Remission With Venlafaxine Extended Release or Selective Serotonin Reuptake Inhibitors in Depressed Patients: A Randomized, Open-Label Study
Background: This randomized, open-label, rater-blinded, multicenter study compared treatment outcomes with the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine extended release (ER) with selective serotonin reuptake inhibitors (SSRIs) in primary care patients with major depressive disorder.
Method: Study data were collected from November 29, 2000, to March 4, 2003. Outpatients who met diagnostic criteria for major depressive disorder according to the Mental Health Screener, a computer-administered telephone interview program that screens for the most common mental disorders, and had a total score on the 17-item Hamilton Depression Rating Scale (HDRS17) ≥ 20 were randomly assigned to receive up to 6 months of open-label venlafaxine ER 75−225 mg/d (n = 688) or an SSRI (n = 697): fluoxetine 20−80 mg/d, paroxetine 20−50 mg/d, citalopram 20−40 mg/d, and sertraline 50−200 mg/d. The primary outcome was remission (HDRS17 score ≤ 7) at study end point using the last-observation-carried-forward method to account for early termination. A mixed-effects model for repeated measures (MMRM) analysis evaluated secondary outcome measures.
Results: Fifty-one percent of patients completed the study. Month 6 remission rates did not differ significantly for venlafaxine ER and the SSRIs (35.5% vs 32.0%, respectively; P = .195). The MMRM analysis of HDRS17 scores also did not differ significantly (P = .0538). Significant treatment effects favoring the venlafaxine ER group were observed for remission rates at days 30, 60, 90, and 135 and a survival analysis of time to remission (P = .006), as well as Clinical Global Impressions-severity of illness scale (P = .0002); Hospital Anxiety and Depression Scale-Anxiety subscale (P = .03); 6-item Hamilton Depression Rating Scale, Bech version (P = .009); and Quick Inventory of Depressive Symptomatology-Self-Report (P = .0003).
Conclusions: Remission rates for patients treated with venlafaxine ER or an SSRI did not differ significantly after 6 months of treatment. Results of most secondary analyses suggested that SNRI treatment had a greater antidepressant effect versus the SSRIs studied.
Prim Care Companion CNS Disord 2011;13(1):e1-e9
© Copyright 2011 Physicians Postgraduate Press, Inc.
Submitted: March 8, 2010; accepted June 17, 2010.
Published online: February 24, 2011 (doi:10.4088/PCC.10m00979blu).
Corresponding author: Michael E. Thase, MD, University of Pennsylvania School of Medicine, Department of Psychiatry, Mood and Anxiety Disorders Treatment and Research Program, 3535 Market St, Ste 670, Philadelphia, PA 19104-3309 ([email protected]).
Major depressive disorder (MDD) is a common, often chronic condition1 with annual costs in the United States estimated at $83.1 billion for inpatient hospitalizations, outpatient programs, suicide attempts, lost productivity, and impaired functioning at work, at home, or in social situations.2 More than half of all MDD patients who seek treatment do so in the primary care setting,3 where up to one-fifth of patients have significant depressive symptoms,4 and rates of MDD range from 6.6%5 to 12.5%.4 As a result, primary care physicians write at least 60% of all prescriptions for antidepressant medications.6
Efforts to improve the treatment of MDD and to reduce the burden of chronic and recurrent depression have led to the development of treatment algorithms such as those made available by the Agency for Healthcare Research and Quality7 and the American Psychiatric Association,8 as well as clinical practice guidelines that emphasize complete symptom remission as the therapeutic goal. Such guidelines represent “best practice” research focusing on the effects of treatments on outcomes, and their use allows primary care physicians to make evidence-based treatment decisions. However, relatively few clinical studies have directly compared the therapeutic effects of newer antidepressant treatments in the routine primary care setting.
Clinical Points
- Most people who receive treatment for major depressive disorder (MDD) are cared for by primary care physicians.
- The selective serotonin reuptake inhibitors (SSRIs) are the most commonly used first-line treatments for patients with MDD.
- Results of the current research suggest that venlafaxine, a serotonin-norepinephrine reuptake inhibitor, may have a modest efficacy advantage compared to SSRIs, which is consistent with evidence from some earlier studies.
Despite the common use of selective serotonin reuptake inhibitors (SSRIs) as first-line agents for treating depression, the published literature suggests that outcomes in actual clinical practice are usually less than optimal.9-13 For example, the ARTIST study (A Randomized Trial Investigating SSRI Treatment), a large (N = 573) 9-month, open-label study designed and powered to compare the effectiveness of 3 widely prescribed SSRIs (paroxetine, fluoxetine, and sertraline), found that only 23% of patients achieved remission after 6 months of therapy.13
Venlafaxine extended release (ER), the first member of the serotonin-norepinephrine reuptake inhibitor (SNRI) class of antidepressants, is one of the principal alternatives to the SSRIs. Similar to the SSRIs, venlafaxine ER has established efficacy in both depression14,15 and anxiety disorders16-20 and has demonstrated a more favorable tolerability and safety profile than the previous standard of first-line pharmacotherapy, the tricyclic antidepressants.21 By virtue of effects on both serotonergic and noradrenergic neurotransmission, some have argued that venlafaxine may have greater efficacy compared with the more selective SSRIs. A number of meta-analyses of a progressively expanding group of studies tend to support this hypothesis,14,22-26 although not all meta-analyses are in agreement.27-30 However relatively few of the studies included in these meta-analyses were conducted exclusively in primary care settings, and results of those studies, which were not powered to detect the modest between-group differences that would be expected in a comparison of active treatments, yielded inconsistent findings.31-34
To further evaluate differences in therapeutic outcomes between the SNRI and SSRI antidepressant classes in a primary care setting, we compared the efficacy and safety of antidepressant treatment with venlafaxine ER with physician’s choice of the SSRIs citalopram, fluoxetine, paroxetine, or sertraline in patients with moderate to severe MDD.
