Background: Clozapine is indicated for treatment-resistant schizophrenia (TRS), but only 30%-60% of patients will respond. There have been studies of clozapine augmentation with oral second-generation antipsychotics with mixed results, but no studies considering the combination with long-acting injectable antipsychotics (LAIAs). This study is the first to attempt to establish the benefits of the combination of clozapine and LAIAs in TRS using a variety of outcome measures of symptomatology and quality of life.
Methods: A mirror-image study design was employed to review outcome measures 2 years pre and post combination of clozapine with a LAIA in a small sample of patients with chronic schizophrenia or schizoaffective disorders followed by the assertive community treatment service in the community. Outcome measures include demographic data, Brief Psychiatric Rating Scale, Clinical Global Impressions Scale-Improvement and Severity, 24-item Behavior and Symptom Identification Scale, World Health Organization Quality of Life Scale, Health of the Nation Outcome Scales, Threshold Assessment Grid, number of admissions, emergency department (ED) visits, and hospital bed days.
Results: Paired sample t tests showed a statistically significant reduction in average ED visits and hospital admissions in the 2 years post combination, with an average 1.8 fewer ED visits (95% CI, 0.58-3.02, P = .024) and a mean reduction of 0.85 hospital admissions (95% CI , 0.363-1.337, P = .008). The reduction in hospital bed days post combination was not statistically significant. Chart reviews found insufficient data for analysis of the remaining outcome measures.
Conclusions: The combination of clozapine and a long-acting injectable antipsychotic appears to reduce health care utilization in terms of ED visits and number of hospital admissions. Larger prospective studies will be required to confirm the results.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Combination of Clozapine With Long-Acting Injectable Antipsychotics in Treatment-Resistant Schizophrenia:
Preliminary Evidence From Health Care Utilization Indices
ABSTRACT
Background: Clozapine is indicated for treatment-resistant schizophrenia (TRS), but only 30%-60% of patients will respond. There have been studies of clozapine augmentation with oral second-generation antipsychotics with mixed results, but no studies considering the combination with long-acting injectable antipsychotics (LAIAs). This study is the first to attempt to establish the benefits of the combination of clozapine and LAIAs in TRS using a variety of outcome measures of symptomatology and quality of life.
Methods: A mirror-image study design was employed to review outcome measures 2 years pre and post combination of clozapine with a LAIA in a small sample of patients with chronic schizophrenia or schizoaffective disorders followed by the assertive community treatment service in the community. Outcome measures include demographic data, Brief Psychiatric Rating Scale, Clinical Global Impressions Scale-Improvement and Severity, 24-item Behavior and Symptom Identification Scale, World Health Organization Quality of Life Scale, Health of the Nation Outcome Scales, Threshold Assessment Grid, number of admissions, emergency department (ED) visits, and hospital bed days.
Results: Paired sample t tests showed a statistically significant reduction in average ED visits and hospital admissions in the 2 years post combination, with an average 1.8 fewer ED visits (95% CI, 0.58-3.02, P = .024) and a mean reduction of 0.85 hospital admissions (95% CI , 0.363-1.337, P = .008). The reduction in hospital bed days post combination was not statistically significant. Chart reviews found insufficient data for analysis of the remaining outcome measures.
Conclusions: The combination of clozapine and a long-acting injectable antipsychotic appears to reduce health care utilization in terms of ED visits and number of hospital admissions. Larger prospective studies will be required to confirm the results.
Prim Care Companion CNS Disord 2020;22(4):19m02560
To cite: Grimminck R, Oluboka O, Sihota M, et al. Combination of clozapine with long-acting injectable antipsychotics in treatment-resistant schizophrenia: preliminary evidence from health care utilization indices. Prim Care Companion CNS Disord. 2020;22(4):19m02560.
To share: https://doi.org/10.4088/PCC.19m02560
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Foothills Medical Centre, University of Calgary, Calgary, Alberta, Canada
bDepartment of Psychiatry, South Health Campus, University of Calgary, Calgary, Alberta, Canada
cDecision Support Team, Addiction and Mental Health, Alberta Health Services—Calgary Zone, Calgary, Alberta, Canada
dDepartment of Psychiatry, Assertive Community Treatment Service, Calgary, Alberta, Canada
*Corresponding author: Rachel Grimminck, MD, FRCPC, DABPN, Department of Psychiatry, University of Calgary Cumming School of Medicine, 1403 – 29 St NW, Calgary, AB, Canada T2N 4N1 ([email protected]).
Schizophrenia is a devastating, serious, and persistent mental illness, costing untold millions of dollars in lost productivity for those afflicted.1 Successful management is complicated by a subset of patients who are deemed “treatment refractory” after failure to appropriately respond to at least 2 trials of different antipsychotics.2 Studies3,4 have suggested that clozapine may be superior to other second-generation antipsychotics (SGAs) in controlling symptoms that are not responsive to conventional or first-generation antipsychotics (FGAs) in patients with chronic schizophrenia. Studies5,6 indicate that approximately 30%-50% of patients with schizophrenia do not respond to older antipsychotics such as chlorpromazine or haloperidol. In such cases, a trial with an SGA is typically warranted. However, because of clozapine’s burden of serious side effects, debate remains as to whether multiple trials involving some or all of the SGAs should be undertaken before treating a patient with clozapine.7
There is consistent evidence that clozapine is the most effective antipsychotic for severe refractory schizophrenia; approximately 30%-60% of people with schizophrenia who fail to respond to other antipsychotics may respond to clozapine.8-12 Although not all studies of clozapine in first-episode patients have found evidence of superiority over other antipsychotics,13 some research14,15 suggests that clozapine can be effective for first-episode patients whose psychotic illness fails to remit during the early months of treatment. Moreover, there are claims of other benefits with the use of clozapine, including improvement in cognitive function,16-19 reduction in suicidality,20,21 relief of caregiver burden,22 decrease in cigarette smoking,23 and, possibly, less need for adjunctive medications.24,25 There is also relatively strong evidence for antihostility and antiaggression with the use of clozapine independent of its efficacy or improvement in side effects such as akathisia.26-29
The current guidelines for the treatment of schizophrenia acknowledge the role of clozapine in the management of treatment-resistant schizophrenia (TRS) and treatment of residual symptoms, persistent violent behaviors, and suicidal thoughts or behaviors.30,31 The Canadian clinical practice guidelines for the treatment of schizophrenia,32 in addition to the preceding indications, also acknowledge the beneficial role of clozapine in patients with tardive dyskinesia and the need for earlier use of clozapine in the course of schizophrenia. The Texas Medication Algorithm Project33 recommends that 2 trials of other antipsychotics should precede a clozapine trial, but earlier use of clozapine should be considered in patients with a history of recurrent suicidality, violence, or comorbid substance abuse. The recommendations suggest that the persistence of positive symptoms > 2 years warrants and > 5 years requires a clozapine trial, independent of number of preceding antipsychotic trials. Furthermore, guidelines and consensus statements have consistently recommended the routine use of a single antipsychotic drug at a standard dose in routine practice.34-39
Despite the robust evidence for efficacy in TRS, the therapeutic response to clozapine may be inadequate. Only 30%-60% of patients with TRS will respond satisfactorily.4,9 The clinical implication is that a significant number of patients with TRS would require additional measures to achieve optimum therapeutic benefits. The period recommended for an adequate trial of clozapine varies from 4 to 12 months.40-43 Schulte42 reviewed studies of plasma drug monitoring and the time to response and concluded that an adequate trial should be a duration of at least 8 weeks. However, response may take longer in a proportion of patients. Meltzer41 reported that 30% of TRS patients treated with clozapine responded by 6 weeks, another 20% by 3 months, and a further 10% to 20% by 6 months. Some of the patients responding at 3 and 6 months had the greatest reductions in BPRS scores at 12 months.41 Therefore, Kerwin and Bolonna43 concluded that it would be reasonable to test clozapine monotherapy for 6 months.
