Results From an Online Survey of Patient and Caregiver Perspectives on Unmet Needs in the Treatment of Bipolar Disorder

ABSTRACT
Objective: To look at the manner in which patients and caregivers perceive the treatment of bipolar disorder compared with the evidence base for bipolar treatment.
Method: Between April 2013 and March 2014, 469 respondents took a 14-question online survey on demographics, medications taken, and perspectives on bipolar treatment and medications. Participants were recruited through social media outlets (Facebook and Twitter accounts) of Global Medical Education (New York, New York) and the blog Bipolar Burble, which has a primary audience of people with bipolar disorder. There were no exclusion criteria to participation, and both patients and health care professionals were encouraged to participate.
Results: Most respondents were taking ≥ 3 medications, and the greatest unmet need in treatment was for bipolar depression. In general, respondent perspectives on the effectiveness of individual medication treatments did not align with the available literature. Weight gain was the greatest side effect concern for both antipsychotics and mood stabilizers.
Conclusions: Our survey demonstrates that there are still many unmet needs in the treatment of bipolar disorder. There is also a mismatch between the evidence base for treatments in bipolar disorder and patient perception of the relative efficacy of different medications. In order to achieve better outcomes, there is a need to provide patients and clinicians greater quality education with regard to the best evidence-based treatments for bipolar disorder.
Prim Care Companion CNS Disord 2014;16(4):doi:10.4088/PCC.14m01655
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: March 13, 2014; accepted May 27, 2014.
Published online: August 28, 2014.
Corresponding author: Prakash S. Masand, MD, 26 E 63rd St, Ste 11-F, New York, NY 10065 ([email protected]).
Bipolar disorder is one of the most severe, chronic, and prevalent mental illnesses and affects about 5.7 million adults in the United States.1 Worldwide, bipolar disorder is the sixth leading cause of disability and can double a person’s risk of early death from a range of medical conditions, including obesity, diabetes, and cardiovascular disease.1
Despite many available treatments, a substantial proportion of patients with bipolar disorder experience partial remission of symptoms or recurrence of symptoms. In a meta-analysis, Geddes et al2 found that lithium, while regarded as a quality, first-line treatment option, only protects patients from a relapse in 60% of cases over 1 or 2 years (as compared to 40% of patients avoiding relapse on placebo). Moreover, while risk of mania was minimized with lithium (14% risk as opposed to 24% for placebo), the risk of a depression relapse was still significant (25% for lithium, 32% for placebo).2
There are several additional treatments approved by the US Food and Drug Administration (FDA) for bipolar mania and mixed episodes. As Geoffroy et al3 noted, there is no evidence of differences in efficacy, but there are differences in tolerability. Extrapyramidal symptom rates, including akathisia, are greater with risperidone, paliperidone, aripiprazole, and ziprasidone, whereas weight gain and metabolic disturbances are greater with olanzapine and quetiapine and less with ziprasidone, aripiprazole, and lurasidone.
Patients typically need more than 1 drug to maintain euthymia, and the practice of adding an atypical antipsychotic to a regimen of lithium, sodium valproate, and/or an antidepressant is still the trend. The agents that target the manic and mixed episodes of bipolar disorder have a stronger evidence base than those for the treatment of depressive episodes, particularly long term.
Bipolar depressive episodes are severely debilitating and disrupt the lives of many patients, yet there are currently few treatment options that have successfully demonstrated efficacy in clinical trials. There are only 3 FDA-approved treatments for acute bipolar depression: quetiapine for bipolar I and II depression as monotherapy, olanzapine-fluoxetine combination for bipolar I depression as monotherapy, and lurasidone for bipolar I as monotherapy and as an adjunct to lithium and valproate in nonresponders. Data on lithium are from poorly designed studies with very small sample sizes. There are negative trials of aripiprazole,4 ziprasidone,5 lamotrigine,5 and pramipexole5 in acute bipolar depression.
As stated by Kulkarni et al,6 relapse and recurrence can occur with depressive or manic symptoms, but symptomatic depressive relapse is more prevalent than symptomatic manic relapse over the course of 2 years at a ratio of approximately 2:1 in patients with bipolar I or schizoaffective disorder. Remission from syndromal mania is more common (92%) than remission from syndromal depression (76.5%).6 These results align with research by Judd et al,7 who conducted a long-term study of patients with bipolar I disorder and found that patients were symptomatically ill 47% of the time, predominantly with depressive symptoms.