METHOD
Study Design
This randomized, open-label, rater-blinded, multicenter study enrolled outpatients with MDD, with or without symptoms of anxiety, who were randomly assigned to receive either venlafaxine ER or an SSRI (fluoxetine, paroxetine, citalopram, or sertraline at the discretion of the prescribing physician) for up to 180 days of treatment. Study data were collected from November 29, 2000, to March 4, 2003. The study protocol was initiated at 92 primary care sites but was discontinued at 5 sites (2 sites for not enrolling any patients, 2 for protocol violations, and 1 because approval was withdrawn by the center’s institutional review board). The protocol and amendments received independent approval from the centers’ ethics committee/institutional review boards before the study began. The study was conducted in accordance with the Declaration of Helsinki and its amendments, and written informed consent was obtained from all study participants before enrollment.
Study Sample
Inclusion criteria. Study participants were male or female outpatients aged ≥ 18 years and judged by the physician to be experiencing an episode of MDD with stable symptoms for the previous 2 months that required initiation of antidepressant treatment or change from the current treatment regimen. Eligible patients met diagnostic criteria for MDD according to the Mental Health Screener, a computer-administered telephone interview program based on the Primary Care Evaluation of Mental Disorders that screens for the most common mental disorders,35,36 and scored ≥ 20 on the 17-item Hamilton Depression Rating Scale (HDRS17), a measure of the severity of depressive symptoms and the accepted standard for evaluating antidepressant treatment outcomes.37
Exclusion and withdrawal criteria. Patients were excluded from the study if they had a known hypersensitivity to venlafaxine or the selected SSRI, history or presence of bipolar disorder, need for hospitalization (eg, acutely suicidal), or had received electroconvulsive therapy within the past 90 days. In addition, patients beginning or changing the intensity of cognitive-behavioral or interpersonal therapy or receiving investigational drugs, psychopharmacologic drugs, transcranial magnetic stimulation, or vagus nerve stimulation were also excluded. Patients previously taking monoamine oxidase inhibitors (including St John’s wort) or fluoxetine were required to undergo a 14-day washout period prior to the baseline evaluation. Patients were withdrawn from the study if dosage reductions below the minimum maintenance dose were required, if a dose-related increase in blood pressure did not respond to dose reduction, or if a patient became pregnant.
Study Procedures
Patients underwent evaluation at screening and baseline. At screening, HDRS17 score and symptoms of MDD according to the Mental Health Screener were assessed by telephone using an interactive voice response system. This method of assessment was chosen to minimize the effects of rater bias on study assessments.35,36 Eligibility based on general signs and symptoms of depression and need for antidepressant pharmacotherapy was confirmed, informed consent was provided, and demographic and patient characteristic data were collected. Study participants completed additional assessments by telephone using an interactive voice response system no more than 3 days before randomization. At the baseline visit, further assessments of depressive symptoms and safety variables (including physical examination, vital signs, and blood chemistry) were performed, and patients continuing to meet all inclusion criteria were randomly assigned to treatment. Past antidepressant treatment history was not systematically assessed.
Study Treatment
On study day 1, physicians administered to patients the following doses of antidepressant medication: venlafaxine ER 37.5 mg/d for 4 days, followed by 75 mg/d thereafter; fluoxetine, paroxetine, or citalopram 20 mg/d; or sertraline 50 mg/d. Treatment was initiated at the lowest effective dose, with increases permitted at day 30 (venlafaxine ER 150 mg/d; fluoxetine, paroxetine, or citalopram 40 mg/d; or sertraline 100 mg/d) and day 60 (venlafaxine ER 225 mg/d, fluoxetine 80 mg/d, paroxetine 50 mg/d, citalopram 40 mg/d, or sertraline 200 mg/d) on the basis of treatment response. Prescriptions were filled by the patient’s pharmacy on an open-label basis. Study staff monitored adherence through review of electronic pharmacy prescription refill lists and the amount of returned medication at study visits and verified that antidepressant dosages were consistent with the protocol. Patients were counseled if they did not adhere or were thought to be at risk for not adhering to the medication regimen.
Clinical Assessments
Safety and effectiveness assessments were obtained on days 1 (baseline), 14, 30, 60, 90, 135, and 180 (final visit). Patient self-report measures used to evaluate effectiveness were the HDRS17; the 6-item Hamilton Depression Rating Scale, Bech version (HDRS6)38; the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR16; a 16-item inventory of the scope and severity of depressive symptoms)39; and the Hospital Anxiety and Depression Scale-Anxiety subscale (HADS-A; a 6-item scale assessing anxiety, tension, and nervousness experienced within “the last few days”).40 The physician-rated Clinical Global Impressions-severity of illness scale41 (CGI-S; 1 item assessing overall illness severity) was also used at all study visits to assess efficacy.
Adverse events (AEs) were recorded at all study visits. Vital signs (resting pulse rate and 2 sitting blood pressure readings) and concomitant medications were recorded on days 1, 14, 30, 60, 90, 135, and 180. Blood chemistry determinations were performed on days 1, 135, and 180; physical examinations were conducted and weight was measured on days 1 and 180.
Statistical Methods
The primary efficacy analyses used data from the intent-to-treat population who had at least 1 postbaseline efficacy assessment, and safety analyses used data from the safety population, which included all randomized patients who received at least 1 dose of study medication.
Planned analyses. The primary end point was remission rate at day 180 or study end point, defined as the proportion of patients whose depressive symptoms had remitted (ie, HDRS17 total score ≤ 7), using the last-observation-carried-forward (LOCF) method to account for the outcomes of patients who withdrew early from study treatment. For patients who withdrew before study completion, LOCF analyses of primary outcome data were performed using last post-dose observed values. Treatment group proportions were compared using a χ2 test. The LOCF method likewise was used in the planned secondary analyses of change in the continuous dependent measures across the 6 months of therapy. HDRS17 total scores at days 60, 90, 135, and 180 were compared between treatment groups using an analysis of variance model with treatment as the main effect. Time to remission was evaluated using Mantel-Cox methods; results were compared between treatment groups and summarized using Kaplan-Meier methods. Time to treatment discontinuation because of an adverse event was compared between treatment groups using Kaplan-Meier methods. All inferential analyses of treatment effects were 2-sided and were performed at the α = .05 level. No adjustments were made for multiple tests. Version 6.12 of the SAS System (SAS Institute, Cary, North Carolina) was used to provide all data summaries, statistical analyses, and data listings.