When there is inadequate response to clozapine, it is imperative to consider a number of factors before adding another agent. These factors include adherence, side effect burden, ongoing substance use, lack of psychosocial rehabilitation, and comorbidities such as depression. Factors influencing clozapine plasma levels may also contribute to inadequate response, poor tolerability, and side effects. These factors include daily dose, age, sex, and drug-drug interactions including the effects of caffeine and cigarette smoking.23,44 The target plasma clozapine level to ensure an adequate trial is generally considered to be 350 ng/mL (1,070 nmol/L), although clinical response is commonly seen at lower levels.45,46 Anecdotal observations by clinicians have noted that change in clinical status from inpatient to outpatient may contribute to loss of therapeutic response to clozapine. This loss of therapeutic response is largely attributed to lifestyle changes, including resumption or increased cigarette smoking, nonadherence, and differences in the level of psychosocial support and supervision.
There are some indications that clozapine augmentation with a second antipsychotic may be more effective for the reduction of negative symptoms rather than positive symptoms.47,48 When choosing the augmenting antipsychotic, consideration should be given to antipsychotics with a complementary receptor profile to clozapine and a side effect profile that minimizes compounding recognized problems with clozapine such as sedation and metabolic side effects.49 Risperidone has been the most studied antipsychotic as an adjunct to clozapine in randomized controlled trials (RCTs).48,50-53 However, RCTs have also investigated the addition of sulpiride, amisulpride, or aripiprazole to clozapine,47,54-56 and there are relevant open trials and case reports57,58 regarding add-on treatment with ziprasidone. The evidence from these studies is inconsistent and largely inconclusive. A Cochrane review59 examined 3 RCTs of clozapine combination with risperidone, sulpiride, ziprasidone, or quetiapine but could not draw a conclusion as to which particular combination was superior. Another Cochrane review60 that specifically addressed sulpiride add-on to clozapine concluded that while the combination may be more effective than clozapine alone in this context, more robust data were required. Meta-analyses61-63 of larger numbers of relevant clinical trials have concluded that the expected benefit is at best modest and may not be evident for at least 10 weeks of treatment.
Despite the obvious potential for increased nonadherence with oral antipsychotic combination treatment, no study has explored the presumed benefits of the combination of long-acting injectable antipsychotics (LAIAs) and clozapine. Benefits of LAIAs over oral antipsychotics include assurance of medication delivery, convenience, early identification of nonadherence, decreased frequency of dosing, and decreased oral polypharmacy. Recommendations have been made for use of LAIAs in all phases of treatment, especially in the setting of partial adherence or nonadherence.64 LAIAs were also shown to prevent hospitalization in a meta-analysis and systematic review of mirror image studies by Kishimoto et al.65 Two reviews66,67 confirm that LAIAs may lead to a reduction in hospitalization and health care utilization as well as improved quality of life.
Thus, the study objective was to evaluate the effectiveness of the combination of LAIAs and clozapine in the management of TRS using a variety of outcome measures of symptomatology, health care utilization, and quality of life.

- The combination of clozapine and a long-acting injectable antipsychotic (LAIA) leads to lower health care utilization including emergency department visits and psychiatric admissions.
- The combination of clozapine with an LAIA showed a trend toward improved quality of life for patients with schizophrenia and schizoaffective disorder.
METHODS
Sample
The study sample comprised outpatients attending the assertive community treatment (ACT) team clinics in Calgary, Alberta, Canada. ACT teams provide intensive outreach services to adults with severe and persistent mental illness who were unable to engage in traditional mental health services. The inclusion criteria were patients over the age of 18 years meeting criteria for schizophrenia or schizoaffective disorder according to the DSM-5 with or without comorbid substance use disorders who have shown a partial response to clozapine or an LAIA and are currently on the combination of clozapine and an LAIA. Patients with comorbid substance use disorders were included given the high level of comorbidity with psychotic disorders and to improve the external validity of the study. Similarly, patients currently taking mood stabilizers, benzodiazepines, anticholinergics, and antidepressants were included to reflect real-world polypharmacy necessary for symptom control in this complex population. Patients were included regardless of their history of nonadherence or partial adherence given that suboptimal adherence is common in chronic psychotic illness. Finally, we included patients whether or not they were mandated to comply with the treatment plan per a community treatment order (CTO) under the mental health act of Alberta. Augmentation with either FGA or SGA LAIA was also included.
Exclusion criteria include age > 65 years, comorbid dementia, or primary diagnosis of substance use. In this mirror image study and chart review, patients on clozapine with poorly controlled symptoms who did not receive an LAIA were not included in the analysis, as the subjects were defined a priori to include patients on combination treatment only.
