Objective: To describe the characteristics of the electrical phenomena of antidepressant discontinuation syndrome known as brain zaps and their effect on quality of life.
Methods: We examined 595 unsolicited posts made by individuals frequenting a popular lay mental health website. The site was accessed between December 13, 2014, and December 12, 2016, and its content was saved in a text document. The posts had been accumulating on the site since December 2014. These posts were analyzed and separated into 648 separate statements regarding antidepressant intake. Of the statements, 378 contained reference to symptoms experienced in the context of antidepressant discontinuation. These posts were further analyzed for specifics of the medications involved, temporal characteristics of the medication intake, associated symptoms, specifics of the “zap” experience itself, and effect of the zaps on quality of life. As this was a convenience sample, only qualitative analysis was performed.
Results: Venlafaxine and paroxetine were reported more frequently, and fluoxetine less frequently, in the sample compared to their frequency of prescription in clinical practice. This finding mirrors the frequency distribution of all withdrawal effects versus antidepressant prescriptions written as reported in the literature. The most likely cause of brain zaps was abrupt discontinuation of the medication, but gradual tapering had only a partial mitigating effect. An unexpected finding was the frequent association of brain zaps with lateral eye movements. The presence of brain zaps was typically transitory, but in a small number of cases it caused significant disability lasting for months or years with no treatment available. Patients’ inability to obtain effective help from prescribers and the perceived lack of interest in this symptom on the part of the medical profession risks fueling antipsychiatry attitudes among patients.
Conclusions: Brain zaps are a poorly understood symptom of antidepressant discontinuation, which require further study for both better prevention and treatment. The apparent association of brain zaps with lateral eye movements may open avenues for investigation of this process.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
An Underappreciated Symptom of Antidepressant Discontinuation

ABSTRACT
Objective: To describe the characteristics of the electrical phenomena of antidepressant discontinuation syndrome known as brain zaps and their effect on quality of life.
Methods: We examined 595 unsolicited posts made by individuals frequenting a popular lay mental health website. The site was accessed between December 13, 2014, and December 12, 2016, and its content was saved in a text document. The posts had been accumulating on the site since December 2014. These posts were analyzed and separated into 648 separate statements regarding antidepressant intake. Of the statements, 378 contained reference to symptoms experienced in the context of antidepressant discontinuation. These posts were further analyzed for specifics of the medications involved, temporal characteristics of the medication intake, associated symptoms, specifics of the “zap” experience itself, and effect of the zaps on quality of life. As this was a convenience sample, only qualitative analysis was performed.
Results: Venlafaxine and paroxetine were reported more frequently, and fluoxetine less frequently, in the sample compared to their frequency of prescription in clinical practice. This finding mirrors the frequency distribution of all withdrawal effects versus antidepressant prescriptions written as reported in the literature. The most likely cause of brain zaps was abrupt discontinuation of the medication, but gradual tapering had only a partial mitigating effect. An unexpected finding was the frequent association of brain zaps with lateral eye movements. The presence of brain zaps was typically transitory, but in a small number of cases it caused significant disability lasting for months or years with no treatment available. Patients’ inability to obtain effective help from prescribers and the perceived lack of interest in this symptom on the part of the medical profession risks fueling antipsychiatry attitudes among patients.
Conclusions: Brain zaps are a poorly understood symptom of antidepressant discontinuation, which require further study for both better prevention and treatment. The apparent association of brain zaps with lateral eye movements may open avenues for investigation of this process.
Prim Care Companion CNS Disord 2018;20(6):18m02311
To cite: Papp A, Onton JA. Brain zaps: an underappreciated symptom of antidepressant discontinuation. Prim Care Companion CNS Disord. 2018;20(6):18m02311.
To share: https://doi.org/10.4088/PCC.18m02311
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of California, San Diego, La Jolla, California
bInstitute for Neural Computation, University of California, San Diego, La Jolla, California
*Corresponding author: Alexander Papp, MD, Department of Psychiatry, University of California, San Diego, 9500 Gilman Dr, La Jolla, CA 92093 ([email protected]).
Brain zaps are sensations perceived as electrical flashes that occur inside the brain typically during decreasing antidepressant levels. The term is a colloquialism that emerged on internet discussion boards and is a succinct name for a phenomenon that is described in less colorfully descriptive definitions in the psychiatric literature as one of the symptoms of antidepressant discontinuation.
Symptoms associated with discontinuation of antidepressants were first observed with tricyclic antidepressants (TCAs).1,2 Their presence was initially seen as a conversion reaction; only after several observations of similar reactions were they theorized as being physiologic due to cholinergic rebound.2 A different discontinuation reaction was observed with selective serotonin reuptake inhibitors (SSRIs).3 In 1997, Zajecka and colleagues3 mentioned reports on the internet of “electric shocks,” which were subsequently called brain zaps on lay websites.
Schatzberg et al4 described an SSRI discontinuation syndrome, defined as a set of symptoms emerging after abrupt discontinuation or during dose reduction. These symptoms are generally mild and transient, are rapidly reversed by the reintroduction of the original medication, and can be minimized by a slow tapering down of the drug.4 The characteristic symptoms were grouped as those of disequilibrium, gastrointestinal symptoms, flu-like symptoms, and sensory and sleep disturbances. The sensation of electric shocks was categorized as a sensory disturbance. It was suggested that a shorter half-life of the agent, a more extended length of the treatment, and treatment-emergent anxiety were risk factors.4 The mechanism of action was theorized to be the down-regulation of serotonin receptors, with potential secondary effects on other neurotransmitters, or some unknown innate sensitivity in individual patients.5 The recommended clinical management was reassurance about the transitory nature of the symptoms, education about the importance of not skipping doses, and tapering the medications slowly. In stubborn cases, it was recommended to switch to fluoxetine and then taper off fluoxetine very slowly.