Mixed-effects model for repeated measures analyses. Secondary analyses were change from baseline of continuous efficacy measures at day 180 using mixed-effects model for repeated measures (MMRM) models for longitudinal analyses of continuous outcomes. The continuous efficacy measures evaluated were HDRS17, HDRS6, CGI-S, HADS-A, and QIDS-SR16. The MMRM models included baseline values for response, treatment group, visit, treatment-by-visit interaction, and day 180 completion status as predictors in the models. These MMRM models were obtained using the unstructured covariance matrix, as this type of structure gives the best model fit for almost all of the end points according to the Akaike information criterion. Effect sizes were calculated using a repeated-measures model, and additional analyses were conducted, which included day 180 completion status by treatment interaction.
The sample size estimate was based on expected remission rates of 45% in the venlafaxine ER sample arm and 35% in the SSRI sample arm and a randomization ratio of 1:1 (venlafaxine ER:SSRI). A sample size of 523 patients per group (venlafaxine ER and total SSRI), calculated using the 2-group continuity χ2 test, would provide 90% power (α = .05, 2-tailed). The study was not designed to make comparisons between venlafaxine ER and individual SSRIs.
RESULTS
Patient Characteristics and Disposition
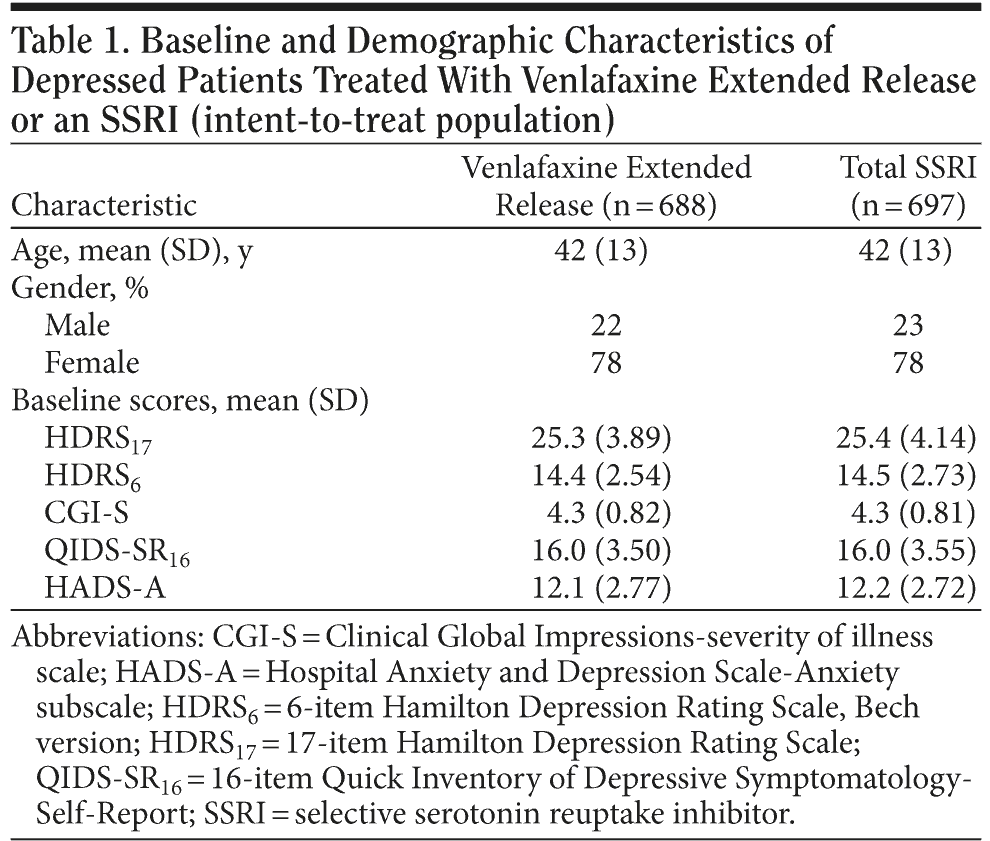
Patient characteristics at baseline were similar for both treatment groups (Table 1). A total of 688 patients were randomized to the venlafaxine ER group and 697 to the SSRI group (fluoxetine, n = 114; paroxetine, n = 131; citalopram, n = 259; and sertraline, n = 193). A total of 675 patients (49%) withdrew from the study (venlafaxine ER, n = 327 [48%]; SSRIs, n = 348 [50%]). The most frequently cited reason for withdrawal in both treatment groups was failure to return (venlafaxine ER, n = 113 [16%]; SSRIs, n = 109 [16%]). With the exception of patient request unrelated to study (which was higher among the patients in the SSRI arm vs the venlafaxine ER arm [7.5% vs 4.8%, respectively; P = .039]), reasons for early withdrawals did not differ significantly between the 2 treatment groups.
Mean time spent on study medication was comparable for the venlafaxine ER (138.8 days) and SSRI (135.8 days) groups (fluoxetine: 138.9 days, paroxetine: 128.5 days, citalopram: 129.9 days, and sertraline: 147.0 days). Mean prescribed daily doses were 129.4 mg venlafaxine ER, 42.3 mg fluoxetine, 35.0 mg paroxetine, 30.4 mg citalopram, and 106.2 mg sertraline. Maximum prescribed daily doses were 156.9 mg venlafaxine ER, 54.7 mg fluoxetine, 40.8 mg paroxetine, 34.9 mg citalopram, and 134.5 mg sertraline. At day 180, 267 (38.3%) SSRI-treated patients and 224 (32.6%) venlafaxine ER-treated patients were at the maximum dose.