Ethics approval was obtained from the Conjoint Health Research Ethics Board at the University of Calgary on June 6, 2014 and renewed yearly thereafter.
Study Design
This retrospective observational study used a mirror image (or pre/post) design to compare measures pre and post the addition of an LAIA to clozapine or vice versa. The mirror date is the date of starting the combination of clozapine and an LAIA. We reviewed data retrospectively on outcome measures 2 years pre and 2 years post combination. In the mirror image study design, the patient serves as their own control with the only new variable being the intervention of the combination treatment. This study design is effective for this complex patient population involved in the highest intensive level of community follow-up pre and post research inclusion, hence the intensive level of care and strategies are controlled for.
Outcome Measures
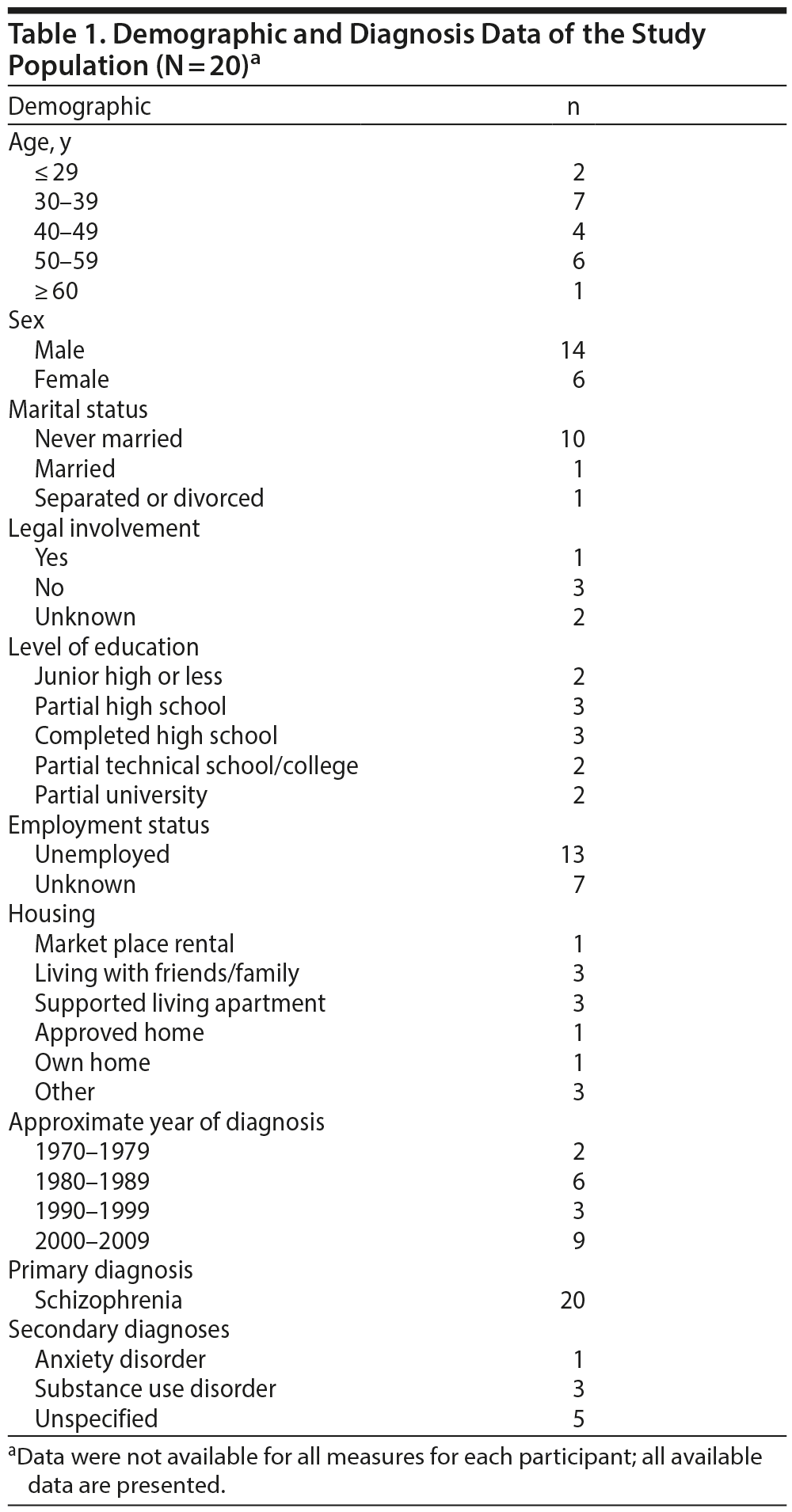
We reviewed charts and ACT databases to obtain basic demographic data such as the patient’s age at the time of starting combination treatment, sex, number of years at ACT, primary diagnosis, secondary diagnosis (if applicable), medical conditions, level of educational attainment, marital status, employment status, current living situation, legal involvement, and CTO status. These data are presented in Table 1.
ACT collects the following outcome measures for clients at intake and then yearly: Brief Psychiatric Rating Scale (BPRS),68 Clinical Global Impressions Scale-Improvement and Severity (CGI-I and CGI-S),69 24-item Behavior and Symptom Identification Scale (BASIS-24),70 World Health Organization Quality of Life Scale (WHO-QOL),71 Health of the Nation Outcome Scales (HoNOS),72 and Threshold Assessment Grid (TAG).73 Data on hospital bed days, number of hospitalizations, and emergency department (ED) visits were available through Alberta Health Services health care utilization data systems.
For this study, outcome measures in the 2 years pre and post combination were included in the statistical analysis. If the outcome measures had not been updated in the 2 years prior to combination treatment, the last instance of this measure was included. Data were included pre and post combination for a mean ± SD of 24 ± 4 months to account for unforeseen circumstances that might have led to a delay in completing scales for treatment planning. In all cases, however, we included only 2 data points pre and post combination.
Statistical Analyses
Paired sample t tests were conducted for within-patient comparison of number of ED visits, number of urgent care visits, number of hospital admissions, and number of days in mental health inpatient units in the 2 years pre and post combination of clozapine and an LAIA. HoNOS data were compared for 5 patients pre and post combination, but data were insufficient for statistical analysis.
