q
21. Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59(11):1052-1060. PubMed doi:10.1016/j.biopsych.2006.01.016
22. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011;72(7):914-928. PubMed doi:10.4088/JCP.09m05571gry
23. Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60(10):668-676. PubMed doi:10.4088/JCP.v60n1005
24. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252. PubMed doi:10.1056/NEJMoa052964
25. Rush AJ, Trivedi MH, Wisniewski SR, et al; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242. PubMed doi:10.1056/NEJMoa052963
26. Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72(5):e18. PubMed doi:10.4088/JCP.8133tx4c
27. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991. PubMed doi:10.1176/appi.ajp.2009.09030312
28. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. PubMed doi:10.1124/jpet.114.213793
29. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231. PubMed doi:10.4088/JCP.14m09688
30. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232-1240. PubMed doi:10.4088/JCP.14m09689
31. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
32. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. PubMed doi:10.1111/j.1755-5949.2009.00102.x
33. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
34. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217-222.
35. Bastien CH, Valliרres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. PubMed doi:10.1016/S1389-9457(00)00065-4
36. Orr WC. Utilization of polysomnography in the assessment of sleep disorders. Med Clin North Am. 1985;69(6):1153-1167. PubMed doi:10.1016/S0025-7125(16)30980-4
37. Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035-1043. PubMed doi:10.1176/ajp.2007.164.7.1035
38. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The Consensus Sleep Diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287-302. PubMed
39. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. PubMed
40. Fava M, Iosifescu DV, Pedrelli P, et al. Reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire. Psychother Psychosom. 2009;78(2):91-97. PubMed doi:10.1159/000201934
41. Wilkinson RT, Houghton D. Field test of arousal: a portable reaction timer with data storage. Hum Factors. 1982;24(4):487-493. PubMed
42. Datto C, Berggren L, Patel JB, et al. Self-reported sedation profile of immediate-release quetiapine fumarate compared with extended-release quetiapine fumarate during dose initiation: a randomized, double-blind, crossover study in healthy adult subjects. Clin Ther. 2009;31(3):492-502. PubMed doi:10.1016/j.clinthera.2009.03.002
43. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed doi:10.1176/appi.ajp.2011.10111704
44. Scharf MB, Roth T, Vogel GW, et al. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55(5):192-199. PubMed
45. Erman MK, Zammit G, Rubens R, et al. A polysomnographic placebo-controlled evaluation of the efficacy and safety of eszopiclone relative to placebo and zolpidem in the treatment of primary insomnia. J Clin Sleep Med. 2008;4(3):229-234. PubMed
46. Zammit GK, McNabb LJ, Caron J, et al. Efficacy and safety of eszopiclone across 6 weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20(12):1979-1991. PubMed doi:10.1185/174234304X15174
47. Erman M, Seiden D, Zammit G, et al. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7(1):17-24. PubMed doi:10.1016/j.sleep.2005.09.004
48. Lee RSC, Hermens DF, Porter MA, et al. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140(2):113-124. PubMed doi:10.1016/j.jad.2011.10.023
49. Wingen M, Bothmer J, Langer S, et al. Actual driving performance and psychomotor function in healthy subjects after acute and subchronic treatment with escitalopram, mirtazapine, and placebo: a crossover trial. J Clin Psychiatry. 2005;66(4):436-443. PubMed doi:10.4088/JCP.v66n0405
50. Quera-Salva MA, Hajak G, Philip P, et al. Comparison of agomelatine and escitalopram on nighttime sleep and daytime condition and efficacy in major depressive disorder patients. Int Clin Psychopharmacol. 2011;26(5):252-262. PubMed doi:10.1097/YIC.0b013e328349b117
51. Landolt HP, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. 2009;29(9):1795-1809. PubMed doi:10.1111/j.1460-9568.2009.06718.x
52. Ishida T, Obara Y, Kamei C. Effects of some antipsychotics and a benzodiazepine hypnotic on the sleep-wake pattern in an animal model of schizophrenia. J Pharmacol Sci. 2009;111(1):44-52. PubMed doi:10.1254/jphs.09142FP
Enjoy this premium PDF as part of your membership benefits!