Treatment Response
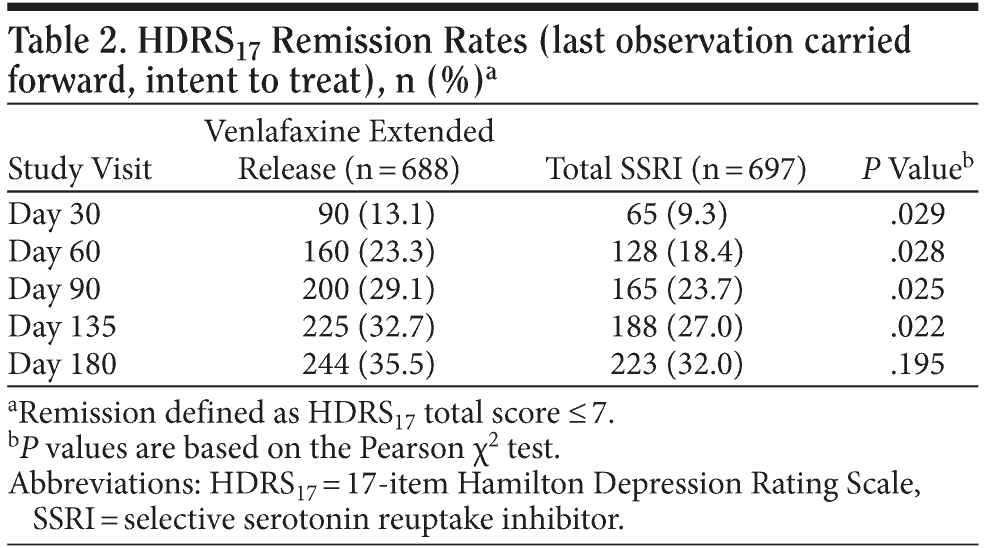
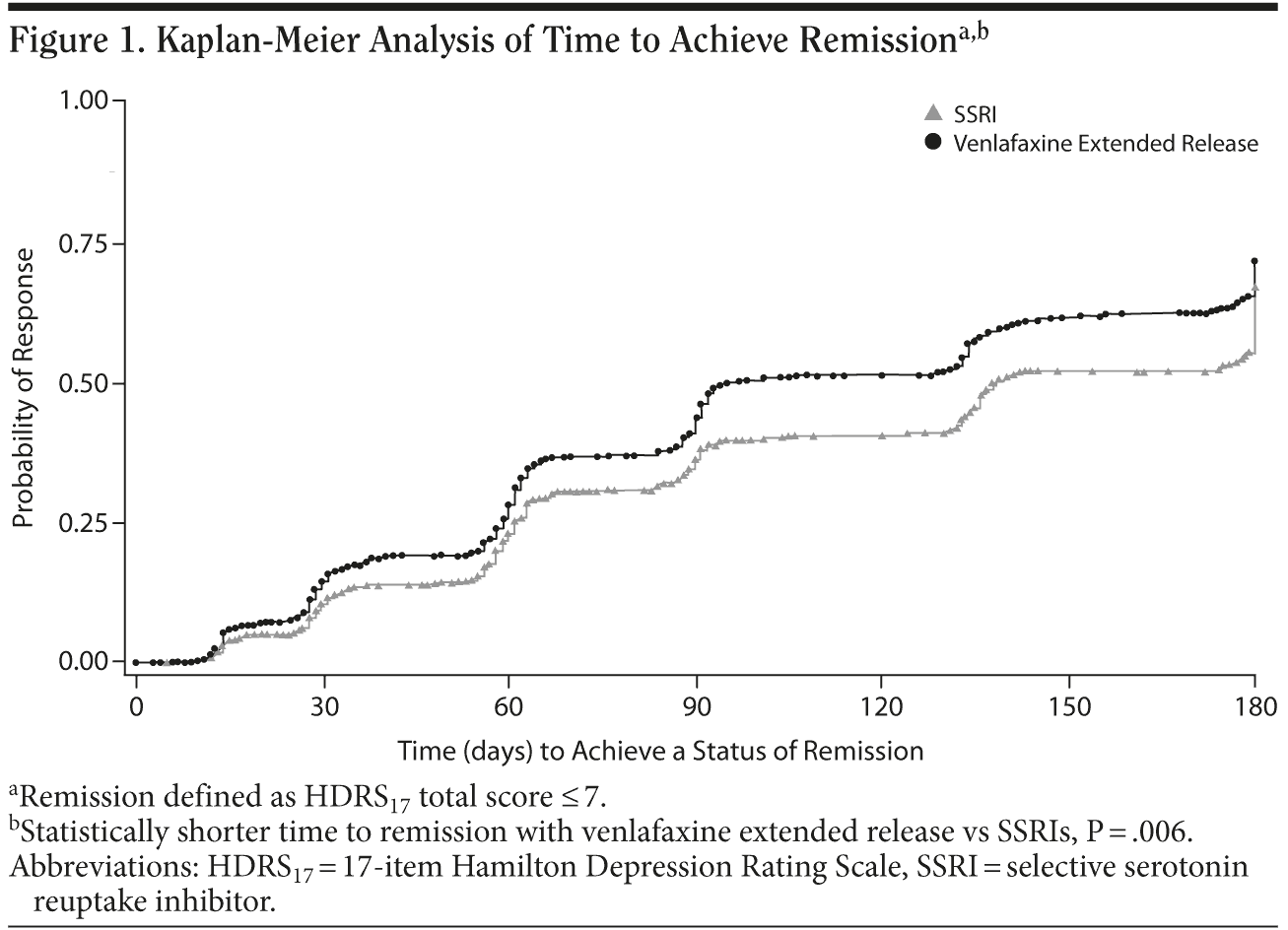
Planned analyses. Remission rates (based on HDRS17 score ≤ 7) at day 180 or study end point using LOCF analysis, the primary efficacy end point, were not significantly different between the venlafaxine ER (35.5%) and SSRI (32.0%) groups (P = .195). However, remission rates at days 30, 60, 90, and 135 were significantly greater for the venlafaxine ER group compared with the SSRI group (Table 2), and patients treated with venlafaxine ER had a statistically significantly shorter time to remission versus the patients treated with SSRIs (P = .006; Figure 1). In addition, across the 180-day study, patients treated with venlafaxine ER had significantly lower mean ± SD HDRS17 scores versus patients treated with an SSRI (11.7 ± 8.76 vs 13.1 ± 9.26 at study day 180, respectively; P = .007).