METHOD
A survey designed to elicit patient, caregiver, and clinician perception of available treatments and of the unmet needs in bipolar illness was administered anonymously using Survey Monkey (www.surveymonkey.com). The survey population was drawn from those with access to the Internet between April 2013 and March 2014. Specifically, participants were recruited through the social media accounts (Facebook and Twitter) of Global Medical Education (New York, New York) and the blog Bipolar Burble (http://natashatracy.com), which has a primary audience of people with bipolar disorder. There were no exclusion criteria to participation, and both patients and health care professionals were encouraged to participate. The number of health care professionals (physicians, nurses, nurse practitioners, social workers, and physician assistants) answering the survey was too small (n = 36) to conduct a meaningful subanalysis. In this article, we only assessed the subsample of respondents (N = 433) who did not identify themselves as health care professionals.

- Survey results on patient and caregiver perception indicate that there are still many unmet needs in the treatment of bipolar disorder
- There is a mismatch between the evidence base for treatments in bipolar disorder and patient perception of the relative efficacy of different medications.
- The effects of direct-to-consumer advertising on patient perception of treatments and their side effects should not be underestimated.
RESULTS
Number of Medications Used by Patients With Bipolar Disorder
Patients in this survey were predominantly taking 2 or 3 medications for bipolar disorder, with more than half taking ≥ 3 medications and 13.3% taking ≥ 5 medications. Only 14.8% of people were taking 1 medication. This result confirms the fact that polypharmacy is the norm, rather than the exception, in the treatment of bipolar disorder.
Unmet Needs in the Treatment of Bipolar Disorder