Rosenbaum and Zajecka6 suggested that every antidepressant should be included in a double-blind, placebo-controlled study of discontinuation events as part of the approval process. Basic studies were also seen as necessary to establish the mechanism of action of the discontinuation phenomena and the specific risk factors for SSRI discontinuation syndrome. Of the antidepressants introduced after SSRI discontinuation syndrome became a known problem, such preclinical studies were only conducted with vortioxetine (in 2016), which found placebo-level discontinuation symptoms.7
A survey by Young and Currie8 in 1997 reported on general practitioners’ and psychiatrists’ knowledge of antidepressant discontinuation syndrome. About one-fourth of the psychiatrists and two-thirds of the general practitioners were found to be unaware of the existence of the phenomenon.8
In 1998, Rosenbaum et al9 published a double-blind study in which treatment with fluoxetine, sertraline, or paroxetine was interrupted with placebo substitution. The syndrome was operationalized by using a specialized questionnaire (Discontinuation-Emergent Signs and Symptoms checklist, DESS) that contained a list of 43 symptoms for which the score was the number of items checked. DESS scores were significantly higher than baseline when sertraline and paroxetine were substituted but not when fluoxetine was used. While the symptom of “electrical sensations” was acknowledged in the article as a symptom of antidepressant discontinuation, it was not included in the DESS.9
In 2000, Black and colleagues,10 in their own proposed diagnostic criteria for SSRI discontinuation syndrome, included “shock-like sensation or paresthesias” and “visual disturbances.” Christmas11 noted in 2005 the contrast between the lack of interest in psychiatry regarding brain zaps and the concern from patients about them. He noted the possibility that as the name (he used “brain shivers”) became increasingly widely known on the internet, people with vaguely similar experiences simply adopted it for their own symptoms, hence the appearance of a regularly occurring phenomenon.11
In 2012, Nielsen et al12 compared symptoms resulting from the discontinuation of benzodiazepines versus SSRIs in a study reviewing 76 articles. They identified 42 symptoms associated with discontinuation, 37 of which were shared by both groups. They noted that when DSM-III13 was replaced by DSM-III-R,14 the bar for diagnosis of dependence was raised substantially. In DSM-III, the diagnosis of dependence required either tolerance or a “substance-specific withdrawal syndrome” (ie, 1 of 2). In DSM-III-R, the number of criteria increased to 3 of 9, and a minimum duration requirement was added. This change occurred at about the same time as it became widely accepted that benzodiazepines lead to dependence and just before the first SSRIs were marketed in 1987-1988. Had SSRIs been marketed under DSM-III, they would have fulfilled the dependence criteria.
In a 2015 review, Fava et al15 observed that despite the widespread use of serotonergic medications, SSRI discontinuation syndrome had attracted scant interest in psychiatry. Paroxetine and later venlafaxine were reported to be the most associated with discontinuation responses, although virtually all antidepressants had been reported to cause such symptoms.
Fava and colleagues15 postulated that the term discontinuation syndrome, as opposed to withdrawal syndrome, has come to be favored in the literature due to pressure by the pharmaceutical industry. The authors15 recommended using the term antidepressant withdrawal syndrome. As this controversy has not been resolved, this article will use the more generic term antidepressant discontinuation.
A few studies have probed antidepressant discontinuation reports for the presence of brain zaps. In 2004, Medawar and Herxheimer16 analyzed side effect reports of patients taking paroxetine made to a drug regulatory agency in the United Kingdom. Of the 1,370 reports labeled as “drug withdrawal reactions,” 4% were descriptions of electric shock sensations. The authors16 also noted that the UK Committee on Safety of Medicines as early as 1993 had collected 78 reports of withdrawal reactions to paroxetine, of which 5 (6.4%) referred to electric shock sensations. Belaise et al17 quoted 12 descriptions of withdrawal experiences by patients from different websites. Of the 12, 5 (42%) contained reference to “electrical” or “zap” sensations. Freifeld and colleagues18 described a methodology of collecting reports of adverse effects by monitoring Twitter feeds that mention the names of various pharmaceutical products. The collection was not specific to antidepressants but, as an example, provided spontaneous reports of brain zaps related to patients taking vilazodone.18 Read et al19 conducted a study with an internet questionnaire that probed adverse reactions of people taking or discontinuing antidepressants. The questionnaire had 1,829 respondents and included questions about the subjective experiences, beliefs, and attitudes related to discontinuation of their antidepressant. Thirteen (< 1%) respondents reported electric shock experiences.19
Only case studies (all published within the past 15 years) focused specifically on brain zaps. Campagne20 reported 3 cases of patients abruptly discontinuing venlafaxine. All 3 cases reported “electrical discharges in the head.” The doses ranged from 37.5 mg to 225 mg daily. There was no difference in the intensity of the discontinuation-related symptomatology depending on the doses.20 Feth and colleagues21 described a patient with “electric shock-like” symptoms. A detailed neurologic examination revealed no abnormalities. The patient interpreted the symptoms as withdrawal and refused to restart the offending agent (escitalopram).21 Cortes and Radhakrishnan22 described the case of a patient who developed brain shivers after abruptly discontinuing venlafaxine. On the basis of the hypothesis that SSRI discontinuation syndrome is a result of noradrenergic imbalance, the patient was prescribed atomoxetine, resulting in the amelioration of the symptoms in a few hours.22

- In some patients, stopping or reducing the dose of an antidepressant can lead to electrical sensations (or brain zaps) perceived as occurring inside the brain.
- Brain zaps can cause varying levels of discomfort and disability.
- The pathomechanism of brain zaps is unknown but seems to be related to lateral eye movement.
METHODS
The aim of this study was to gather information as reported spontaneously by internet users about the specific symptoms experienced while having brain zaps. For this purpose, we tallied the posts made by visitors to a website, Mental Health Daily,23 which was discovered while doing an internet search with the term brain zaps.
Mental Health Daily is a sprawling and popular website devoted to a myriad of mental health issues. The site ranks in popularity within the same general range as similar websites run by laypersons (among 10,000-20,000 of the most popular). The website is a forum dedicated to posting about brain zaps, which makes it uniquely relevant to our topic.