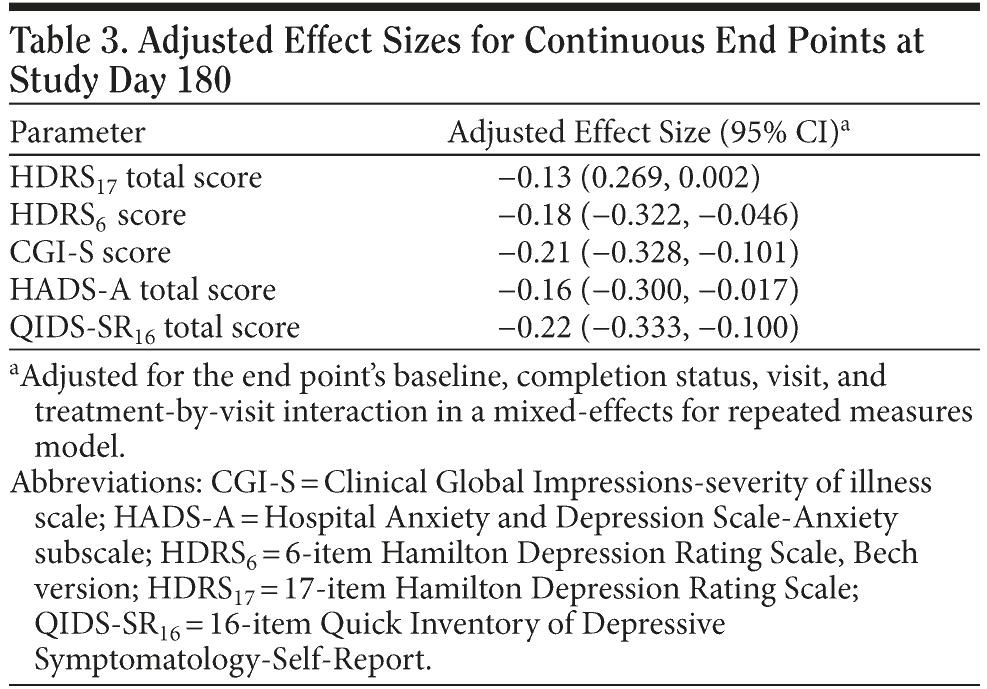
Mixed-effects model for repeated measures analyses. MMRM analyses demonstrated that the treatment effect for HDRS17 remission end points did not differ significantly among patients treated with venlafaxine ER versus those treated with SSRIs (MMRM model adjusted mean change from baseline [95% CI]: −15.33 [−16.05, −14.60] vs −14.32 [−15.06, −13.58], respectively; P = .0538). However, a statistically significant treatment effect was observed for the venlafaxine ER versus SSRI treatment groups for the CGI-S (MMRM model adjusted treatment difference for mean change [95% CI]: −0.27 [−0.41, −0.13]; P = .0002); HADS-A total (−0.38 [−0.73, −0.04]; P = .03); HDRS6 (−0.83 [−1.45, −0.21]; P = .009); and QIDS-SR16 (−1.01 [−1.56, −0.47]; P = .0003) scores. Completion status did not differ significantly among the treatment groups for any of the secondary measures, as indicated by nonsignificant P values for the completion-by-treatment-interaction effect. The QIDS-SR16 and CGI-S were associated with the greatest adjusted effect sizes (−0.22 [−0.333, −0.100] and −0.21 [−0.328, −0.101], respectively; Table 3).
Safety and Tolerability
The majority of patients reported ≥ 1 AE. The frequencies of experiencing any treatment-emergent AEs were comparable between the venlafaxine ER (77.6%) and SSRI (78.5%) groups. Treatment-emergent AEs with an incidence ≥ 5% in the venlafaxine ER or SSRI group, respectively, were headache (17% and 15%), insomnia (17% and 15%), nausea (16% and 11%), dry mouth (10% and 7%), fatigue (10% and 9%), dizziness (8% and 6%), increased sweating (8% and 3%), somnolence (7% and 6%), diarrhea (6% and 9%), constipation (7% and 2%), sinusitis (6% and 6%), and upper respiratory tract infection (6% and 6%). An AE was the primary reason for study discontinuation for 183 patients (13%), including 104 patients (15%) in the venlafaxine ER treatment group and 82 patients (12%) in the total SSRI treatment group (P = .403). The most common AEs leading to study discontinuation were nausea, insomnia, and headache.
Four serious AEs leading to death were reported among the patients treated with SSRIs (3 taking citalopram and 1 taking paroxetine); none were considered treatment related. An attempted suicide was reported as a serious AE for 4 patients, 3 in the venlafaxine ER group, and 1 in the SSRI group (citalopram); in each case, the investigator deemed the attempt unrelated to study treatment. In total, 73 patients experienced serious AEs, 44 (6.4%) in the venlafaxine ER group and 29 (4.0%) in the SSRI group. No single serious AE was reported for more than 1% of patients within any group. The only serious AE considered treatment related was 1 study medication overdose (which was not one of the attempted suicides mentioned above) considered “possibly related” by the investigator.
During the course of the study, 5 patients (0.7%) treated with venlafaxine ER and 7 patients (1.0%) treated with SSRIs had clinically significant sustained hypertension, defined as an increase of diastolic blood pressure ≥ 10 mm Hg from baseline and diastolic blood pressure ≥ 90 mm Hg at 3 consecutive visits. Clinically significant increased diastolic blood pressure (mean postbaseline diastolic blood pressure ≥ 105 mm Hg and increase from baseline ≥ 15 mm Hg) was observed in 4 (0.6%) venlafaxine ER-treated patients and 8 (1.1%) SSRI-treated patients. Clinically significant decreased diastolic blood pressure (mean postbaseline diastolic blood pressure ≤ 50 mm Hg and decrease from baseline ≥ 15 mm Hg) was observed in 3 (0.4%) venlafaxine ER-treated patients and 1 (0.1%) SSRI-treated patient. Clinically significant increased systolic blood pressure (mean postbaseline systolic blood pressure ≥ 180 mm Hg and increase from baseline ≥ 20 mm Hg) was observed in 1 (0.1%) venlafaxine ER-treated patient and 7 (1%) SSRI-treated patients, and clinically significant decreased systolic blood pressure (mean postbaseline systolic blood pressure ≤ 90 mm Hg and decrease from baseline ≥ 20 mm Hg) was observed in 4 (0.6%) venlafaxine ER-treated patients and 5 (0.7%) SSRI-treated patients. No patients experienced sustained increases or decreases in diastolic blood pressure or systolic blood pressure, and no patient had a serious AE related to cardiovascular abnormalities.
