Background: The objective of this study was to estimate how commonly patients with pharmacologically treated depression (PTD) do not receive adequate doses of antidepressant (AD) medications. Such prescribing would have epidemiologic and clinical implications. Patients with PTD have treatment-resistant depression (TRD) if they do not benefit from ≥ 2 AD medications taken with reasonable compliance for adequate durations at adequate doses. Some database studies of TRD do not assess AD medication dose and would, therefore, overestimate TRD incidence unless physicians treating PTD patients routinely prescribe AD medications at adequate doses before changing medications.
Methods: Using data from 3 US health services databases from September 1, 2010, through December 31, 2014, we created PTD cohorts and defined an AD medication era as a sequence of dispensings with ≤ 30 days between the end of the days’ supply of each dispensing and the start of the next. We classified AD medication eras according to whether they had ≥ 1 dispensing at or above the minimum therapeutic dose.
Results: The proportion of AD medication eras with ≥ 1 dose at or above the minimum therapeutic dose varied from 59.6% in the Medicaid database to 66.0% in a database of privately insured patients.
Conclusions: In the population at risk for TRD, a substantial proportion of AD medication dispensing eras do not reach the minimum therapeutic dose. TRD incidence is likely to be overestimated in database studies that do not take account of dose. Clinicians should be aware that AD medication regimens are often stopped without reaching the minimum therapeutic dose, which may cause unnecessary switching.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Database Studies of Treatment-Resistant Depression Should Take Account of Adequate Dosing

ABSTRACT
Background: The objective of this study was to estimate how commonly patients with pharmacologically treated depression (PTD) do not receive adequate doses of antidepressant (AD) medications. Such prescribing would have epidemiologic and clinical implications. Patients with PTD have treatment-resistant depression (TRD) if they do not benefit from ≥ 2 AD medications taken with reasonable compliance for adequate durations at adequate doses. Some database studies of TRD do not assess AD medication dose and would, therefore, overestimate TRD incidence unless physicians treating PTD patients routinely prescribe AD medications at adequate doses before changing medications.
Methods: Using data from 3 US health services databases from September 1, 2010, through December 31, 2014, we created PTD cohorts and defined an AD medication era as a sequence of dispensings with ≤ 30 days between the end of the days’ supply of each dispensing and the start of the next. We classified AD medication eras according to whether they had ≥ 1 dispensing at or above the minimum therapeutic dose.
Results: The proportion of AD medication eras with ≥ 1 dose at or above the minimum therapeutic dose varied from 59.6% in the Medicaid database to 66.0% in a database of privately insured patients.
Conclusions: In the population at risk for TRD, a substantial proportion of AD medication dispensing eras do not reach the minimum therapeutic dose. TRD incidence is likely to be overestimated in database studies that do not take account of dose. Clinicians should be aware that AD medication regimens are often stopped without reaching the minimum therapeutic dose, which may cause unnecessary switching.
Prim Care Companion CNS Disord 2018;20(4):18m02274
To cite: Fife D, Blacketer C, Reps JM, et al. Database studies of treatment-resistant depression should take account of adequate dosing. Prim Care Companion CNS Disord. 2018;20(4):18m02274.
To share: https://doi.org/10.4088/PCC.18m02274
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Epidemiology, Janssen Research and Development, Titusville, New Jersey
*Corresponding author: Daniel Fife, MD, Janssen Research and Development, 1125 Trenton Harbourton Rd, Titusville, NJ 08560 ([email protected]).
Definitions of treatment-resistant depression (TRD) vary,1-11 but the core concept is that a patient with pharmacologically treated depression (PTD) is considered to have TRD if he or she did not benefit from at least 2 antidepressant (AD) regimens that were taken with reasonable compliance at or above the minimum therapeutic doses and at adequate durations that would normally be effective.1 We were developing a protocol for a study of the epidemiology of TRD in the United States based on administrative claims data and, as part of the literature review, we noted that though several recent studies explicitly considered the adequacy of the dose of AD medication3 or the titration of the AD medication dose,5,11 others did not.6,9 We thought the latter approach was reasonable because we assumed that a physician who was treating a depressed patient with an AD medication would raise the dose of that medication to at least the minimal therapeutic dose before changing to a different AD medication. However, we also thought there were several possible reasons, eg, concerns about observed or anticipated adverse effects, that would make it desirable to have evidence to support or refute that assumption before building it into our protocol by ignoring dose.