Even though these patients were receiving extensive treatment, it was clear that there were many additional unmet needs. When asked what is the greatest unmet need in the treatment of bipolar disorder, 33.3% responded "treatment of depression," while 19.5% responded "treatment access," 20.3% "treatment affordability," 19.3% "relapse prevention," 4.5% "treatment of mania," and 3.2% "treatment of hypomania."
Medication Effectiveness
When it came to assessing the overall effectiveness of traditional mood stabilizers, lithium, and anticonvulsants, respondents were split on what they considered to be most effective. In fact, 97 respondents chose "I don’ t know" in terms of the most effective mood-stabilizing treatment, which was more than the best-ranked treatment—lithium—with 73 respondents judging it most effective.
When all votes, from most effective to least effective, were taken into consideration, lamotrigine, carbamazepine, and lithium were ranked similarly and were reported as considerably more effective than topiramate or valproate.
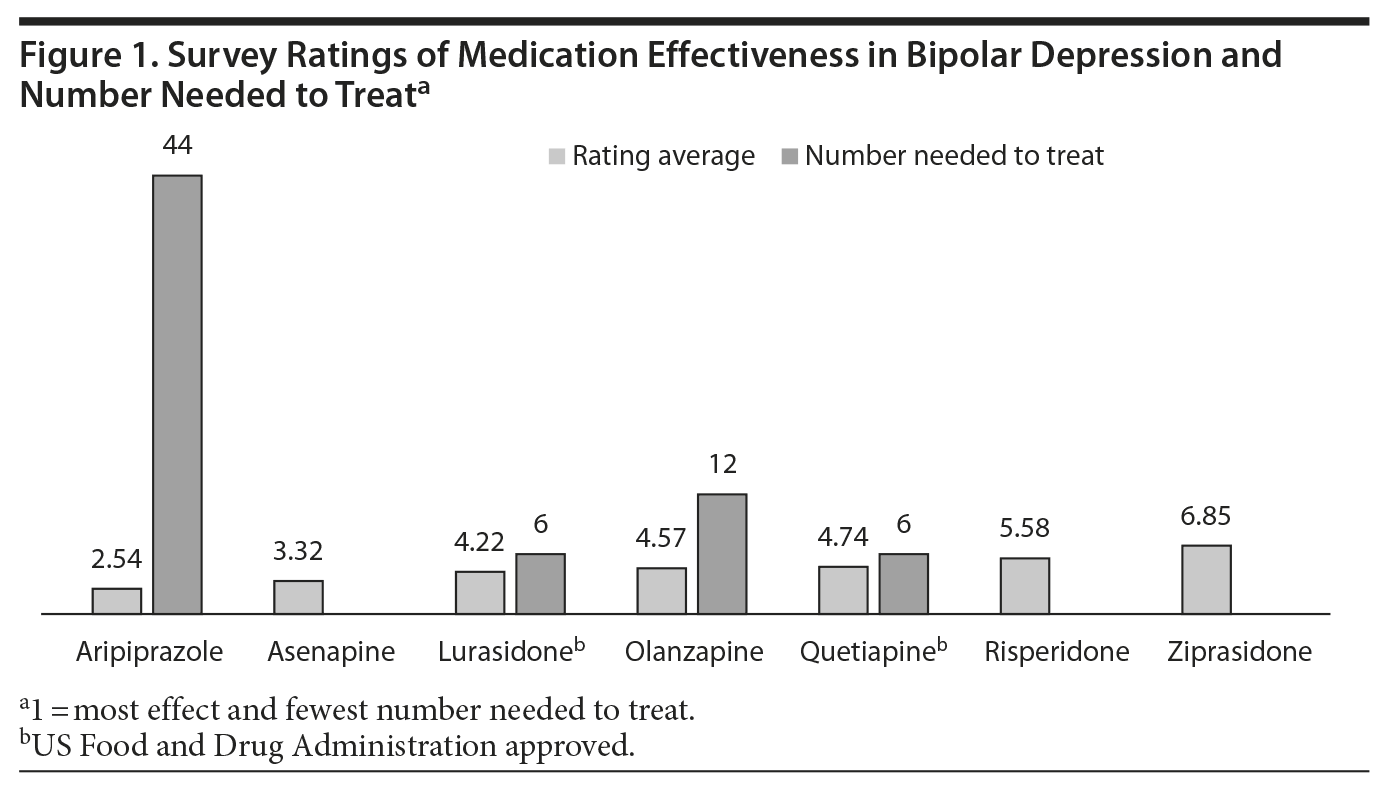
When it came to ranking antipsychotics on their effectiveness in treating bipolar depression, however, the results were much clearer. While 143 respondents did not have an opinion, the 303 who did respond overwhelmingly chose aripiprazole as the most effective treatment for bipolar depression, followed by, in order, asenapine, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone.
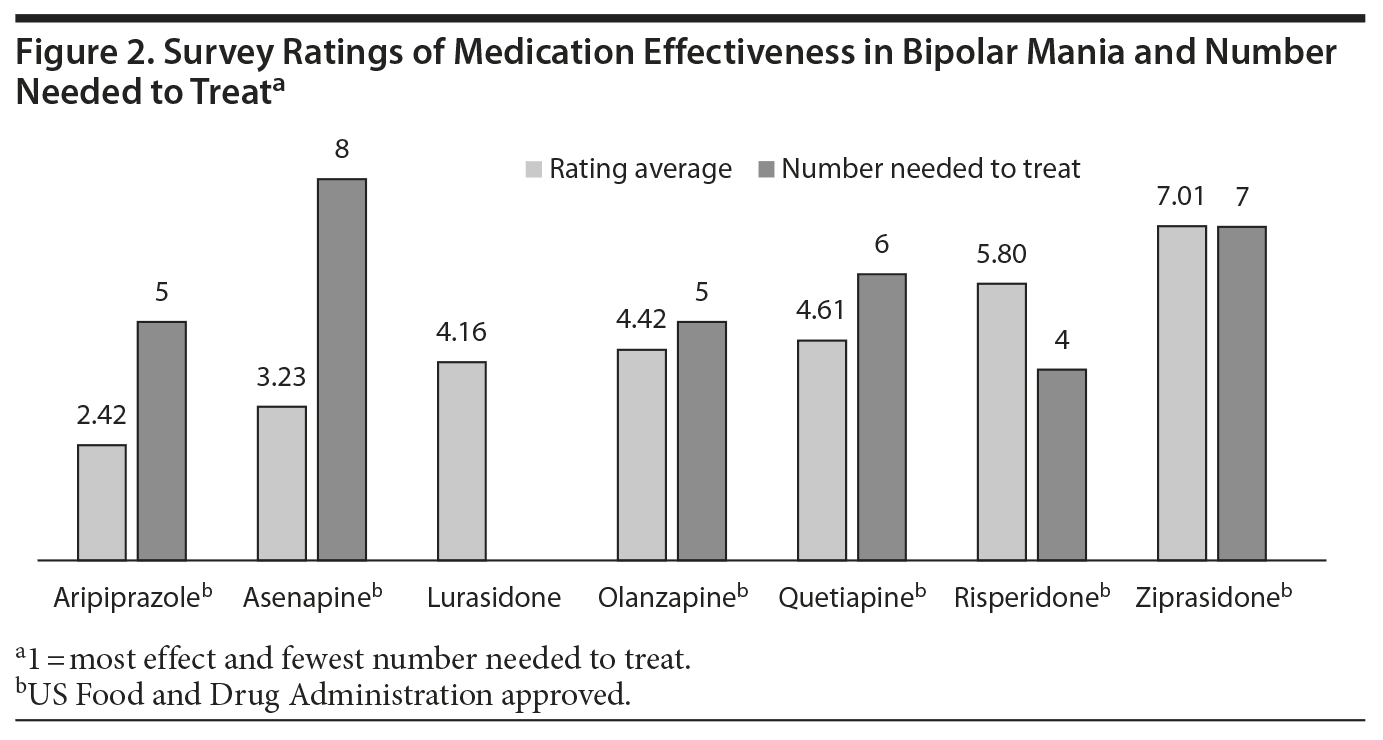
The perception of bipolar mania treatment effectiveness was also quite clear. In this case, 133 respondents did not have an opinion, while most respondents chose aripiprazole as the most effective bipolar mania treatment. The next treatment rated most effective was asenapine, followed by, in order, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone.
Medication Nonadherence and Relapse
The fact that no single drug is considered most effective may partially explain why medication nonadherence is so prevalent. In our survey, 18.8% of participants felt that medication nonadherence occurred in 30% to 70% of patients with bipolar disorder, and 15.9% felt it happened in more than 70% of cases.