RESULTS
Study Population
Twenty ACT patients were currently treated with a combination of clozapine and an LAIA. Detailed demographic data are outlined in Table 1. No patients were lost to follow-up. The mean age was 43 years. The primary diagnosis was schizophrenia for all patients. The average year of diagnosis was 1994 with a range of 1979 to 2009. The mean number of years at ACT was 4.85 years with a range of 1 to 10 years. Eight patients were on a CTO at some point during the study period. Medical conditions included traumatic brain injury (2 patients), thyroid disorders (2 patients), seizure disorders (3 patients), and hypertension (1 patient). Social supports were mostly a combination of professional and personal or professional only for most clients for whom data were available. One patient was involved in volunteer work in the year prior to the combination. No patients were students during the study period.
All patients post combination were taking clozapine with a depot antipsychotic. Fifteen patients were on an atypical antipsychotic depot (risperidone: 8, paliperidone: 6, and aripiprazole: 1) and 5 were on a typical antipsychotic depot (zuclopenthixol: 3, flupenthixol: 1, and fluphenazine: 1).
With respect to smoking and alcohol and drug use pre and post combination, there appears to be a trend toward reduction in alcohol and drug use post combination. Given that only 3 patients (Table 1) were diagnosed with a substance use disorder, the sample size was too small to determine if the presence or absence of a substance use disorder influenced outcomes related to the medication combination.
Health Care Utilization
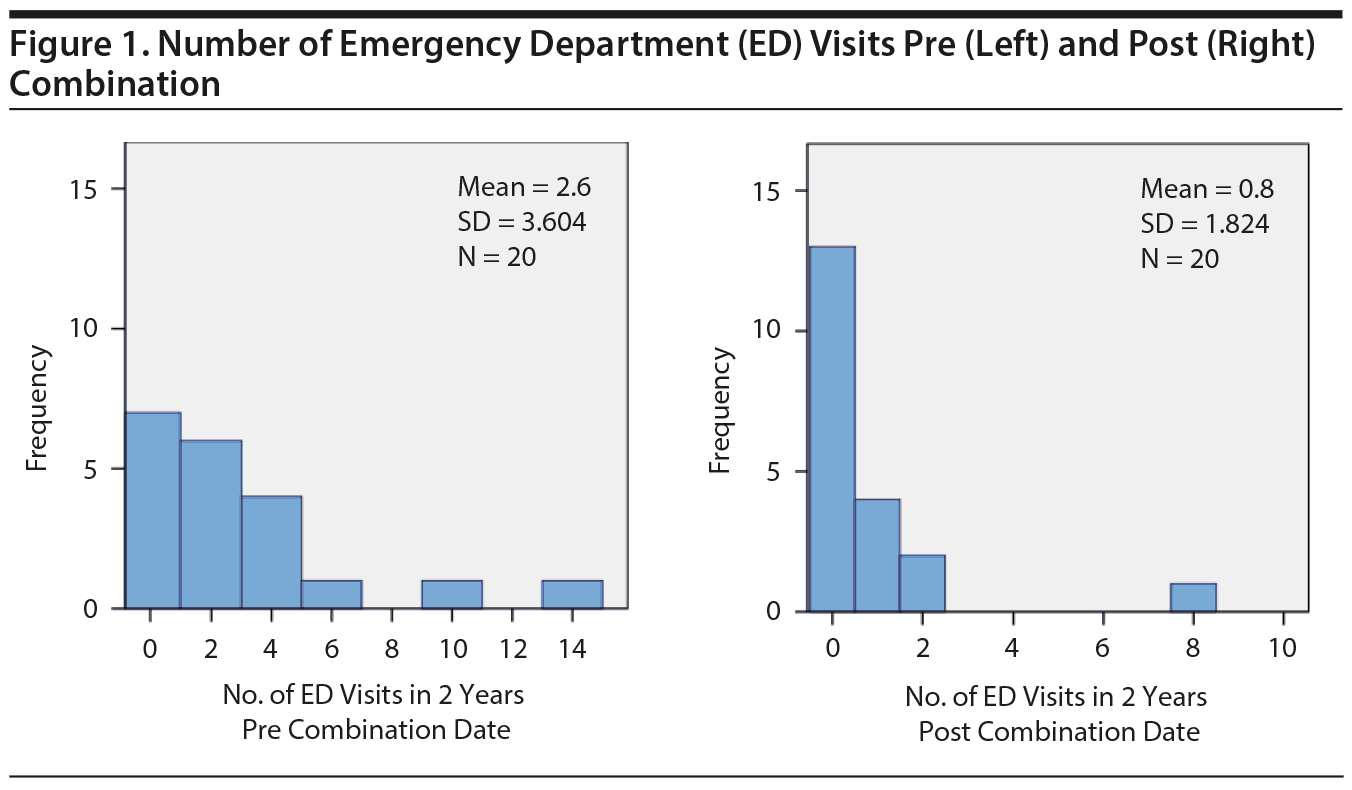
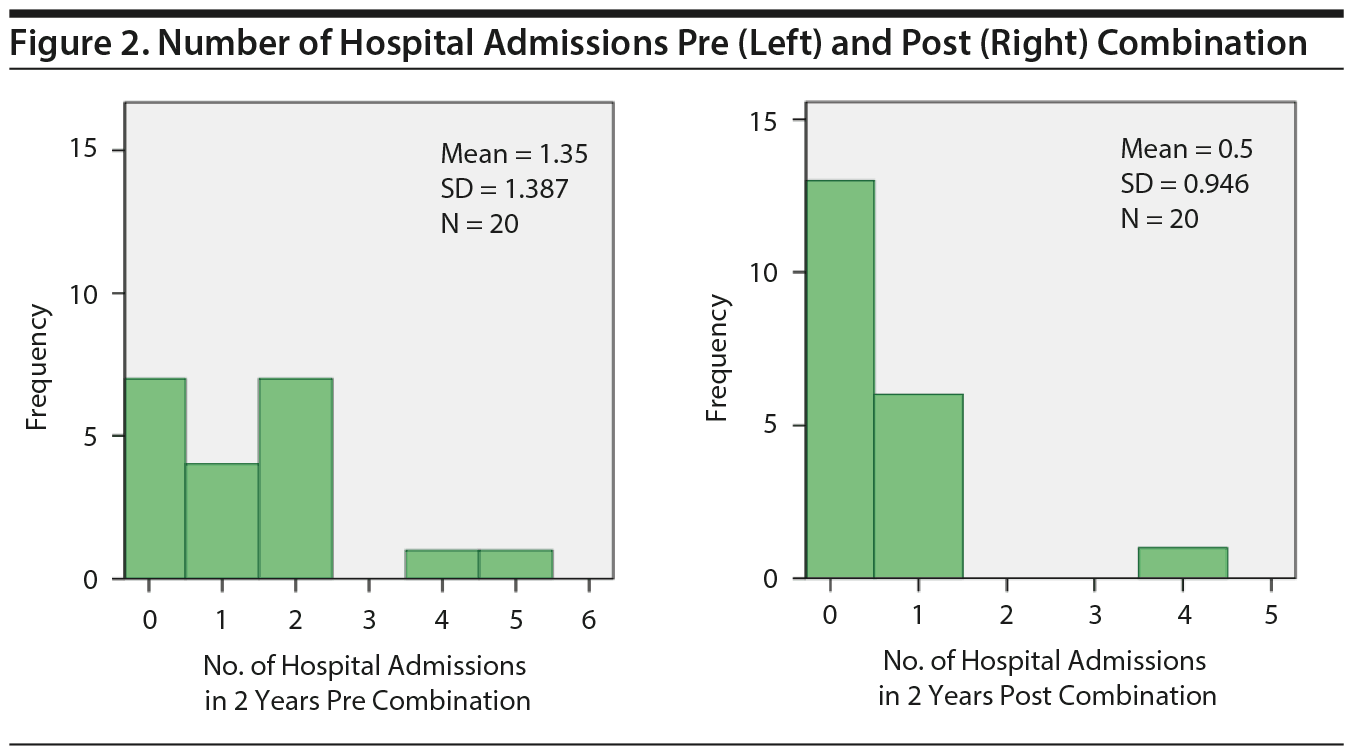
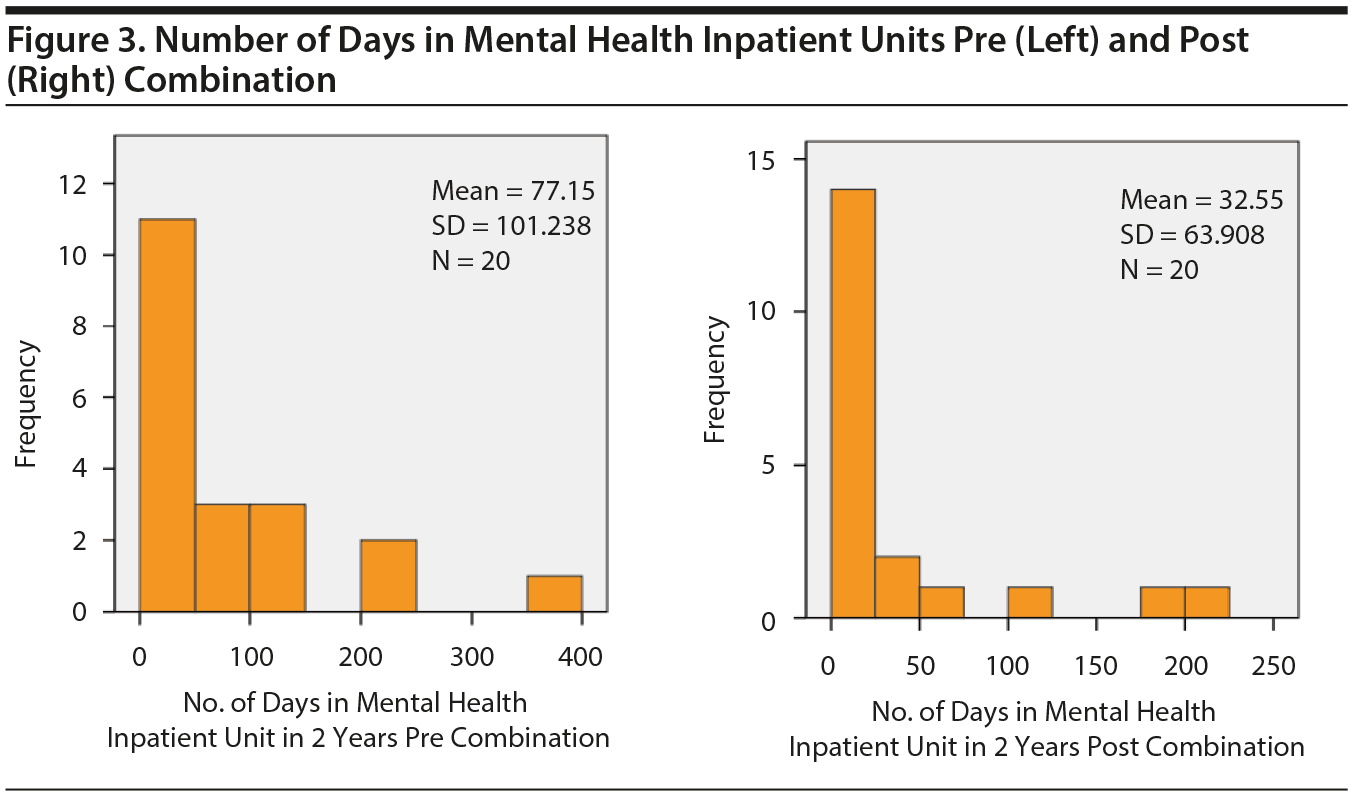
Paired sample t tests showed a statistically significant reduction in mean number of ED visits in the 2 years post combination with a mean of 1.8 fewer ED visits (Figure 1) (95% CI , 0.58-3.02, P = .024). There was also a statistically significant reduction in number of hospital admissions in the 2 years post combination with a mean reduction of 0.85 admissions (Figure 2) (95% CI , 0.363-1.337, P = .008). The reduction in hospital bed days post combination was not statistically significant; however, there was a decrease in hospital bed days, which is shown in Figure 3.