Since it is challenging to accurately estimate the daily dose of medications from pharmacy dispensing records, we sought guidance for the design of the protocol. So, we examined the question with an analysis that could be readily performed on the basis of open-source tools developed within the Observational Health Data Sciences and Informatics (OHDSI) collaborative.12,13
METHODS
This study was based on data from September 1, 2010 (the study start date), through December 31, 2014 (the study end date), in 3 US health services databases: Truven MarketScan Multi-State Medicaid (MDCD), Truven MarketScan Medicare Supplemental Beneficiaries (MDCR), and Truven MarketScan Commercial Claims and Encounters (CCAE). We defined a cohort of patients with PTD as those who had a dispensing of an AD medication between January 1, 2011, and December 31, 2011 (the date of that dispensing was the index date); had a diagnosis of depression within 30 days of their index date; were ≥ 10 years of age at their index date; had at least 120 days of continuous observation prior to their index date with no diagnosis of depression or dispensing of AD or antipsychotic medication; and had no prior diagnosis of any excluded condition: psychosis, mania (including bipolar disease), or dementia. The episode of PTD began on the index date and ended if the subject had 120 days with no diagnosis of depression and no dispensing of an AD or antipsychotic medication, the subject left the database (ignoring breaks of < 30 days), the subject received an excluded diagnosis, or the subject reached the study end date. A subject could have more than 1 PTD episode.
Although clinical trials often define TRD as a subset of major depressive disorder (MDD) and confirm the diagnosis of MDD with standardized screening tools, it seemed unlikely that practitioners would use such tools. We, therefore, followed the approach of several of the database studies cited previously6,9,11 and included a wider range of depression diagnoses: MDD (ICD-9 296.2x, 296.3x), neurotic depression (ICD-9 300.4), and depressive disorder not otherwise classified (ICD-9 311).

- At least one-third of initial antidepressant regimens are stopped or changed without reaching the minimum effective dose.
- If an antidepressant regimen is ineffective and not limited by factors such as adverse effects, clinicians should consider whether the dose is adequate before switching to a different medication.
We defined a medication in terms of the active drug substance and defined an AD medication era as a sequence of dispensings of an AD medication during an episode of PTD with no more than 30 days between each dispensing of the medication and the end of the days’ supply of the previous dispensing. The units of tabulation were these drug eras, and we defined an AD medication era as reaching an adequate dose if at least 1 dispensing in that era had a daily dose at or above the minimum effective dose for that medication on the basis of the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire.14 The AD medications included in the present study were limited to the extensive list in the publication by Desseilles et al.14 Because we were assessing adequacy of dose based on the individual dispensings without taking account of the possibility that several concurrent or nearly concurrent dispensings of the same medication might be combined, we also estimated the proportion of AD medication dispensing days for which the same medication was dispensed to the same patient 2 or more times on the same day, whether at the same dose or at different doses.