Patient relapse was ascribed to medication nonadherence due to intolerable side effects by 23.3% of respondents. More respondents (43.2%), however, identified life stressors as the most common cause for bipolar relapse. Lack of medication effectiveness was identified by 22.9% of respondents, lack of access to psychotherapies by 9.6%, and substance abuse by 3%.
Medication Side Effect Concerns
Another factor that may explain the high levels of medication nonadherence is the concern over side effects. When asked their concerns over traditional mood stabilizer side effects, the most common response, by far, was weight gain at 46.4%, followed by concerns over effects the medications have on the liver or kidneys at 36.1%. Gastrointestinal side effects, effects on the unborn child, sexual side effects, and rash or other dermatologic effects were also noted but at much lower levels (6.5%, 6.2%, 3.4%, 1.4%, respectively).
Several side effects of antipsychotic treatment were considered most concerning, with weight gain selected by 33.3% of respondents. Other side effects selected by more than 9% of respondents were metabolic side effects (like diabetes) at 19%, parkinsonian symptoms at 17%, sedation at 14%, and akathisia or restlessness at 9.7%. Other side effects that a much smaller number of respondents were concerned about included effects on the unborn child and sexual side effects (4% and 3%, respectively).
Demographics
Respondents who took the survey were predominantly female (80%) and between the ages of 30 and 59 years. More than half were married or in a long-term partnership, and 82.4% were patients, while the remaining respondents were caregivers.
DISCUSSION
As noted in the editorial by Chengappa and Goodwin,8 there are significant unmet needs in the treatment of bipolar disorder that result in an increased burden of disease. Available data suggest that caregiver burden is high and largely neglected in bipolar disorder.9 In this study, we aimed to highlight some of these needs as perceived by patients and caregivers.
