Objective: To examine demographic characteristics of patients who experience seclusion and restraint (S&R) events in a pediatric psychiatric inpatient setting and assess whether sleep time 24 hours prior and after the occurrence of an S&R event was different from average sleep time during hospitalization.
Methods: Charts from an acute care inpatient child and adolescent psychiatric unit from 2012 to 2014 were reviewed. A paired samples t test was performed to look for significant differences in sleep time 24 hours prior to S&R versus average sleep times for the same patients during hospitalization.
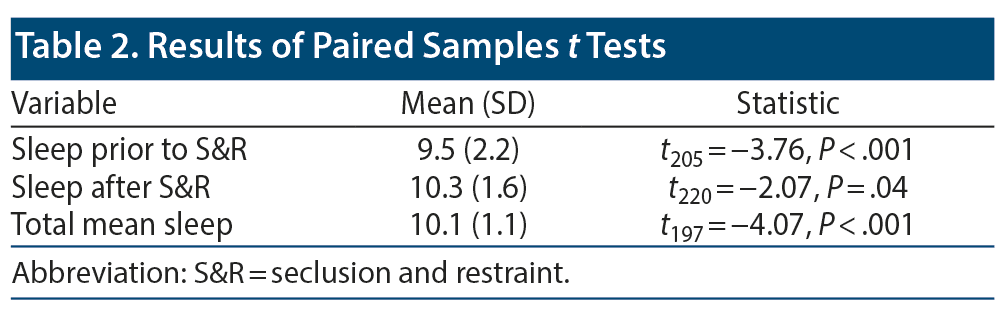
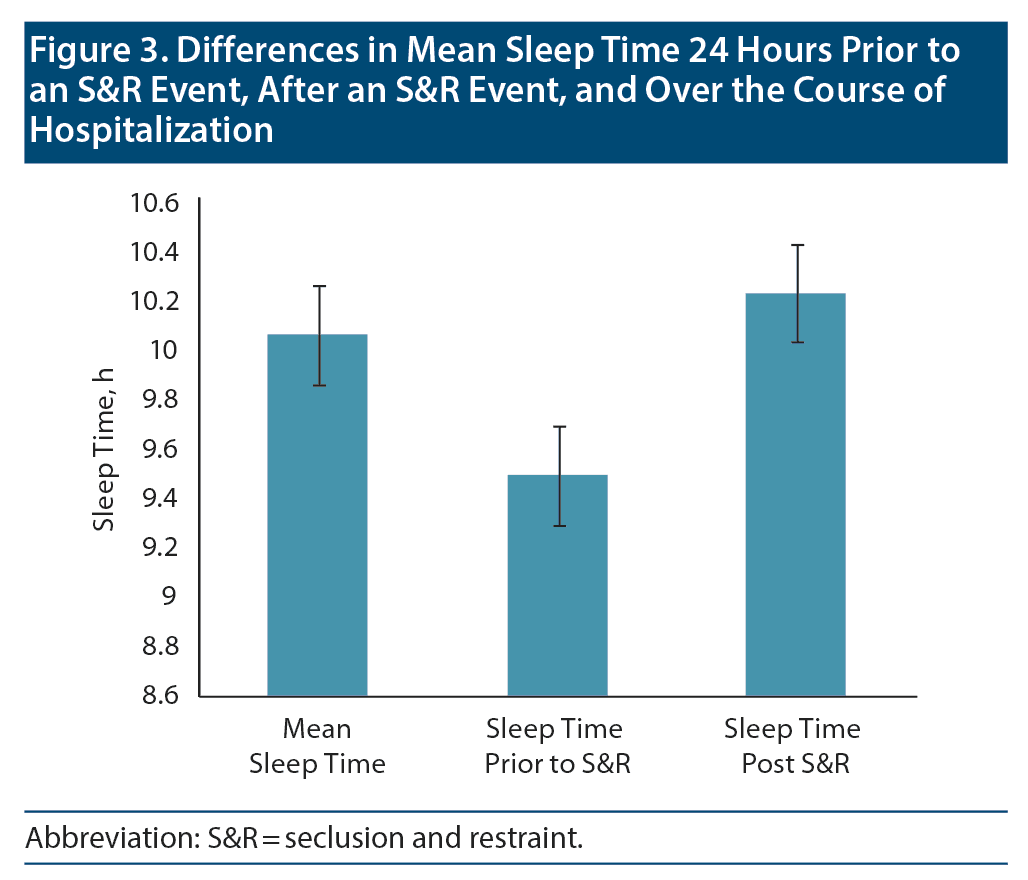
Results: A total of 232 S&R events occurred between 2012 and 2014. Of the incidents, 172 involved children who were ≤ 12 years old, and 178 incidents involved male patients. A paired sample t test revealed a significant mean (SD) decrease in sleep time prior to S&R (9.5 [2.24]) compared to average sleep time during hospitalization (10.07 [1.08], t205 = −3.722, P < .01).