RESULTS
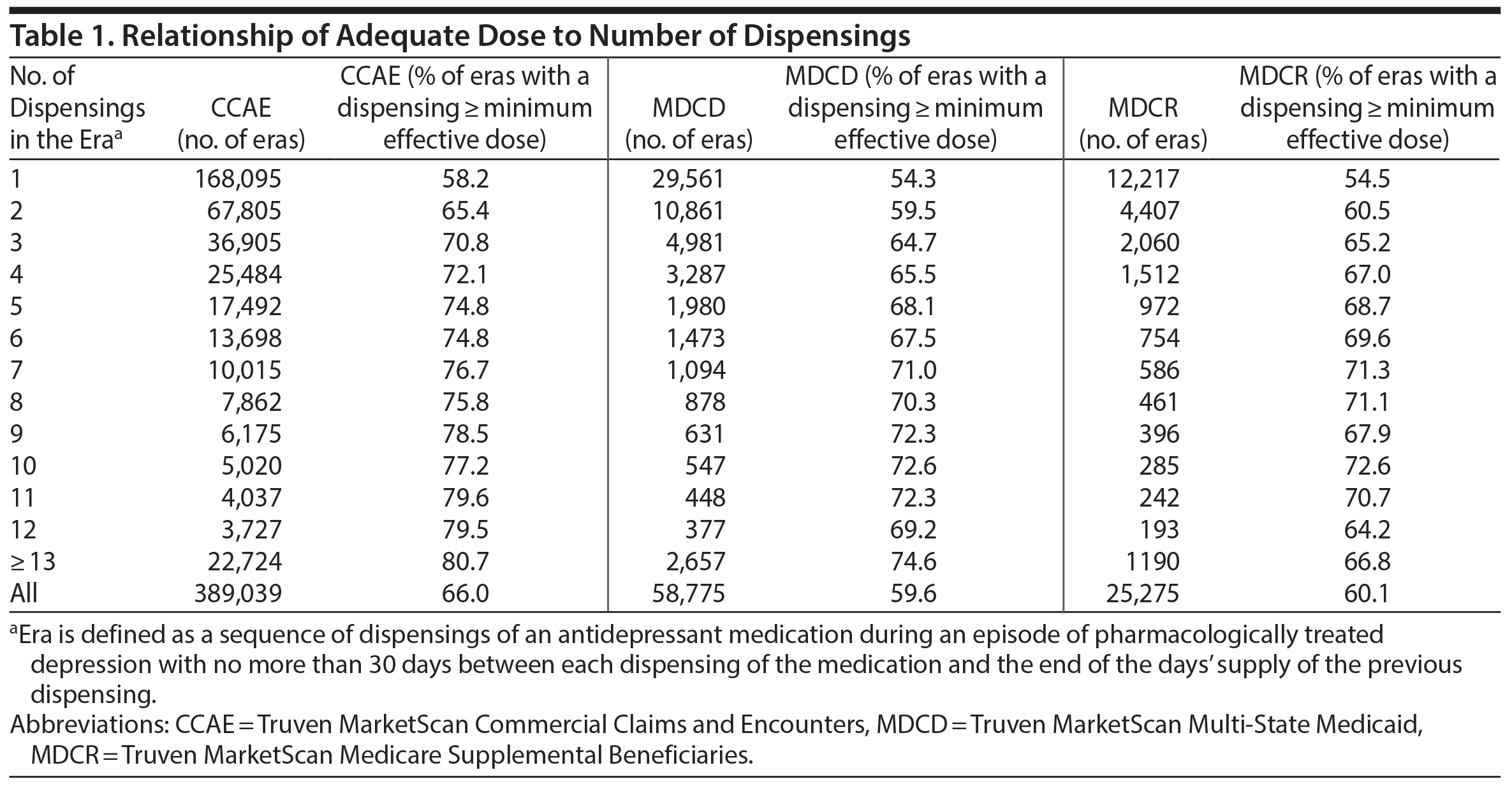
The proportion of AD medication eras with at least 1 daily dose at or above the minimum therapeutic dose varied from 59.6% in the Medicaid database to 66.0% in a database of privately insured patients (Table 1). The proportion of AD medication dispensing days for which the same medication was dispensed 2 or more times was 1.2% in the CCAE database, 1% in the MDCR database, and 2.3% in the MDCD database.
DISCUSSION
Across the 3 databases, at least one-third of all AD medication eras had no dispensings at or above the minimum effective dose. The likelihood that an AD medication would have a dispensing at or above the minimum effective dose increased with the number of dispensings, but even in AD medication eras with relatively large numbers of dispensings, more than 20% of eras had no dispensings at or above the minimum effective dose. If higher doses were frequently achieved via concurrent or near-concurrent dispensings of the same AD medication, we would expect to see a substantial frequency of 2 or more dispensings of the same AD medication on the same day, but only approximately 1% of AD medication dispensings involved 2 or more dispensings of the same AD medication on the same day. Thus, these findings argue persuasively that, in the population at risk for TRD, a substantial proportion of AD medication dispensing eras do not reach the minimum effective dose, and, consequently, the incidence of TRD is likely to be overestimated in studies that do not take account of dose.