Psychopathology and Quality of Life
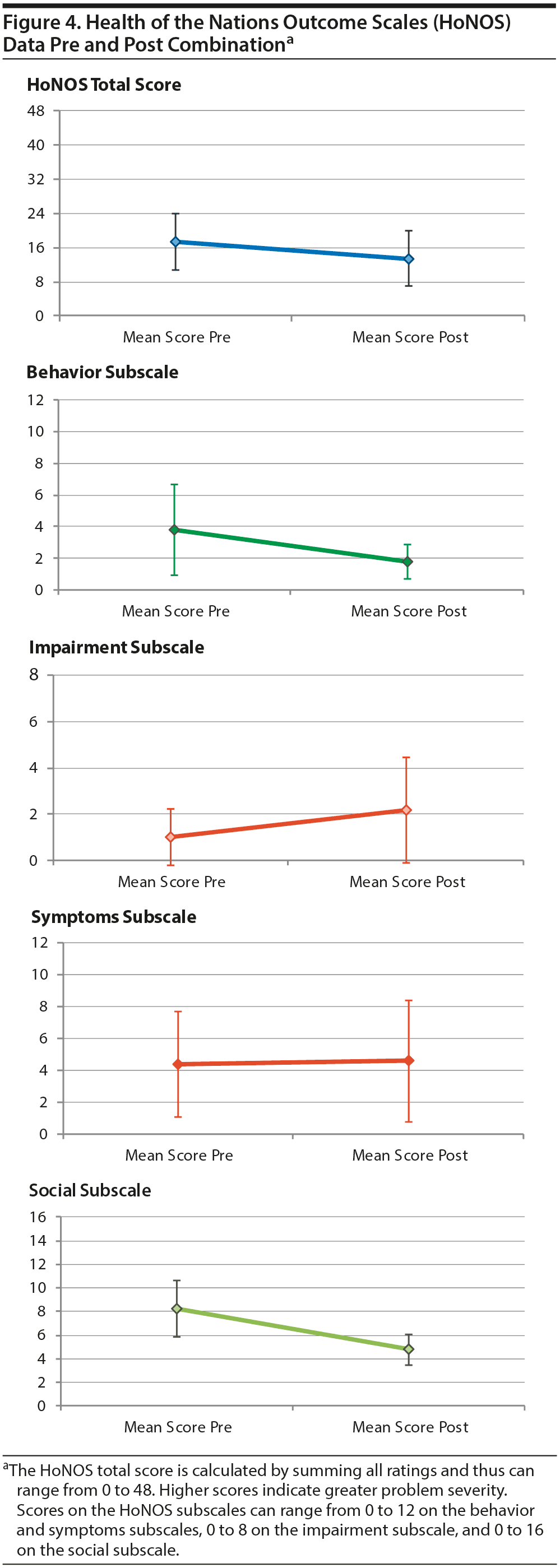
HoNOS is tracked at admission and discharge to hospital and outpatient programs. Similarly, presentations to the ED, hospital admissions, and hospital bed days are tracked systematically. The outcome measures for psychopathology and quality of life are tracked by clinicians at ACT when the patient is admitted to ACT and ideally yearly thereafter. Data were available for all 20 patients with respect to ED visits, hospital admissions, and hospital bed days in the 2 years pre and post combination. However, HoNOS data were available for 14 of the 20 clients, but only 5 had at least 1 data point within both the pre and post combination periods. There were significant missing data for the psychopathology and quality of life measures. A detailed chart review found insufficient data for analysis of BPRS, CGI-I, CGI-S, BASIS-24, WHO-QOL, and TAG scores.
Figure 4 illustrates HoNOS total and subscale scores pre and post combination for a total of 5 patients for whom data were available 1 year pre and 1 year post combination. The total mean HoNOS score decreased in the 1 year post combination, as did the behavior and social subscales of the HoNOS. The symptoms subscale, which includes hallucinations and delusions, depressed mood, and other mental and behavioral problems, was essentially unchanged pre and post combination. The impairment subscale, which includes cognitive and physical impairments, was slightly worse post combination, which may be related to side effects from antipsychotic medication including sedation and weight gain.
DISCUSSION
The combination of an LAIA and clozapine appears to decrease health care utilization, particularly number of ED visits and inpatient admissions. In Calgary, 1 ED visit costs approximately Can $257.40 (US $189.81) for nursing costs alone (based on 2013 data, as more recent figures were unavailable). Physician and allied health care provider costs are not systematically tracked in Alberta Health Services. Admission costs, on average, Can $810.62 (US $597.80) per psychiatric hospital bed day (range, Can $618.55-$1181.31 [US $456.08-871.01]) for nursing and overhead expenses alone, again not including physician and allied health practitioner costs.
As Figure 4 illustrates, there was an overall improvement in mean total HoNOS score as well as in the behavior and social subscale scores. The improvement in the HoNOS behavior subscale scores may be attributed to the suicide and aggression reduction properties of clozapine.27-30 The improvement in the HoNOS social subscale scores indicates patients on this combination appear to be managing better in the community and experiencing enhanced quality of life. Combining clozapine with an LAIA can allow for lower clozapine doses and improved tolerability. This combination also reduces oral antipsychotic polypharmacy, which may also improve adherence. For this group of patients, the symptoms scale was unchanged, which may reflect the chronic nature of psychopathology with a psychotic disorder as well as medication side effects. The impairment subscale is a difficult category to interpret given that it includes both cognitive and physical difficulties. Overall, the mean impairment score rose, which may reflect aging and physical side effects. However, the 2-year window post combination may be too brief to accurately reflect improvement given that clozapine can take 6 to 12 months to reach full efficacy.
Limited data were available from the charts with respect to remaining measures of psychopathology and quality of life. However, it appears this patient population remains chronically psychotic even while on this combination, but their acuity may be reduced, thus leading to few presentations to acute care and possibly greater quality of life.
Limitations to our study include the retrospective nature of the study design, a small sample size, and the limited amount of data available with respect to quality of life. Potential confounding factors include other interventions the patients were receiving, which may have contributed to decreased health care utilization; these include but are not limited to CTOs, interventions of the ACT team including closer follow-up after combination initiation, and the effects of other medications. Nonadherence or partial adherence with medications or changes in adherence also may have affected the results. Finally, the effects of comorbid depression and anxiety were not explored in this study.