DISCUSSION
To our knowledge, this was the first comparison of venlafaxine ER and doctor’s choice of SSRI treatment (ie, citalopram, fluoxetine, paroxetine, or sertraline) in primary care patients with MDD. The study design allowed the prescribing physician to choose the specific SSRI treatment in order to maximize the ecological validity of the study. In addition, patients with moderate to severe depression were chosen for this trial because of evidence of greater antidepressant signal detection (ie, larger drug-vs-placebo differences) in controlled studies of such populations.42,43 The study also was large enough to ensure adequate statistical power to detect between-group differences in remission rates of 10% or larger.
With these issues in mind, it is noteworthy that the primary analysis, a comparison of intent-to-treat remission rates at month 6 using the LOCF method to account for attrition, found that the difference in remission rates between the venlafaxine ER and SSRI treatment groups was not statistically significant. However, it is also noteworthy that results of comparisons of remission rates at earlier time points, a survival analysis of time to remission, and MMRM analyses of most secondary outcome measures, including the HDRS6, CGI-S, HADS-A, and QIDS-SR16, demonstrated significant differences. In addition, measures of safety and tolerability were comparable between the venlafaxine ER and SSRI treatment groups.
The patient population and study design of the current study are similar to those in the ARTIST study.12 Both sought to evaluate differences between antidepressant agents in a primary care setting using methods designed to more closely resemble “real-world” practices than a standard clinical trial. Although this study and the ARTIST study used different primary outcomes (remission vs Short Form-36 Mental Component Summary scale) and statistical methods for primary end points (LOCF vs MMRM), the findings of both studies have similar clinical relevance. The ARTIST investigators found essentially no differences in efficacy between the individual SSRIs studied44; similarly, the current study failed to demonstrate statistically significant differences for the primary efficacy end point (ie, remission rate at study end point) between venlafaxine ER and the SSRIs as a group. Remission rates in the current study were higher than those observed in the ARTIST study, which is possibly the result of different definitions of remission (HDRS17 score ≤ 7 vs Symptom Checklist-20 score ≤ 6, respectively).13
The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study (N = 1,074) assessed the efficacy of venlafaxine ER compared with fluoxetine in the long-term treatment of recurrent MDD using a multiphase study design.45 The PREVENT trial included a 10-week acute phase of double-blind treatment with venlafaxine ER or fluoxetine; a 6-month, double-blind, continuation phase in responders to treatment; and 2 consecutive, 12-month maintenance phases of double-blind treatment with venlafaxine ER or placebo. Similar to the current study, findings of the PREVENT trial showed no significant differences in rates of remission following treatment with venlafaxine ER compared with fluoxetine at the end of the 6-month continuation phase (72% vs 69%, respectively). However, the remission rates following 6 months of continuation treatment were much higher in the PREVENT study, most likely because the study design required patients to be responders at the end of the acute phase in order to enroll in the continuation phase and possibly because of the longer duration of treatment (10 weeks during the acute phase plus 6 months during the continuation phase in the PREVENT study vs 6 months total in this study), more frequent study visits during the first 2 months of treatment (which may have increased patient adherence and encouraged patients to remain in the study), or differences in the patient populations (PREVENT enrolled only patients with recurrent depression).45
The primary analysis of the current study was limited by a low rate of study completion and the protocol-defined primary analysis of remission rates at study end point using LOCF data, which is not optimal for a study of 6 months’ duration. Although the LOCF method of accounting for attrition is generally considered conservative and has been used by regulatory agencies such as the US Food and Drug Administration (FDA) for decades, it is also widely considered to be outmoded, particularly for studies of longer duration and with higher attrition rates.46,47 In retrospect, the Kaplan-Meier survival analysis of time to remission, which was a planned secondary analysis in this study, would have been more appropriate for the primary efficacy analysis.
The overarching aim of this study, namely to conduct an adequately powered, pragmatic comparative effectiveness study of antidepressant therapy in primary care practice, necessitated use of a research design that has several inherent, interpretive limitations. For example, a placebo control group is not used in this type of clinical trial, which prioritizes generalizability and external validity, and the absence of a placebo control group precludes an assessment of the absolute efficacy of the treatment strategies. It is possible that neither venlafaxine ER nor the SSRIs would have been significantly more effective than a placebo control group. The open-label administration of antidepressant therapy, which is used to increase ecological validity, leaves open the possibility that the expectations of the treating physicians, or even the patients, may have influenced outcomes. With respect to the question of efficacy, large numbers of placebo-controlled studies have been conducted with the study medications and, although the results of several recent meta-analyses have suggested that the mean drug-versus-placebo differences of these medications are likely to be smaller than commonly thought,48-50 it was our assessment that a placebo control group was not necessary to achieve the aims of this study.
The potential for bias resulting from open-label administration of study medications is arguably of greater concern, especially because the study was sponsored by the manufacturer of venlafaxine ER. To minimize the possibility of sponsorship bias, the treatment protocol permitted use of the full FDA-approved dosing range of all study medications, and care was taken to ensure that the dosing titration schedules were comparable for both medication strategies. The potential impact for bias also was minimized by using interactive voice response system technology to collect the HDRS17 ratings (ie, they were done without knowledge of treatment assignment). In fact, among the outcome measures, only the CGI-S was completed by the unblinded study physician.
As the number of clinical studies that have directly investigated the effects of the newer antidepressant therapies in the primary care setting is limited,31-34 physicians making evidence-based treatment decisions lack comprehensive data. This fact is especially troublesome given that primary care physicians are responsible for the majority of antidepressant prescriptions. A recent study funded by the federal Substance Abuse and Mental Health Services Administration found that primary care physicians were responsible for prescribing 62% of antidepressant medications during the study period.51 The authors concluded that general practice physicians will likely continue to play a large role in prescribing psychotropic medications and emphasized the importance of ensuring the quality of psychiatric treatment in the primary care setting.51
In this regard, although intent-to-treat remission rates for patients with moderate to severe MDD treated with venlafaxine ER or doctor’s choice of SSRI in the current study did not differ significantly after 6 months of therapy, findings of the secondary analyses suggested that treatment with the SNRI venlafaxine ER had a greater antidepressant effect compared with the SSRIs studied. Taken together, the overall pattern of results suggests that venlafaxine ER could be considered by primary care practitioners when selecting among first-line antidepressant therapies.