The finding that AD medication regimens are often stopped before reaching therapeutic doses as described in Desseilles et al14 suggests that the possibility of inadequate dose should be considered before a patient receiving an AD medication that appears ineffective and whose dose is not limited by observed or anticipated adverse effects is switched to a different AD medication.
The main limitation of this study is that the databases used for it, and for the cited studies of TRD, do not offer information about the reasons for stopping an AD medication, which might include the end of clinical depression or observed or anticipated adverse events. Among its strengths are that its criteria for clinically effective dose and for TRD are based on prior publications and the findings are observed in 3 different health care databases.
Submitted: January 13, 2018; accepted May 18, 2018.
Published online: July 26, 2018.
Potential conflicts of interest: All authors are employees of Janssen Research and Development, and some have stock, stock options, or pension rights. Janssen Research and Development markets several psychiatric medications, some of which are used in the treatment of depression.
Funding/support: The sponsor’s (Janssen Research and Development) financial support of the work consisted of providing support in the form of salaries for all the authors.
Role of the sponsor: The sponsor’s standard operating procedures require that the study undergo an internal company review before submission for publication. That internal company review is limited to roles not responsible for sales or marketing functions. The sponsor had no additional role in the study design, data collection, analysis, or preparation of the manuscript.
Acknowledgments: The authors would like to thank Ms Gayle Murray of Janssen Research and Development, Titusville, New Jersey, for her editorial assistance. Ms Murray has no other conflicts of interest related to the subject of this article.
Additional information: The data for these analyses were made available to the authors by third-party license from Truven MarketScan, a commercial data provider in the United States. The authors have a license for analysis of the Truven MarketScan CCAE data. Under the licensing agreement, the authors cannot provide the raw data themselves. Other researchers could access the data by purchase through Truven MarketScan, and the inclusion criteria specified in the Methods section would allow them to identify the same cohort of patients we used for these analyses. Interested individuals may see http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases for more information on accessing Truven MarketScan data. We confirm that no authors had special privileges to access data from Truven MarketScan via third-party license and that other researchers would be able to access the data in the same manner as the authors.
REFERENCES
1. European Medicines Agency. Guideline on Clinical Investigation of Medicinal Products in the Treatment of Depression. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_
guideline/2013/05/WC500143770.pdf. 2013.
2. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707. PubMed CrossRef
3. Corey-Lisle PK, Birnbaum HG, Greenberg PE, et al. Identification of a claims data "signature" and economic consequences for treatment-resistant depression. J Clin Psychiatry. 2002;63(8):717-726. PubMed CrossRef
4. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659. PubMed CrossRef
5. Gibson TB, Jing Y, Smith Carls G, et al. Cost burden of treatment resistance in patients with depression. Am J Manag Care. 2010;16(5):370-377. PubMed
6. Kubitz N, Mehra M, Potluri RC, et al. Characterization of treatment-resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS One. 2013;8(10):e76882. PubMed CrossRef
7. Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord. 2012;139(2):126-140. PubMed CrossRef
8. Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(suppl 8):17-25. PubMed
9. Olchanski N, McInnis Myers M, Halseth M, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512-522. PubMed CrossRef
10. Ruhé HG, van Rooijen G, Spijker J, et al. Staging methods for treatment-resistant depression: a systematic review. J Affect Disord. 2012;137(1-3):35-45. PubMed CrossRef
11. Corey-Lisle PK, Birnbaum H, Greenberg P, et al. Economic impact of olanzapine plus fluoxetine combination therapy among patients treated for depression: a pilot study. Psychopharmacol Bull. 2003;37(3):90-98. PubMed
12. Cimino JJ, Johnson SB, Hripcsak G, et al. Managing vocabulary for a centralized clinical system. Medinfo. 1995;8(pt 1):117-120. PubMed
13. Galletti G, Portella L, Tagawa ST, et al. Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential. Mol Diagn Ther. 2014;18(4):389-402. PubMed CrossRef
14. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154. PubMed CrossRef
This PDF is free for all visitors!