As the individuals posting comments on a website cannot be seen either as patients or as participants, they will be referred to as “posters,” as is customary on the internet. The posts were made anonymously and with no discernible demographic information. We examined 595 posts, which were analyzed into 648 statements (a single post sometimes described 2 or 3 experiences, and these were treated separately). They were entered into a large spreadsheet wherein the rows represented the individual posts and the columns the various pieces of information extracted, such as the name of the medication and the symptom described. The posts were generated between December 13, 2014, and December 12, 2016, and fell into 3 broad categories: (1) Comments about brain zaps associated with taking medications designated as antidepressant: 378 posts. These posts were examined in detail. (2) Comments about brain zaps associated with taking medications not designated as antidepressants: 63 posts. These posts were mentioned briefly. (3) Miscellaneous posts, such as general comments, encouragements, and questions or reports involving medications considered addictive, such as benzodiazepines. These posts contained no pertinent information and were not examined.
Due to the nature of the source, all data are unverified and range from the expected to the potentially implausible. Some of the numerical data are approximations, eg, statements like “a few” and “5-6” were grouped into categories that seemed the most reasonable. The numbers are meant to convey trends and not to be seen as precise data. We examined the posts from 3 angles: the specifics of medication taking, the specifics of the zap experience itself, and the impact of the zap experience on quality of life and opinion of the psychiatric profession.
RESULTS
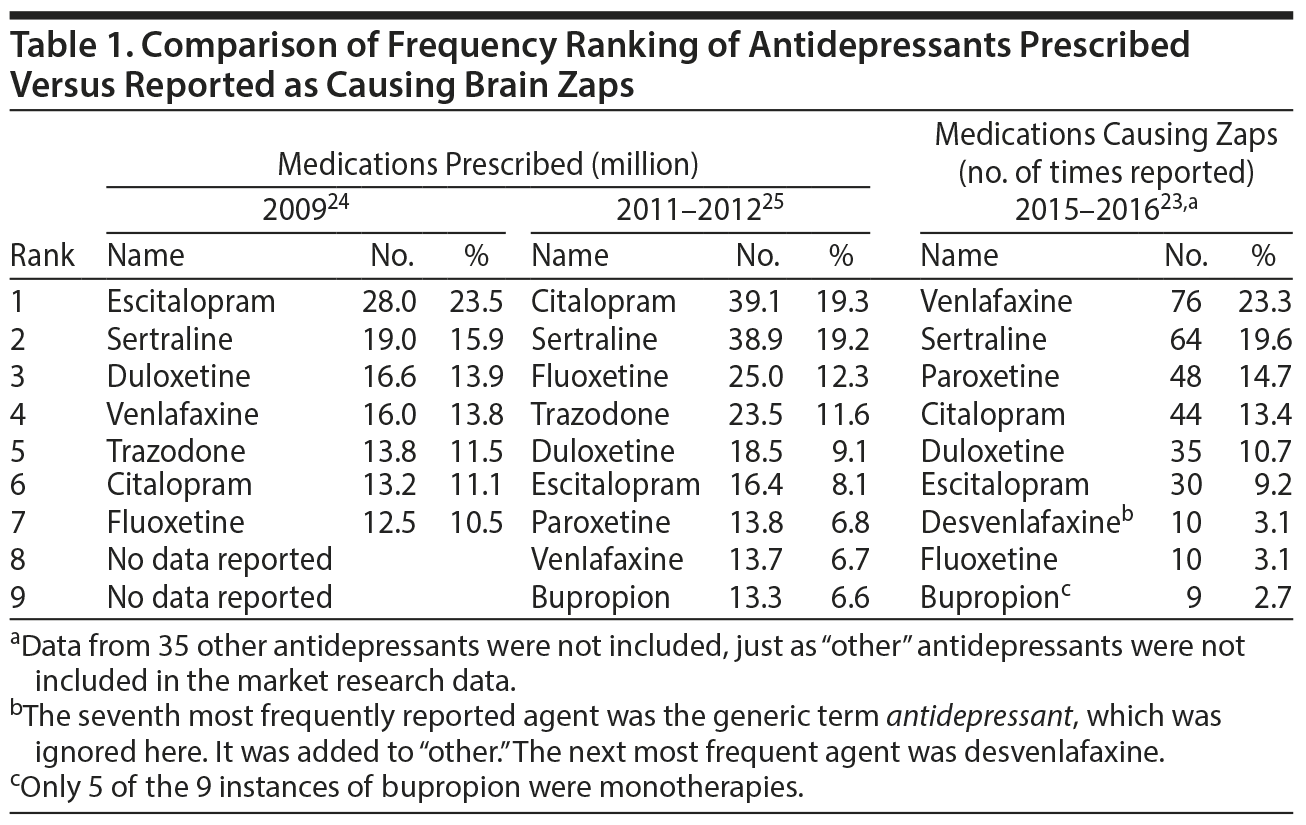
The spontaneous account of the posters roughly paralleled the findings reported in the scientific literature regarding the likelihood of an antidepressant causing withdrawal effects. Market research data from 2 sources provided information about the frequency of antidepressant prescriptions written in the United States.24,25 These data predate the brain zap data collection by a few years, but it is assumed that prescription trends did not change significantly between 2012 and 2015. The number of prescriptions ranking is contrasted with the brain zap reports ranking obtained from our sample in Table 1.
Venlafaxine contributed to 23.3% of the 9 most frequently prescribed drugs in our sample, and together with desvenlafaxine, to over a quarter (26.4%) of the reports. Fluoxetine, one of the most frequently prescribed antidepressants, was mentioned in only 3.1% of the posts. Sertraline, escitalopram, and duloxetine were reported roughly in proportion to the frequency of prescribing.