Conclusions: The study results reveal a statistically significant reduction in sleep time 24 hours prior to an S&R event compared to average sleep duration during hospitalization. The association between sleep times and subsequent problem behaviors in an inpatient setting require further evaluation.
Objective: To examine demographic characteristics of patients who experience seclusion and restraint (S&R) events in a pediatric psychiatric inpatient setting and assess whether sleep time 24 hours prior and after the occurrence of an S&R event was different from average sleep time during hospitalization.
Methods: Charts from an acute care inpatient child and adolescent psychiatric unit from 2012 to 2014 were reviewed. A paired samples t test was performed to look for significant differences in sleep time 24 hours prior to S&R versus average sleep times for the same patients during hospitalization.
Results: A total of 232 S&R events occurred between 2012 and 2014. Of the incidents, 172 involved children who were ≤ 12 years old, and 178 incidents involved male patients. A paired sample t test revealed a significant mean (SD) decrease in sleep time prior to S&R (9.5 [2.24]) compared to average sleep time during hospitalization (10.07 [1.08], t205 = −3.722, P < .01).
Conclusions: The study results reveal a statistically significant reduction in sleep time 24 hours prior to an S&R event compared to average sleep duration during hospitalization. The association between sleep times and subsequent problem behaviors in an inpatient setting require further evaluation.
Prim Care Companion CNS Disord 2020;22(6):20m02612
To cite: Albers C, Nair N, Shankar R. Association between sleep times and seclusion and restraint events in an inpatient pediatric psychiatric hospital: a retrospective chart review. Prim Care Companion CNS Disord. 2020;22(6):20m02612.
To share: https://doi.org/10.4088/PCC.20m02612
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Missouri Health Care, Columbia, Missouri
*Corresponding author: Cecilia Albers, MD, Department of Psychiatry, University of Missouri Health Care, 3 Hospital Drive, Columbia, MO 65212 ([email protected]).
Sleep is a fundamental human need, but it can be altered or disrupted in a variety of ways. There is limited research on how being admitted to an inpatient psychiatric facility affects children’s sleep. However, research based on children and adults admitted to medical units has shown that children admitted to critical care medical units experience decreased quality and duration of sleep.1 Studies2 have also shown that children admitted to inpatient units experience more nighttime awakenings. Many of these factors such as increased light levels and talking, which are commonly cited as reasons for poor sleep, are also present on an inpatient psychiatric unit.1,3
Although there is lot of research on how changes in sleep affect aggression and other behavioral conditions, there is little research on sleep as a potential target to reduce seclusion and restraint (S&R), given that sleep changes are common to many psychiatric conditions that lead to inpatient admission. Changes in sleep in patients with mental illness may occur in several forms such as difficulty falling asleep or waking up too early, restless or nonrestorative sleep, and daytime sleepiness or increased total sleep time.4 Depression is often associated with decreased sleep times and frequent awakenings. There is also some evidence that the increase in depressive symptoms seen in adolescents may be related to decreased sleep periods caused by both biological and social changes.5 Although sleep changes are not a diagnostic criterion for children with conduct disorder (CD) and oppositional defiant disorder (ODD), there is evidence that children with these disorders experience sleep changes. A study6 found that children with ODD/CD had decreased sleep duration compared to age-matched peers with attention-deficit/hyperactivity disorder (ADHD). Parents also reported an increase in behaviors when sleep time was decreased.6 Sleep disturbances and behavioral concerns are both commonly seen in children with autism spectrum disorder (ASD) as well. A study7 showed a significant relationship between sleep disturbances, particularly night awakenings, and physical aggression, irritability, inattention, and hyperactivity. A decrease in sleep time is also seen in psychotic disorders.8 Decreased sleep times are associated with an increased risk of development of psychosis in high-risk adolescents.8 In patients with schizophrenia, insomnia is a commonly occurring comorbid disorder and is related to severity of symptoms.9,10 Anxiety is also associated with an increase in sleep latency. Decreased sleep times are associated with an increased report of subjective anxiety.11
Even relatively small changes in sleep can lead to worsening mood, and these changes become greater over time. A study12 of young adults showed that reducing sleep time to less than 5 hours per night resulted in an increase in reports of mood disturbance and irritability. Cumulative sleep increase has also been associated with an increase in negative emotional responses to daytime disruptions. A single night of sleep disturbance has been correlated with an increase in impulsivity, anger, anxiety, and stress even in response to low-stress negative stimuli.13
S&R is an intervention with limited research in children and adolescents. The only indication for the use of S&R in a psychiatric setting is to prevent or stop dangerous behavior to self or others and to prevent disorganization or serious disruption of the treatment program as a result of significant safety concerns. It is an intervention with many possible negative outcomes including death.14-20 For these reasons, reduction of S&R is ideal. Several studies have identified risk factors associated with S&R in adults and children. In adults, factors such as involuntary admission, male sex, younger age, severity of symptoms, and uncooperativeness were risk factors for S&R.21,22 In children and adolescents, a wide variety of patient risk factors for S&R have been identified, such as younger age, attempts to harm others, female sex, lower psychosocial functioning, use of agitation medications, and longer admissions.23-25 Risk factors related to the facility have also been identified, such as lack of access to acute agitation medications and being a large publicly funded residential treatment center.26,27
METHODS
This study was a retrospective chart review of S&R events that occurred on an acute inpatient pediatric psychiatric unit of an academic hospital located in Missouri (from 2012 to 2014). This study was approved by the University of Missouri Institutional Review Board with full waiver of consent.