Our survey shows that most patients with bipolar disorder are taking ≥ 3 medications, but that it is common (13.3%) for a patient with bipolar disorder to be taking ≥ 5 medications. Our results are similar to those of Levine et al,10 who found that nearly 50% of study participants received ≥ 3 psychotropic agents, and no associations were noted between demographic parameters including age, gender, marital or educational status, and psychotropic prescriptions. As suspected, despite that level of treatment, the greatest unmet need was in the area of the treatment of bipolar depression (identified by 33.3%), with treatment access, treatment affordability, and relapse prevention being identified by about 1 in 5 respondents. In a survey of psychiatrists, Chengappa and Williams11 also found effective treatment options to be a major barrier to the effective management of bipolar disorder. Clinically effective treatments are also thought to lower the burden on caregivers of those with bipolar disorder.12
It was clear from the survey data that respondents had different views on treatment efficacy than is supported in the evidence-based literature. In our survey, lamotrigine, carbamazepine, and lithium were ranked more effective than topiramate and valproate. However, this result may be due to the number of people with experience with those medications rating the more popular medications highly. Similar findings are seen in the crowdsourcing site PatientsLikeMe (http://www.patientslikeme.com). On PatientsLikeMe, patients rated lithium and lamotrigine more efficacious in bipolar treatment (rated major-moderately effective for 64% and 71% of patients, respectively) than carbamazepine (58%) and topiramate (54%), but this result aligns with the number of people who completed ratings.13 Lithium had 165 evaluations and lamotrigine had 262 evaluations, while carbamazepine had only 17 and topiramate had only 11. (Valproate was ranked almost as effective [62%] as lamotrigine but had only 19 evaluations.)13
When survey participants were asked about the effectiveness of treating bipolar depression, aripiprazole was thought to be the most effective treatment, although there are 2 negative double-blind, placebo-controlled trials of aripiprazole in bipolar depression,4 and it is not FDA approved for that use (Figure 1). It is also notable that asenapine was selected as the second most effective treatment, as it is also not FDA approved for the treatment of bipolar depression. This finding may suggest a need for double-blind, placebo-controlled trials to demonstrate its efficacy in bipolar depression.
Lurasidone, which is FDA approved for both monotherapy14 and adjunctive therapy15 with lithium and valproate for the treatment of bipolar depression and has the best risk-benefit ratio of all approved treatments, was rated as the third most effective treatment.16
Aripiprazole was also considered to be the most effective in treating bipolar mania even though there are very few differences in the effect sizes of risperidone, olanzapine, and quetiapine compared to aripiprazole. Interestingly, lurasidone was considered to be the third most effective treatment even though the drug is not FDA approved for that use since it has not been studied in large double-blind, placebo-controlled studies (Figure 2). Lurasidone has demonstrated efficacy for subsyndromal manic symptoms in patients with bipolar depression. Moreover, all of the other antipsychotics approved for schizophrenia that have been studied for acute mania have demonstrated efficacy.
There clearly is a mismatch between the evidence base for treating bipolar illness and patient and caregiver perception of efficacy arguing for more education about this issue. The mismatch may also be due to a selection bias since clinicians may be choosing olanzapine and risperidone for the more severely ill patients given tolerability and safety concerns, leading to a diminished perception of efficacy relative to aripirazole, which may be chosen for mildly ill patients.
By far, the most concerning side effect for respondents was weight gain from both antipsychotics (33.3%) and mood stabilizers (46.4%). It is interesting to note that more respondents were concerned about weight gain on mood stabilizers than weight gain on antipsychotics, when, indeed, weight gain with many second-generation antipsychotics such as olanzapine, quetiapine, and clozapine is as problematic as with the traditional mood stabilizers according to Hasnain and Vieweg.16 Since the antipsychotics are more heavily advertised than the generic mood stabilizers, perception of the risk-benefit ratio may be skewed.
Parkinsonian symptoms were a primary concern to 17% of the respondents regarding antipsychotic treatment, which, though not surprising given that the second-generation antipsychotics, by definition, cause less extrapyramidal symptoms, is still a significant number. Clinicians need to be less complacent about extrapyramidal symptoms with the newer antipsychotics since they can be a source of great morbidity and even suicidality.
While a significant number of respondents did not know about the prevalence of medication nonadherence, the second most common answer with regard to nonadherence was a rate of 30%-70%, perhaps indicating that many of the respondents themselves had been medication nonadherent at 1 or more times. This finding is within the documented range of 36%-80% nonadherence according to Sylvia et al.17 Only 7.5% of people thought that medication nonadherence occurred in less than 20% of patients.
Life stressors were stated to be the most common cause of relapse by 43.2% of the respondents. There is extensive literature to support this perception. In fact, psychoeducation and therapies like cognitive-behavioral therapy and interpersonal therapy have been shown to significantly decrease relapse and recurrence rates when added to medication in patients with bipolar illness.18 Unfortunately, they are underutilized because of the paucity of trained therapists, cost, and reimbursement issues.