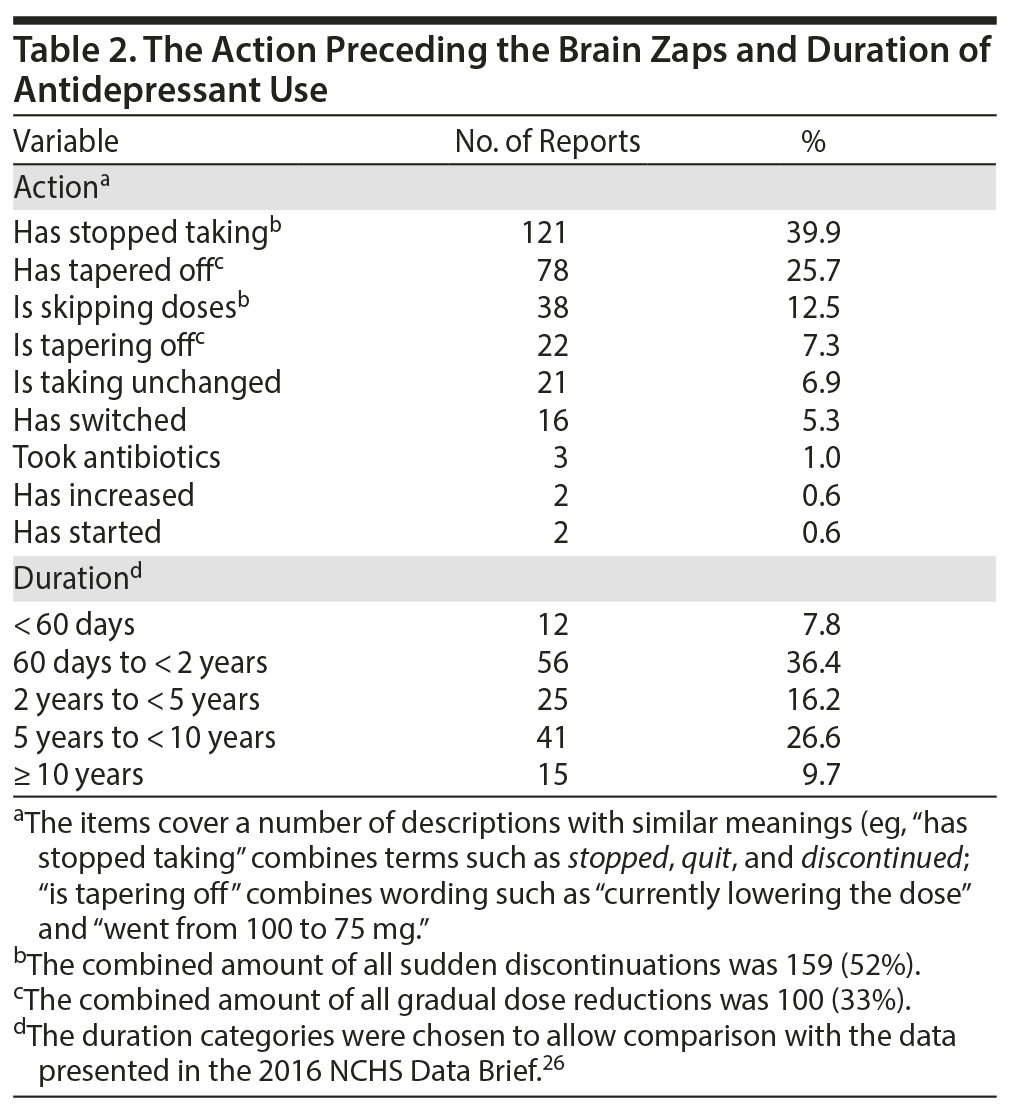
The most frequently reported change was abrupt stopping, followed by tapering (Table 2). The duration of taking antidepressants before the onset of brain zaps ranged from 2 days to 25 years, with 2 years or more reported in a little over half of the cases (Table 2).
Most patients experienced brain zaps for less than a year (122, 77% of the 159 reports), about half of whom (37% of 159 reports) experienced them for a month or less. There were 24 reports of patients experiencing brain zaps between 5 and 30 years (37, 23% of 159 reports). The most frequently reported time lags between the last dose of the medication and the first instance of a brain zap were “immediate” and “while taking,” followed by “1-2 weeks” and “20-36 hours.”