In conclusion, the combination of clozapine and a LAIA appears to reduce health care utilization in terms of ED visits and number of hospital admissions. Future research will investigate the effects of this combination on psychopathology and health-related quality of life outcomes in this patient population in a prospective manner.
Submitted: October 27, 2019; accepted February 12, 2020.
Published online: July 16, 2020.
Potential conflicts of interest: Dr Oluboka has served on advisory boards or similar committees for Janssen, Pfizer, Lundbeck, Bristol-Myers Squibb, Sunovion, and Otsuka; participated in clinical trials or studies for Otsuka and Lundbeck; received honoraria or other fees for Janssen, Lundbeck, Pfizer, Bristol-Myers Squibb, Otsuka, and Sunovion; and received research grants from AstraZeneca, Lundbeck, and Otsuka. Drs Grimminck and Yeung, Mr Sihota, and Ms Rutherford report no conflicts of interest related to the subject of this article.
Funding/support: This study was supported by the University of Calgary Department of Psychiatry Mental Health Research Funding Competition for Trainees in 2014 in the amount of $5,000. The University of Calgary is located in Calgary, Alberta, Canada.
Role of the sponsor: The supporters had no role in the design, analysis, interpretation, or publication of this study.
Previous presentation: These findings have been presented in 2 separate 15-minute presentations at the University of Calgary Sebastian K. Littman Research Day; March 13, 2015; Calgary, Alberta, Canada, and Alberta Psychiatric Association Annual Conference; March 19-22, 2015; Banff, Alberta, Canada, as well as in a poster format at International College of Neuropsychopharmacology Collegium Internationale Neuropsychopharmacologicum; July 3-5, 2016; Seoul, South Korea.
Acknowledgments: The authors thank Brian Marriott, MSc (Addiction and Mental Health, Alberta Health Services Calgary Zone, Alberta, Canada), for help with cost-saving approximations; Tolulope Sajobi, PhD (Community Health Sciences, Clinical Neurosciences, University of Calgary, Calgary, Alberta, Canada), for input with respect to the statistical analysis; and the ACT staff who helped by ensuring charting was up to date and complete. Mr Marriott and Dr Sajobi report no conflicts of interest related to the subject of this article.
REFERENCES
1. Gutierrez-Recacha P, Chisholm D, Haro JM, et al. Cost-effectiveness of different clinical interventions for reducing the burden of schizophrenia in Spain. Acta Psychiatr Scand suppl. 2006;114(432):29-38. PubMed CrossRef
2. Kerwin R. When should clozapine be initiated in schizophrenia? some arguments for and against earlier use of clozapine. CNS Drugs. 2007;21(4):267-278. PubMed CrossRef
3. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry. 2002;159(2):255-262. PubMed CrossRef
4. Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158(4):518-526. PubMed CrossRef
5. Altamura AC, Bassetti R, Cattaneo E, et al. Some biological correlates of drug resistance in schizophrenia: a multidimensional approach. World J Biol Psychiatry. 2005;6(suppl 2):23-30. PubMed CrossRef
6. Kinon BJ, Kane JM, Johns C, et al. Treatment of neuroleptic-resistant schizophrenic relapse. Psychopharmacol Bull. 1993;29(2):309-314. PubMed
7. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56. PubMed
8. Essali A, Al-Haj Haasan N, Li C, et al. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;(1):CD000059. PubMed
9. Iqbal MM, Rahman A, Husain Z, et al. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33-48. PubMed CrossRef
10.Kane JM. Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry suppl. 1992;160(17):41-45. PubMed CrossRef
11.Tandon R, Belmaker RH, Gattaz WF, et al; Section of Pharmacopsychiatry, World Psychiatric Association. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res. 2008;100(1-3):20-38. PubMed CrossRef
12.Kane JM, Marder SR, Schooler NR, et al. Clozapine and haloperidol in moderately refractory schizophrenia: a 6-month randomized and double-blind comparison. Arch Gen Psychiatry. 2001;58(10):965-972. PubMed CrossRef
13.Woerner MG, Robinson DG, Alvir JM, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516. PubMed CrossRef
14.Agid O, Remington G, Kapur S, et al. Early use of clozapine for poorly responding first-episode psychosis. J Clin Psychopharmacol. 2007;27(4):369-373. PubMed CrossRef
15.Szymanski S, Masiar S, Mayerhoff D, et al. Clozapine response in treatment-refractory first-episode schizophrenia. Biol Psychiatry. 1994;35(4):278-280. PubMed CrossRef
16.Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159(6):1018-1028. PubMed CrossRef
17.Purdon SE, Labelle A, Boulay L. Neuropsychological change in schizophrenia after 6 weeks of clozapine. Schizophr Res. 2001;48(1):57-67. PubMed CrossRef
18.Remington G. Tardive dyskinesia: eliminated, forgotten, or overshadowed? Curr Opin Psychiatry. 2007;20(2):131-137. PubMed CrossRef
19.Small JG, Milstein V, Marhenke JD, et al. Treatment outcome with clozapine in tardive dyskinesia, neuroleptic sensitivity, and treatment-resistant psychosis. J Clin Psychiatry. 1987;48(7):263-267. PubMed
20.Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190. PubMed CrossRef
21.Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91. PubMed CrossRef
22.Conley RR. Optimizing treatment with clozapine. J Clin Psychiatry. 1998;59(suppl 3):44-48. PubMed
23.de Leon J. Atypical antipsychotic dosing: the effect of smoking and caffeine. Psychiatr Serv. 2004;55(5):491-493. PubMed CrossRef
24.Chong SA, Remington GJ, Bezchlibnyk-Butler KZ. Effect of clozapine on polypharmacy. Psychiatr Serv. 2000;51(2):250-252. PubMed CrossRef
25.Glick ID, Zaninelli R, Hsu C, et al. Patterns of concomitant psychotropic medication use during a 2-year study comparing clozapine and olanzapine for the prevention of suicidal behavior. J Clin Psychiatry. 2004;65(5):679-685. PubMed CrossRef
26.Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514. PubMed CrossRef
27.Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228. PubMed CrossRef
28.Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629. PubMed CrossRef
29.Volavka J, Citrome L. Heterogeneity of violence in schizophrenia and implications for long-term treatment. Int J Clin Pract. 2008;62(8):1237-1245. PubMed CrossRef
30.Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103. PubMed CrossRef
31.National Collaborating Centre for Mental Health (UK). Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care (Update). Leicester, UK: British Psychological Society. 2009.