Our survey shows that perceptions about medication treatment efficacy and tolerability are often at odds with the evidence base in the literature. Is this a function of direct-to-consumer or other forms of marketing? Or is it a function of inadequate education of physicians around efficacy data? The answer is probably both. Aripiprazole is heavily advertised for major depression as an adjunct (for which it is FDA approved), which may lead to a halo effect for its perceived efficacy in bipolar depression.
The effects of direct-to-consumer marketing should not be underestimated, as this controversial form of advertising has been shown to influence illness conversation, side effect perception, and prescription requests. Proponents of direct-to-consumer advertising state that consumers can make more informed choices about the products advertised with the additional information, while critics say that direct-to-consumer ads lead to overprescribing of more expensive medications and possibly even overdiagnosis. And, because individuals with bipolar disorder are more likely to visit health- and support-related websites, they are more likely than the average consumer to see these direct-to-consumer ads.
Dieringer et al19 reported on responsiveness to direct-to-consumer advertising, and a correlation was made to the number of prescription medications a patient was taking. In their study, 25% of the study’s general population was responsive to direct-to-consumer marketing, but of those taking ≥ 5 prescription medications, 45% were found responsive to direct-to-consumer marketing. As shown in our study, patients with bipolar disorder tend to be taking multiple medications, with 13.3% taking ≥ 5 medications.
Similarly, Bell et al20 found that online depression support group members were shown to be responsive to online marketing, with 52.4% visiting the official drug websites after seeing a direct-to-consumer ad. More telling, though, is that 39.9% of people talked to their doctor and 20.3% made an advertising-induced prescription request after seeing such advertising. This patient behavior also affects doctors’ behaviors, as Kravitz et al21 showed that when patients request medication, whether specifically or generally, doctors are between 20% and 45% more likely to prescribe antidepressants in cases of depression than when no request is made. Consumers may be underestimating the risks of these medications. This underestimation could partially be because many pharmaceutical websites do not list even the top 3 side effects, as noted by Davis et al.22 Another study by Davis23 also found that the less complete the risk statement, the more positively consumers rated the drugs. Thus, in the absence of information from their doctor, decisions may be made from advertising material alone.
The limitations of our survey include the following: the respondents were self-identified bipolar patients, and diagnosis and medical details were self-reported and were not verified; the assumption that the respondents had personal experience with the medications surveyed versus perceptions based on advertisements and discussion with fellow patients and colleagues; the possibility of gender bias influencing results since the majority of our respondents were female, whereas bipolar disorder is equally distributed between the genders; and use of a selected sample of Facebook and Twitter followers and blog readers of the authors versus a broader sample.
CONCLUSIONS
Clinicians often believe that bipolar disorder has a better prognosis compared to other chronic psychiatric illnesses. Our survey demonstrates that there are still many unmet needs in the treatment of bipolar disorder. There is also a mismatch between the evidence base for treatments in bipolar disorder and patient perception of the relative efficacy of different medications. In order to achieve better outcomes, there is a need to provide patients and clinicians with greater quality education with regard to the best evidence-based treatments for bipolar disorder.
Drug names: aripiprazole (Abilify), asenapine (Saphris), carbamazepine (Tegretol, Epitol, and others), clozapine (Clozaril, FazaClo, and others), fluoxetine (Prozac and others), lamotrigine (Lamictal and others), lithium (Lithobid, Eskalith, and others), lurasidone (Latuda), olanzapine (Zyprexa and others), paliperidone (Invega), pramipexole (Mirapex and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), topiramate (Topamax and others), ziprasidone (Geodon and others).
Author affiliations: Global Medical Education, Inc, New York, New York (Dr Masand), and self-employed freelancer, Victoria, British Columbia, Canada (Ms Tracy).
Potential conflicts of interest: Dr Masand has served as a consultant to Forest, Lundbeck, Takeda, Pamlab, Pfizer, and Sunovion; has received grant/research support from Forest; has received honoraria from and served on the speakers or advisory boards of Forest, GlaxoSmithKline, Lundbeck, Merck, Pamlab, Pfizer, Sunovion, and Takeda; and is a stock shareholder in Global Medical Education. Ms Tracy reports no conflicts of interest related to the subject of this article.
Funding/support: None reported.