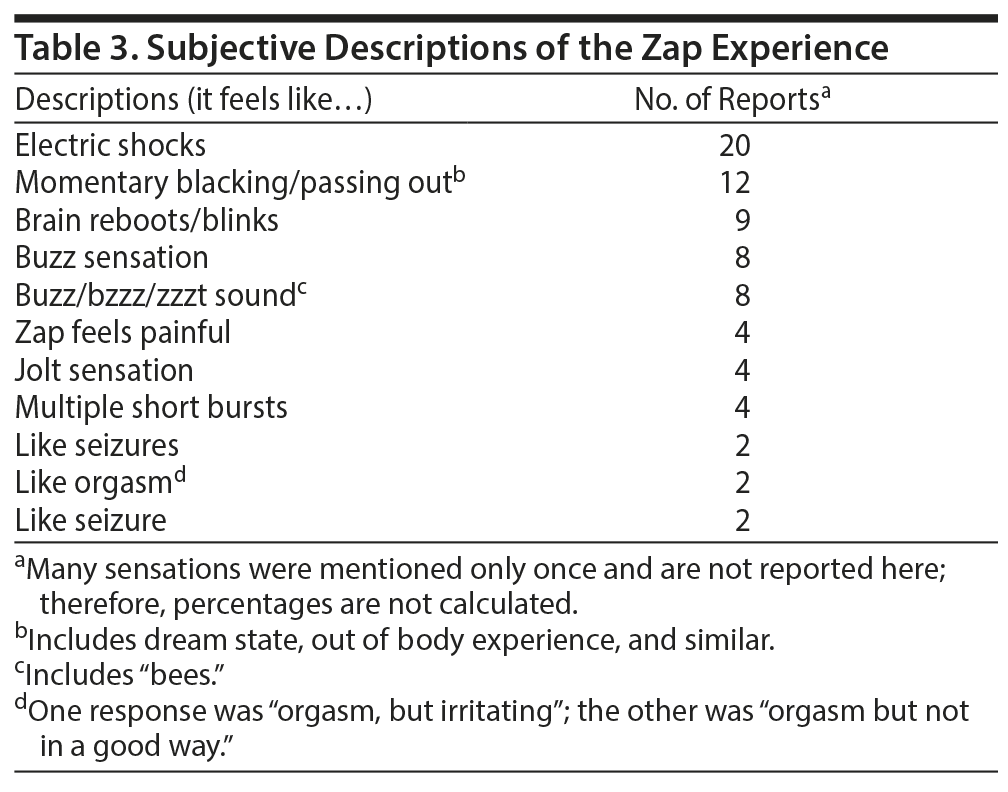
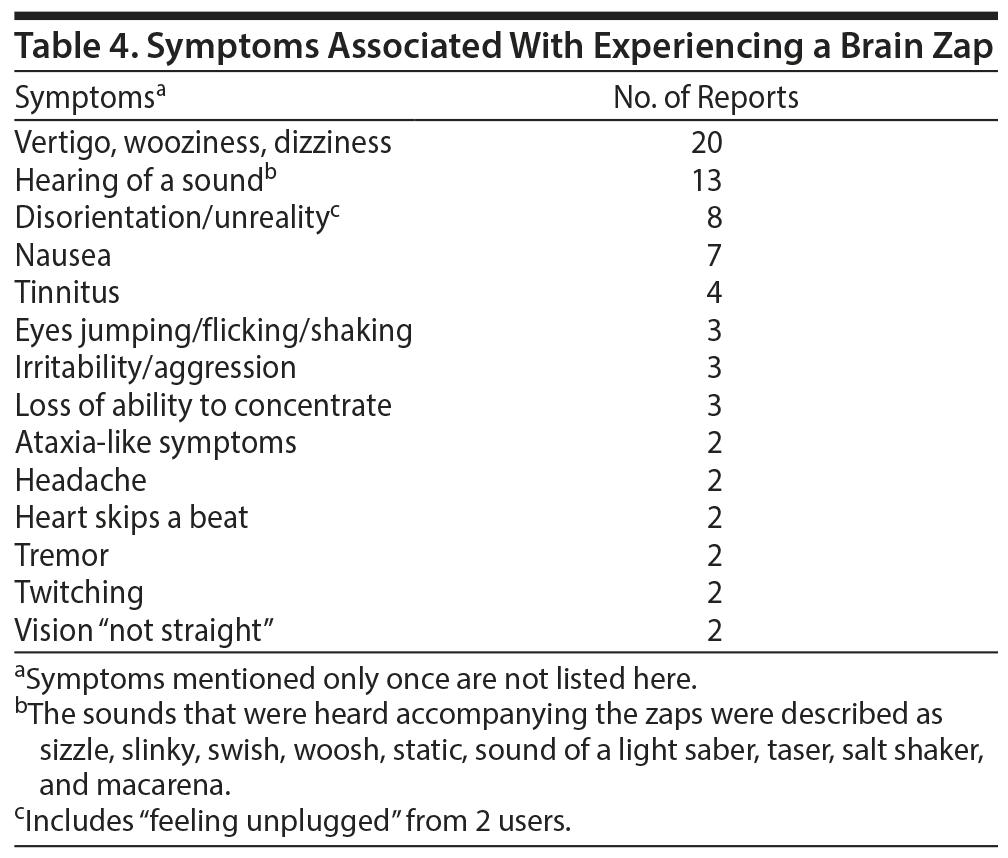
Very few posters made specific statements about the length of a brain zap, most likely because the term zap itself conveys the very brief experience. The most frequent description was “a split second.” Other descriptions were few seconds, 2 seconds, one-half-5 seconds, and 2-30 seconds, with the higher numbers described as rare extremes. The subjective experience was most often likened to an electric shock felt inside the skull. There were several reports of experiences that seem like momentary dissociations (Table 3). The zap experience most often was accompanied by vertigo, as well as the hearing of a sound, including people reporting “hearing their eyes move” (Table 4).