Drug names: citalopram (Celexa, Lexapro, and others), fluoxetine (Prozac and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Author affiliations: Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia (Dr Thase); Pfizer, Collegeville, Pennsylvania (Dr Ninan and Mr Musgnung); and Department of Psychiatry, University of Texas Southwestern Medical School, Dallas (Dr Trivedi).
Potential conflicts of interest: Dr Thase has served as a consultant to or on the advisory boards of AstraZeneca, Bristol-Myers Squibb, Dey Pharma, Eli Lilly, Forest, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, H. Lundbeck A/S, MedAvante, Merck, Neuronetics, Novartis, Otsuka, Ortho-McNeil, Pamlab, Pfizer, Schering-Plough, Shire, Supernus, Takeda, and Transcept; has received grant/research support from Agency for Healthcare Research and Quality, Eli Lilly, GlaxoSmithKline, National Institute of Mental Health, and Sepracor; has served on the speakers bureaus of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck, and Pfizer; has equity holdings in MedAvante; and receives royalty income from American Psychiatric Publishing, Guilford Publications, Herald House, and W.W. Norton. Dr Thase’s wife is employed by Embryon (formerly Advogent). Dr Ninan and Mr Musgnung are employees of Pfizer. Dr Trivedi has served as a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Evotec, GlaxoSmithKline, Janssen, Johnson & Johnson, Medtronic, Neuronetics, Otsuka, Pfizer, and Shire and has received grant/research support from Agency for Healthcare Research and Quality, National Institute of Mental Health, National Institute on Drug Abuse, and Targacept.
Funding/support: The clinical trial and analyses included in this article were supported by Pfizer, formerly Wyeth Research. Medical writing and editing support for the preparation of this manuscript was supported by Pfizer, formerly Wyeth Research. Writing support was provided by Callie Grimes, PhD, and medical editing support was provided by Jennifer Karpinski, BA, both of Embryon, LLC, Somerville, New Jersey, A Division of Advanced Health Media, LLC (formerly Medesta Publications Group, A Business of Advogent). Dr Grimes and Ms Karpinksi report no other conflicts of interest relevant to the subject of this article.
Previous presentation: Portions of the results presented in this article have been presented as a poster at various annual meetings: World Federation of Societies of Biological Psychiatry Annual Meeting, February 9-13, 2004, Sydney, Australia; World Congress of Women’s Mental Health Annual Meeting, March 17-20, 2004, Washington, DC; American Psychiatric Association Annual Meeting, May 1-6, 2004, New York, New York; New Clinical Drug Evaluation Unit Annual Meeting, June 1-4, 2004, Phoenix, Arizona; American Academy of Physicians Assistants Annual Meeting, June 1-6, 2004, Las Vegas, Nevada; College of International Neuropsychopharmacology Annual Meeting, June 20-24, 2004, Paris, France; British Association for Psychopharmacology Annual Meeting, July 25-28, 2004, Harrogate, United Kingdom; American Academy of Family Physicians/World Congress of Family Doctors Annual Meeting, October 13-17, 2004, Orlando, Florida; American College of Clinical Pharmacy Annual Meeting, October 24-27, 2004, Dallas, Texas; American Osteopathic Association Annual Meeting, November 7-11, 2004, San Francisco, California; World Psychiatric Association Annual Meeting, November 10-13, 2004, Florence, Italy; US Psychiatric and Mental Health Congress Annual Meeting, November 18-21, 2004, San Diego, California; American College of Neuropsychopharmacology Annual Meeting, December 12-16, 2004, San Juan, Puerto Rico; Biennial World Organization of Family Doctors-Asia Pacific Regional Conference, May 27-31, 2005, Kyoto, Japan; 470 Predictors: American College of Neuropsychopharmacology Annual Meeting, December 11-15, 2005, Waikoloa, Hawaii; College of Psychiatric and Neurologic Pharmacists Annual Meeting, April 23-26, 2006, Baltimore, Maryland; American Psychiatric Association Annual Meeting, May 20-25, 2006, Toronto, Canada; New Clinical Drug Evaluation Unit Annual Meeting, June 12-15, 2006, Boca Raton, Florida; Collegium Internationale Neuro-Psychopharmacologicum Annual Meeting, July 9-13, 2006, Chicago, Illinois.
REFERENCES
1. Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(8):694-700. PubMed doi:10.1001/archpsyc.55.8.694
2. Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465-1475. PubMed doi:10.4088/JCP.v64n1211
3. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system: epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94. PubMed
4. Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. Gen Hosp Psychiatry. 1994;16(4):267-276. PubMed doi:10.1016/0163-8343(94)90006-X
5. Simon GE, VonKorff M. Recognition, management, and outcomes of depression in primary care. Arch Fam Med. 1995;4(2):99-105. PubMed doi:10.1001/archfami.4.2.99
6. Simon GE, VonKorff M, Wagner EH, et al. Patterns of antidepressant use in community practice. Gen Hosp Psychiatry. 1993;15(6):399-408. PubMed doi:10.1016/0163-8343(93)90009-D
7. Clinical Practice Guidelines Number 5: Depression in Primary Care: Overview of Mood Disorders. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsarchive&part=A14485#A14600. Accessed October 1, 2010.
8. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157(4 suppl):1-45. PubMed
9. Areסn PA, Alvidrez J. Treating depressive disorders: who responds, who does not respond, and who do we need to study? J Fam Pract. 2001;50(6):E2. PubMed
10. Simon GE, Lin EH, Katon W, et al. Outcomes of “inadequate” antidepressant treatment. J Gen Intern Med. 1995;10(12):663-670. PubMed doi:10.1007/BF02602759
11. Simon GE, Von Korff M, Rutter CM, et al. Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry. 2001;58(4):395-401. PubMed doi:10.1001/archpsyc.58.4.395
12. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA. 2001;286(23):2947-2955. PubMed doi:10.1001/jama.286.23.2947
13. Corey-Lisle PK, Nash R, Stang P, et al. Response, partial response, and nonresponse in primary care treatment of depression. Arch Intern Med. 2004;164(11):1197-1204. PubMed doi:10.1001/archinte.164.11.1197
14. Smith D, Dempster C, Glanville J, et al. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. Br J Psychiatry. 2002;180(5):396-404. PubMed doi:10.1192/bjp.180.5.396
15. Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178(3):234-241. PubMed doi:10.1192/bjp.178.3.234
16. Allgulander C, Hackett D, Salinas E. Venlafaxine extended release (ER) in the treatment of generalised anxiety disorder: twenty-four-week placebo-controlled dose-ranging study. Br J Psychiatry. 2001;179(1):15-22. PubMed doi:10.1192/bjp.179.1.15
17. Gelenberg AJ, Lydiard RB, Rudolph RL, et al. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: a 6-month randomized controlled trial. JAMA. 2000;283(23):3082-3088. PubMed doi:10.1001/jama.283.23.3082
18. Liebowitz MR, Gelenberg AJ, Munjack D. Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry. 2005;62(2):190-198. PubMed doi:10.1001/archpsyc.62.2.190
19. Rickels K, Pollack MH, Sheehan DV, et al. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157(6):968-974. PubMed doi:10.1176/appi.ajp.157.6.968
20. Liebowitz MR, Asnis G, Mangano R, et al. A double-blind, placebo-controlled, parallel-group, flexible-dose study of venlafaxine extended release capsules in adult outpatients with panic disorder. J Clin Psychiatry. 2009;70(4):550-561. PubMed doi:10.4088/JCP.08m04238
21. Machado M, Iskedjian M, Ruiz I, et al. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Curr Med Res Opin. 2006;22(9):1825-1837. PubMed doi:10.1185/030079906X132415
22. Nemeroff CB, Entsuah R, Benattia I, et al. Comprehensive Analysis of Remission (COMPARE) with venlafaxine versus SSRIs. Biol Psychiatry. 2008;63(4):424-434. PubMed doi:10.1016/j.biopsych.2007.06.027
23. Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry. 2008;63(7):699-704. PubMed doi:10.1016/j.biopsych.2007.08.010
24. Stahl SM, Entsuah R, Rudolph RL. Comparative efficacy between venlafaxine and SSRIs: a pooled analysis of patients with depression. Biol Psychiatry. 2002;52(12):1166-1174. PubMed doi:10.1016/S0006-3223(02)01425-7
25. Bauer M, Tharmanathan P, Volz HP, et al. The effect of venlafaxine compared with other antidepressants and placebo in the treatment of major depression: a meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2009;259(3):172-185. PubMed doi:10.1007/s00406-008-0849-0
26. Thase ME, Shelton RC, Khan A. Treatment with venlafaxine extended release after SSRI nonresponse or intolerance: a randomized comparison of standard- and higher-dosing strategies. J Clin Psychopharmacol. 2006;26(3):250-258. PubMed doi:10.1097/01.jcp.0000219922.19305.08
27. Kornstein SG, Li D, Mao Y, et al. Escitalopram versus SNRI antidepressants in the acute treatment of major depressive disorder: integrative analysis of four double-blind, randomized clinical trials. CNS Spectr. 2009;14(6):326-333. PubMed
28. Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25(1):161-175. PubMed doi:10.1185/03007990802622726
29. Weinmann S, Becker T, Koesters M. Re-evaluation of the efficacy and tolerability of venlafaxine vs SSRI: meta-analysis. Psychopharmacology (Berl). 2008;196(4):511-520, discussion 521-522. PubMed doi:10.1007/s00213-007-0975-9
30. Kennedy SH, Andersen HF, Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31(2):122-131. PubMed
31. Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50(1):57-64. PubMed doi:10.1159/000078225
32. Alves C, Cachola I, Brandao J. Efficacy and tolerability of venlafaxine and fluoxetine in outpatients with major depression. Prim Care Psychiatry. 1999;5:57-63.
33. McPartlin GM, Reynolds A, Anderson C, et al. A comparison of once-daily venlafaxine XR and paroxetine in depressed outpatients treated in general practice. Prim Care Psychiatry. 1998;4:127-132.
34. Tylee A, Beaumont G, Bowden MW, et al. A double-blind, randomized, 12-week comparison study of the safety and efficacy of venlafaxine and fluoxetine in moderate to severe major depression in general practice. Prim Care Psychiatry. 1997;3:51-58.
35. Kobak KA, Taylor LH, Dottl SL, et al. A computer-administered telephone interview to identify mental disorders. JAMA. 1997;278(11):905-910. PubMed doi:10.1001/jama.278.11.905
36. Kobak KA, Taylor LH, Dottl SL, et al. Computerized screening for psychiatric disorders in an outpatient community mental health clinic. Psychiatr Serv. 1997;48(8):1048-1057. PubMed
37. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
38. Bech P, Gram LF, Dein E, et al. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51(3):161-170. PubMed doi:10.1111/j.1600-0447.1975.tb00002.x
39. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
40. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. PubMed doi:10.1111/j.1600-0447.1983.tb09716.x
41. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976:217-222.
42. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982, discussion 983. PubMed
43. Khan A, Brodhead AE, Kolts RL, et al. Severity of depressive symptoms and response to antidepressants and placebo in antidepressant trials. J Psychiatr Res. 2005;39(2):145-150. PubMed doi:10.1016/j.jpsychires.2004.06.005
44. Mallick R, Chen J, Entsuah AR, et al. Depression-free days as a summary measure of the temporal pattern of response and remission in the treatment of major depression: a comparison of venlafaxine, selective serotonin reuptake inhibitors, and placebo. J Clin Psychiatry. 2003;64(3):321-330. PubMed doi:10.4088/JCP.v64n0315
45. Keller MB, Trivedi MH, Thase ME, et al. The Prevention of Recurrent Episodes of Depression with Venlafaxine for Two Years (PREVENT) study: outcomes from the acute and continuation phases. Biol Psychiatry. 2007;62(12):1371-1379. PubMed doi:10.1016/j.biopsych.2007.04.040
46. Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat. 2001;11(1-2):9-21. PubMed doi:10.1081/BIP-100104194
47. Mallinckrodt CH, Sanger TM, Dubé S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry. 2003;53(8):754-760. PubMed doi:10.1016/S0006-3223(02)01867-X
48. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53. PubMed doi:10.1001/jama.2009.1943
49. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5(2):e45. PubMed doi:10.1371/journal.pmed.0050045
50. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252-260. PubMed doi:10.1056/NEJMsa065779
51. Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60(9):1167. PubMed doi:10.1176/appi.ps.60.9.1167