REFERENCES
1. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939. PubMed doi:10.1001/jamapsychiatry.2013.1394
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;161(2):217-222. PubMed doi:10.1176/appi.ajp.161.2.217
3. Geoffroy PA, Bellivier F, Henry C. Treatment of manic phases of bipolar disorder: critical synthesis of international guidelines [article in French]. Encephale. 2014. PubMed
4. Yatham LN. A clinical review of aripiprazole in bipolar depression and maintenance therapy of bipolar disorder. J Affect Disord. 2011;128(suppl 1):S21-S28. PubMed doi:10.1016/S0165-0327(11)70005-2
5. Selle V, Schalkwijk S, Vסzquez GH, et al. Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics. Pharmacopsychiatry. 2014;47(2):43-52. PubMed doi:10.1055/s-0033-1363258
6. Kulkarni J, Filia S, Berk L, et al. Treatment and outcomes of an Australian cohort of outpatients with bipolar I or schizoaffective disorder over twenty-four months: implications for clinical practice. BMC Psychiatry. 2012;12(1):228. PubMed doi:10.1186/1471-244X-12-228
7. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. PubMed doi:10.1001/archpsyc.59.6.530
8. Chengappa KR, Goodwin GM. Characterizing barriers, challenges and unmet needs in the management of bipolar disorder. Bipolar Disord. 2005;7(suppl 1):5-7. PubMed doi:10.1111/j.1399-5618.2005.00188.x
9. Ogilvie AD, Morant N, Goodwin GM. The burden on informal caregivers of people with bipolar disorder. Bipolar Disord. 2005;7(suppl 1):25-32. PubMed doi:10.1111/j.1399-5618.2005.00191.x
10. Levine J, Chengappa KN, Brar JS, et al. Psychotropic drug prescription patterns among patients with bipolar I disorder. Bipolar Disord. 2000;2(2):120-130. PubMed doi:10.1034/j.1399-5618.2000.020205.x
11. Chengappa KR, Williams P. Barriers to the effective management of bipolar disorder: a survey of psychiatrists based in the UK and USA. Bipolar Disord. 2005;7(suppl 1):38-42. PubMed doi:10.1111/j.1399-5618.2005.00193.x
12. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168. PubMed doi:10.1176/appi.ajp.2013.13070984
13. Patientslikeme. Live better, together! http://www.patientslikeme.com. Accessed May 5, 2014.
14. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-177. PubMed doi:10.1176/appi.ajp.2013.13070985
15. Citrome L, Ketter TA, Cucchiaro J, et al. Clinical assessment of lurasidone benefit and risk in the treatment of bipolar I depression using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2014;155:20-27. PubMed doi:10.1016/j.jad.2013.10.040
16. Hasnain M, Vieweg WV. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med. 2013;125(5):117-129. PubMed doi:10.3810/pgm.2013.09.2706
17. Sylvia LG, Reilly-Harrington NA, Leon AC, et al. Medication adherence in a comparative effectiveness trial for bipolar disorder [published online ahead of print October 3, 2013]. Acta Psychiatr Scand. 2014;129(5):359-365. PubMed doi:10.1111/acps.12202
18. Vieta E, Pacchiarotti I, Valent× M, et al. A critical update on psychological interventions for bipolar disorders. Curr Psychiatry Rep. 2009;11(6):494-502. PubMed doi:10.1007/s11920-009-0075-0
19. Dieringer NJ, Kukkamma L, Somes GW, et al. Self-reported responsiveness to direct-to-consumer drug advertising and medication use: results of a national survey. BMC Health Serv Res. 2011;11(1):232. PubMed doi:10.1186/1472-6963-11-232
20. Bell RA, Taylor LD, Kravitz RL. Do antidepressant advertisements educate consumers and promote communication between patients with depression and their physicians? Patient Educ Couns. 2010;81(2):245-250. PubMed doi:10.1016/j.pec.2010.01.014
21. Kravitz RL, Epstein RM, Feldman MD, et al. Influence of patients’ requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. JAMA. 2005;293(16):1995-2002. PubMed doi:10.1001/jama.293.16.1995
22. Davis JJ, Cross E, Crowley J. Pharmaceutical websites and the communication of risk information. J Health Commun. 2007;12(1):29-39. PubMed doi:10.1080/10810730601091326
23. Davis JJ. Riskier than we think? the relationship between risk statement completeness and perceptions of direct to consumer advertised prescription drugs. J Health Commun. 2000;5(4):349-369. PubMed doi:10.1080/10810730050199141