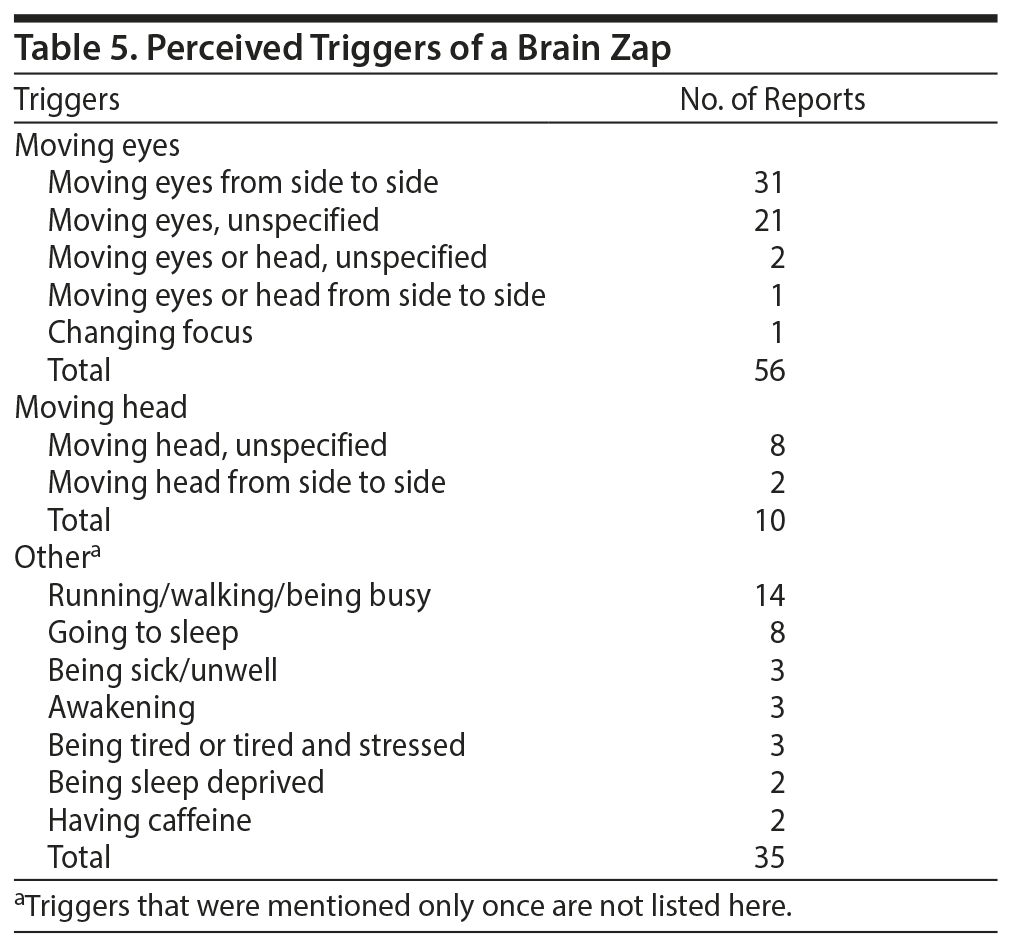
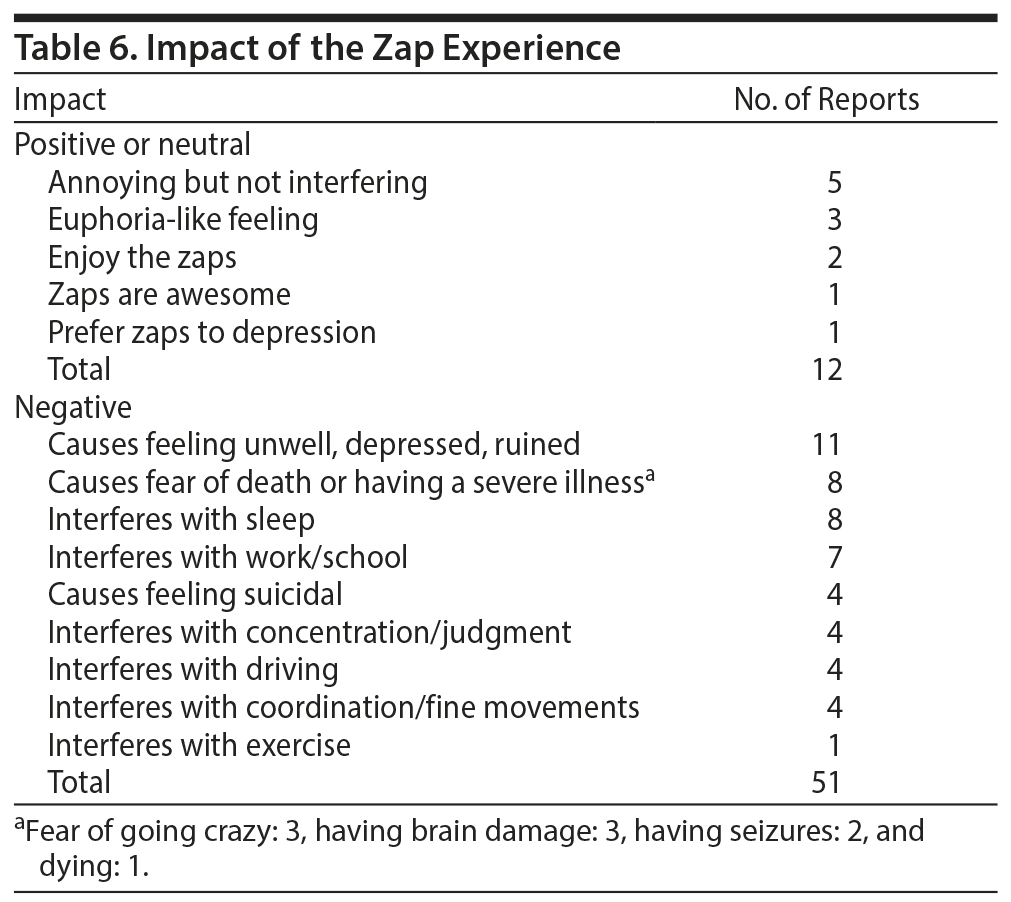
Posters did not always mention a specific trigger for the zaps, but among the posts that did mention a trigger, movements of the eyes or head were surprisingly common (Table 5). About one-sixth of the posts described the valence of their experience and its impact on their lives (Table 6) unambiguously. In what appears to be a minority of the cases, experiencing brain zaps resulted in significant disability.
In the few instances that having zaps was compared to being depressed, the following pattern was observed: rather have brain zaps than be depressed (3 posts) and rather be depressed than have brain zaps or take antidepressants again (6 posts).