32.Canadian Psychiatric Association. Clinical Practice Guidelines. Treatment of Schizophrenia. Can J Psychiatry. 2005;50(suppl 1):7S-57S. PubMed
33.Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68(11):1751-1762. PubMed CrossRef
34.American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Schizophrenia. Second Edition. Psychiatry online website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2004. Accessed June 2020.
35.Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93. PubMed CrossRef
36.The Canadian Psychiatric Association. Canadian clinical practice guidelines for the treatment of schizophrenia. Can J Psychiatry. 1998;43(suppl 2):25S-40S. PubMed
37.American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997;154(suppl 4):1-63. PubMed CrossRef
38.Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for the Treatment of Schizophrenia and Related Disorders. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for the Treatment of Schizophrenia and Related Disorders. Aust N Z J Psychiatry. 2005;39(1-2):1-30. PubMed CrossRef
39.Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication (CR 190). Royal College of Psychiatrists website. https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr190.pdf?sfvrsn=54f5d9a2_2. Accessed June 12, 2020.
40.Conley RR, Carpenter WT Jr, Tamminga CA. Time to clozapine response in a standardized trial. Am J Psychiatry. 1997;154(9):1243-1247. PubMed CrossRef
41.Meltzer HY. Treatment of the neuroleptic-nonresponsive schizophrenic patient. Schizophr Bull. 1992;18(3):515-542. PubMed CrossRef
42.Schulte P. What is an adequate trial with clozapine? therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet. 2003;42(7):607-618. PubMed CrossRef
43.Kerwin RW, Bolonna A. Management of clozapine-resistant schizophrenia. Adv Psychiatr Treat. 2005;11(2):101-106. CrossRef
44.Rostami-Hodjegan A, Amin AM, Spencer EP, et al. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol. 2004;24(1):70-78. PubMed CrossRef
45.Dettling M, Sachse C, Brockmöller J, et al. Long-term therapeutic drug monitoring of clozapine and metabolites in psychiatric in- and outpatients. Psychopharmacology (Berl). 2000;152(1):80-86. PubMed CrossRef
46.Flanagan RJ. Therapeutic monitoring of antipsychotic drugs. CPD Clin Biochem. 2006;7:3-18.
47.Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720-731. PubMed CrossRef
48.Josiassen RC, Joseph A, Kohegyi E, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162(1):130-136. PubMed CrossRef
49.Barnes TRE; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. PubMed CrossRef
50.Anil YaÄŸcioÄŸlu AE, Kivircik Akdede BB, Turgut TI, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66(1):63-72. PubMed CrossRef
51.Akdede BB, Anil YaÄŸcioÄŸlu AE, Alptekin K, et al. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. J Clin Psychiatry. 2006;67(12):1912-1919. PubMed CrossRef
52.Honer WG, Thornton AE, Chen EY, et al; Clozapine and Risperidone Enhancement (CARE) Study Group. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354(5):472-482. PubMed CrossRef
53.Freudenreich O, Henderson DC, Walsh JP, et al. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92(1-3):90-94. PubMed CrossRef
54.Shiloh R, Zemishlany Z, Aizenberg D, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine: adouble-blind, placebo-controlled study. Br J Psychiatry. 1997;171(6):569-573. PubMed CrossRef
55.Assion HJ, Reinbold H, Lemanski S, et al. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine: a randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry. 2008;41(1):24-28. PubMed CrossRef
56.Fleischhacker WW, Heikkinen ME, Olié JP, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13(8):1115-1125. PubMed CrossRef
57.Ziegenbein M, Kropp S, Kuenzel HE. Combination of clozapine and ziprasidone in treatment-resistant schizophrenia: an open clinical study. Clin Neuropharmacol. 2005;28(5):220-224. PubMed CrossRef
58.Henderson DC, Fan X, Copeland PM, et al. Ziprasidone as an adjuvant for clozapine- or olanzapine-associated medical morbidity in chronic schizophrenia. Hum Psychopharmacol. 2009;24(3):225-232. PubMed
59.Barber S, Olotu U, Corsi M, et al. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev. 2017;3:CD006324. PubMed
60.Wang J, Omori IM, Fenton M, et al. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2010;(1):CD008125. PubMed
61.Paton C, Whittington C, Barnes TR. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacol. 2007;27(2):198-204. PubMed CrossRef
62.Barbui C, Signoretti A, Mulר S, et al. Does the addition of a second antipsychotic drug improve clozapine treatment? Schizophr Bull. 2009;35(2):458-468. PubMed CrossRef
63.Taylor DM, Smith L. Augmentation of clozapine with a second antipsychotic—a meta-analysis of randomized, placebo-controlled studies. Acta Psychiatr Scand. 2009;119(6):419-425. PubMed CrossRef
64.Malla A, Tibbo P, Chue P, et al. Long-acting injectable antipsychotics: recommendations for clinicians. Can J Psychiatry. 2013;58(suppl 1):30S-35S. PubMed CrossRef
65.Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. PubMed CrossRef
66.Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7(7):1171-1180. PubMed CrossRef
67.Manchanda R, Chue P, Malla A, et al. Long-acting injectable antipsychotics: evidence of effectiveness and use. Can J Psychiatry. 2013;58(suppl 1):5S-13S. PubMed CrossRef
68.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799-812. CrossRef
69.Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217-222.
70.Eisen SV, Normand SLT, Belanger AJ, et al. The Revised Behavior and Symptom Identification Scale (BASIS-R): reliability and validity. Med Care. 2004;42(12):1230-1241. PubMed CrossRef
71.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551-558. PubMed CrossRef
72.Wing JK, Beevor AS, Curtis RH, et al. Health of the Nation Outcome Scales (HoNOS): research and development. Br J Psychiatry. 1998;172(1):11-18. PubMed CrossRef
73.Slade M, Powell R, Rosen A, et al. Threshold Assessment Grid (TAG): the development of a valid and brief scale to assess the severity of mental illness. Soc Psychiatry Psychiatr Epidemiol. 2000;35(2):78-85. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!