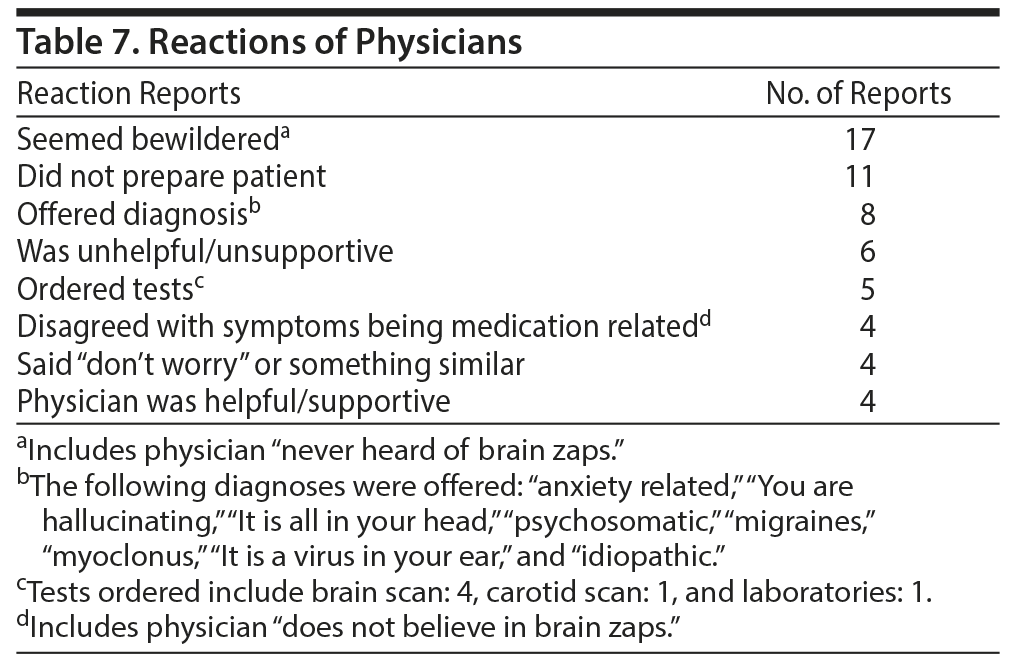
Several patients described their experience with their physicians. The descriptions of these experiences are summarized in Table 7.
Close to 50 different methods were described in the posts as attempts to alleviate the symptoms. These were activities such as exercising or relaxation and various ingestible agents ranging from chocolate to medications prescribed for other reasons. Most of the agents were mentioned only once. About half of the reports were positive, and the other half were negative in seemingly random distribution. The most frequent agents, based on recommendations of lay sites including Mental Health Daily, were various combinations of omega-3 fatty acids, B-complex preparations, and magnesium. Eight reports were negative, and 8 were partially or fully positive. Three reports of using 5-hydroxytryptophan and 2 reports of using nonsteroidal anti-inflammatory drugs were positive.
The posters’ reactions to their experiences were expressed in diverse statements, which are summarized here for their main message.
Criticizing drug companies: 13 comments. These comments ranged from criticizing “Cymbalta” that comes in a formulation that does not allow proper tapering to stating “never trust the drug companies.” Five posters wanted to sue drug companies.
Criticizing the medical profession: 5 comments. These comments ranged from expressing surprise that physicians do not seem to know about this side effect to the sentiment expressed in “I despise the psychiatric profession.” There was a general sentiment that both physicians and pharmaceutical companies choose profit over patients.
Expressing surprise about the lack of research on brain zaps: 7 comments. These comments ranged from “I hope some researcher reads all these posts” to expressing hope that class action lawsuits will force drug companies to do “compulsory research.”
Many of the posts contained statements in which the posters were lamenting their situation. These statements were typically made by the same posters who made the negative impact reports in Table 6. A few examples are as follows: “Pity so many of us have to suffer unnecessarily,” “I can’ t imagine going another 6 months with this,” “I wish I’ d never gone on this dreadful drug,” and “Three years have been stolen from me because of Prozac.”
DISCUSSION
Examination of data derived from Mental Health Daily revealed findings similar to earlier reports, as well as some findings that were unexpected. The difference in frequency distribution of medications taken versus withdrawal effects reported on the site roughly mirrors the difference in distributions reported in other studies.3-5,10,26 The importance of this finding is that it suggests that the source of our dataset, even though it represents a convenience sample, is reasonably similar to other groups of antidepressant users, and, therefore, our conclusions are likely to be meaningful. Further support for the same is provided by the observation that the overall distribution of the length of medication taking mirrors the data in the NCHS Data Brief.26
Previously published studies4,5,9 suggest that only medication half-life and abruptness of discontinuation are associated with more discontinuation events; however, our data showed that sertraline, despite having a long half-life, was the second most frequent medication associated with brain zaps. This finding indicates that half-life is perhaps not the only factor influencing brain zap events. Even though it has been suggested that gradual tapering had a dampening effect on the severity of antidepressant discontinuation symptoms,4,15 our data show that brain zaps can occur even in the presence of proper tapering techniques.
Both the existing literature4,5 and our findings suggest that brain zaps occur only for a few weeks; nevertheless, rare instances of extremely long durations have also been reported, especially on website accounts. Our data mirror the reports of longer durations, but since many posters sought out the website while still suffering from the symptoms, the total length of time reported may be skewed toward the briefer durations.
The most frequently used substance to alleviate zap symptoms was omega-3 fatty acid, but, overall, the efficacy was inconsistent and thus could not be recommended as an effective treatment. Since the pathophysiologic mechanisms offered to date in the literature are all hypothetical,15 it is not currently known how best to treat brain zaps.
Besides the SSRI discontinuation syndrome typically described in the literature, such as vertigo or nausea, we found some heretofore unreported symptoms as well, such as hearing a sound concurrently with the zaps or a sense of momentary disorientation or unreality. The sounds were invariably described as having a static or sizzling quality, with quite a few posters stating that they heard their eyes move. One of the more surprising findings from this data set, one that also has not been described in the literature, is the report of lateral eye movements acting as a trigger for the zaps—it was the most frequently described trigger on this website. Even in the case of the second most frequently reported trigger, running/walking, the movement of the head or eyes can be assumed to be involved, thus further reinforcing the involvement of eye movements. In the few instances when behaviors reducing the severity of brain zaps were reported, the actions specifically involved not moving, or fixating, the eyes. The only vaguely similar report in the literature came from Zajecka et al.3 Of the 15 case reports reviewed in the article,3 4 contained “electric sensations” and 2 contained experiences involving some unspecified abnormalities in eye movements. All 6 reports came from different patients. No connections were postulated to exist between the 2 phenomena.3
If the interest in Alzheimer’s disease symptoms is low in clinical and academic psychiatry, there is even less interest in patients’ subjective experience. Notable attention is given to the topic in publications hostile to mainstream psychiatry (eg, Whitfield27).
The ad hoc expressions of negative attitude that we found on the site echo the more formalized positions of the antipsychiatry movement, which some of our patients who feel wronged by us may feel compelled to turn to. As Nasrallah28 warns, it is important not to dismiss the ideas put forth by antipsychiatry as irrelevant, even if they seem exasperating. Instead, we should strive to understand their origination and draw motivation from them to continue the progress toward better diagnostic models and treatment approaches.
SUMMARY
Brain zaps are an infrequent, but a fairly unique and poorly understood, symptom of antidepressant discontinuation. The speed by which the biological activity of the antidepressant diminishes in the brain after discontinuation appears to mediate some but not all of the effect.
The mechanism by which individuals can experience a momentary electrical shock inside their brain is unknown. People who suffer from brain zaps can experience varying levels of functional impairment, typically very little but at times disabling. Most of the time the symptoms last for weeks to months, but some decades-long durations have also been reported. Lateral eye movements seem to be involved in triggering brain zaps. This finding may open avenues for future objective studies.
The limitation of this study is the nature of the database, which is a convenience sample of spontaneous self-reports. While the numbers obtained are not suitable for statistical analysis, they still express trends and can be used to plan more systematic analyses of the problem.
To date, little specific attention has been devoted to brain zaps from the psychiatric community, which has caused serious grievances among patients experiencing them. Further study seeking systematic and statistically analyzable data about this phenomenon is needed to advance our understanding and provide help to those who experience brain zaps.
Submitted: April 5, 2018; accepted August 28, 2018.
Published online: December 20, 2018.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors thank David Janowsky, MD, Department of Psychiatry, University of California, San Diego, for his constructive criticism and encouragement. Dr Janowsky reports no conflicts of interest related to the subject of this article.
REFERENCES
1. Kramer JC, Klein DF, Fink M. Withdrawal symptoms following discontinuation of imipramine therapy. Am J Psychiatry. 1961;118(6):549-550. PubMed CrossRef
2. Dilsaver SC. Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113-117. PubMed CrossRef
3. Zajecka J, Tracy KA, Mitchell S. Discontinuation symptoms after treatment with serotonin reuptake inhibitors: a literature review. J Clin Psychiatry. 1997;58(7):291-297. PubMed CrossRef
4. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation Consensus Panel. J Clin Psychiatry. 1997;58(suppl 7):5-10. PubMed
5. Schatzberg AF, Haddad P, Kaplan EM, et al; Discontinuation Consensus Panel. Possible biological mechanisms of the serotonin reuptake inhibitor discontinuation syndrome. J Clin Psychiatry. 1997;58(suppl 7):23-27. PubMed
6. Rosenbaum JF, Zajecka J. Clinical management of antidepressant discontinuation. J Clin Psychiatry. 1997;58(suppl 7):37-40. PubMed
7. Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242-252. PubMed CrossRef
8. Young AH, Currie A. Physicians’ knowledge of antidepressant withdrawal effects: a survey. J Clin Psychiatry. 1997;58(suppl 7):28-30. PubMed
9. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. PubMed CrossRef
10. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261. PubMed
11. Christmas DMB. “Brain shivers”: from chat room to clinic. Psychiatric Bulletin. 2005;29(6):219-221. CrossRef
12. Nielsen M, Hansen EH, G׸tzsche PC. What is the difference between dependence and withdrawal reactions? a comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction. 2012;107(5):900-908. PubMed CrossRef
13. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Third Edition. Washington, DC: American Psychiatric Association; 1980.
14. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Third Edition, Revised. Washington, DC: American Psychiatric Association; 1987.
15. Fava GA, Gatti A, Belaise C, et al. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84(2):72-81. PubMed CrossRef
16. Medawar C, Herxheimer A. A comparison of adverse drug reaction reports from professionals and users, relating to risk of dependence and suicidal behavior with paroxetine. Int J Risk Saf Med. 2004;16(2003/2004):5-19.
17. Belaise C, Gatti A, Chouinard V-A, et al. Patient online report of selective serotonin reuptake inhibitor-induced persistent postwithdrawal anxiety and mood disorders. Psychother Psychosom. 2012;81(6):386-388. PubMed CrossRef
18. Freifeld CC, Brownstein JS, Menone CM, et al. Digital drug safety surveillance: monitoring pharmaceutical products in Twitter. Drug Saf. 2014;37(5):343-350. PubMed CrossRef
19. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73. PubMed CrossRef
20. Campagne DM. Venlafaxine and serious withdrawal symptoms: warning to drivers. MedGenMed. 2005;7(3):22. PubMed
21. Feth N, Cattapan-Ludewig K, Jaquenoud Sirot E. Electric sensations: neglected symptom of escitalopram discontinuation. Am J Psychiatry. 2006;163(1):160. PubMed CrossRef
22. Cortes JA, Radhakrishnan R. A case of amelioration of venlafaxine-discontinuation “brain shivers” with atomoxetine. Prim Care Companion CNS Disord. 2013;15(2):PCC.12l01427. PubMed CrossRef
23. Mental Health Daily website. http://mentalhealthdaily.com/2014/11/29/brain-zaps-causes-treatments-for-electrical- shock-sensations/. Accessed November 19, 2018.
24. American’s Most Popular Mind Medicines. Forbes website. https://www.forbes.com/2010/09/16/prozac-xanax-valium-business-healthcare-psychiatric-drugs.html. Accessed November 19, 2018.
25. Top antidepressant drugs in the United States based on prescriptions dispensed in 2011-2012 (in 1,000s). Statista website. https://www.statista.com/statistics/242651/top-depression-drugs-in-the-us-based-on-prescriptions-dispensed-2011-2012/. Accessed November 19, 2018.
26. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. NCHS Data Brief. 2011;(76):1-8. PubMed
27. Whitfield CL. Psychiatric drugs as agents of trauma. Int J Risk Saf Med. 2010;22(4):195-207.
28. Nasrallah HA. The antipsychiatry movement: who and why. Curr Psychiatr. 2011;10(12):4, 6, 56.
This PDF is free for all visitors!