Setting
The setting of the study was a single 17-bed child and adolescent psychiatric inpatient unit in an academic center. The unit serves patients from Missouri and neighboring states. Staff members are dedicated to the child and adolescent psychiatry unit and comprise nursing staff, social workers, mental health technicians, an occupational and recreation therapist, 2 child and adolescent psychiatrists, and resident/fellow trainees. Staff and physicians are trained on S&R, and new employees are also oriented to the protocol. The inpatient unit also has a performance improvement specialist who regularly monitors all S&R on the unit including orders and documentation and also provides education and training to staff to ensure national regulatory standards are followed. The unit follows the American Academy of Child and Adolescent Psychiatry practice parameters and the standards set by the Joint Commission on Accreditation of Healthcare Organization when patients are placed in S&R.28
Sampling
S&R events were identified based on the presence of a physician order being placed for S&R. All S&R events that occurred between 2012 and 2014 were included. The inclusion criterion was any patient that was in S&R, and reasons for S&R included physical aggression to self or others, physical destruction of property, extreme disruptive behaviors, and suicidal behaviors (danger to self and inability to stay safe even with staff support). Patients are released from S&R when they are able to display safe behaviors and contract for safety.

- Sleep is an important biological process that is often altered by psychiatric illness and can be disrupted in a variety of ways during hospitalization.
- Assessment of sleep concerns should be a goal of all treatment teams and may lead to a reduction in the use of seclusion and restraint on the inpatient unit.
Sleep times 24 hours prior to an S&R event that occurred on the day of admission were unavailable and hence were excluded from further analysis. If a patient had multiple S&R events on the same day, all of the S&R events were included and were identified as an order that was placed in the electronic medical record along with the corresponding S&R documentation.
Data Extraction
The Chronic Behavioral and Affective Dysregulations Syndromes (CBADS)29 is an instrument developed at the University of Missouri and approved by the university institutional review board. The CBADS collected 51 data points about each patient at the time of admission including age, diagnosis, date of admission, race, living situation, history of prior inpatient and outpatient treatment, psychiatric history, medical history, and family psychiatric history. Data were gathered by retrospective electronic chart review of admission notes from 2012 to 2014 for patients who had experienced at least 1 S&R during their hospitalization. Research personnel were trained to acquire CBADS and S&R data. In addition to demographic information, data were gathered about each S&R event. All documentation that was created on the day of the S&R including progress notes, nursing documentation, medication list, and S&R notes were reviewed for information about the S&R event. The notes were used to determine the time, type, reason, and duration of S&R.
In addition, the electronic medical record was also used to gather information on sleep times 24 hours prior to and after the S&R event. Sleep time information was gathered by patient care technicians as part of the usual care of patients. Technicians observed the patients visually to determine if they appeared to be awake or asleep and recorded it on paper. Those data were then reported as a total every 8 hours in the electronic medical record. For each patient, the average sleep time over the entire course of the hospitalization was also calculated along with all sleep that occurred the day before each S&R event including day sleeping and naps.
Statistical Analysis
For the purpose of analysis, Axis I diagnosis at the time of admission was divided into 6 groups: group 1 comprised mood disorders such as major depressive disorder and bipolar disorder. Group 2 comprised patients who were admitted because of anxiety disorder-related Axis I diagnosis. Patients who had an Axis I diagnosis of autism, pervasive developmental disorders, and other neurodevelopmental disorders at the time of admission were in group 3. Group 4 comprised patients with Axis I diagnoses of ADHD or ODD at time of admission. Posttraumatic stress disorder patients were included in group 5, and all other diagnoses were included in group 6.
A paired samples t test was performed in SPSS version 25 (IBM Corp, Armonk, New Jersey) to look for significant differences in sleep time 24 hours prior to S&R, sleep time post S&R event, and average sleep time documented during the course of hospitalization. Missing data were excluded on a case-by-case basis by the analysis software.
RESULTS
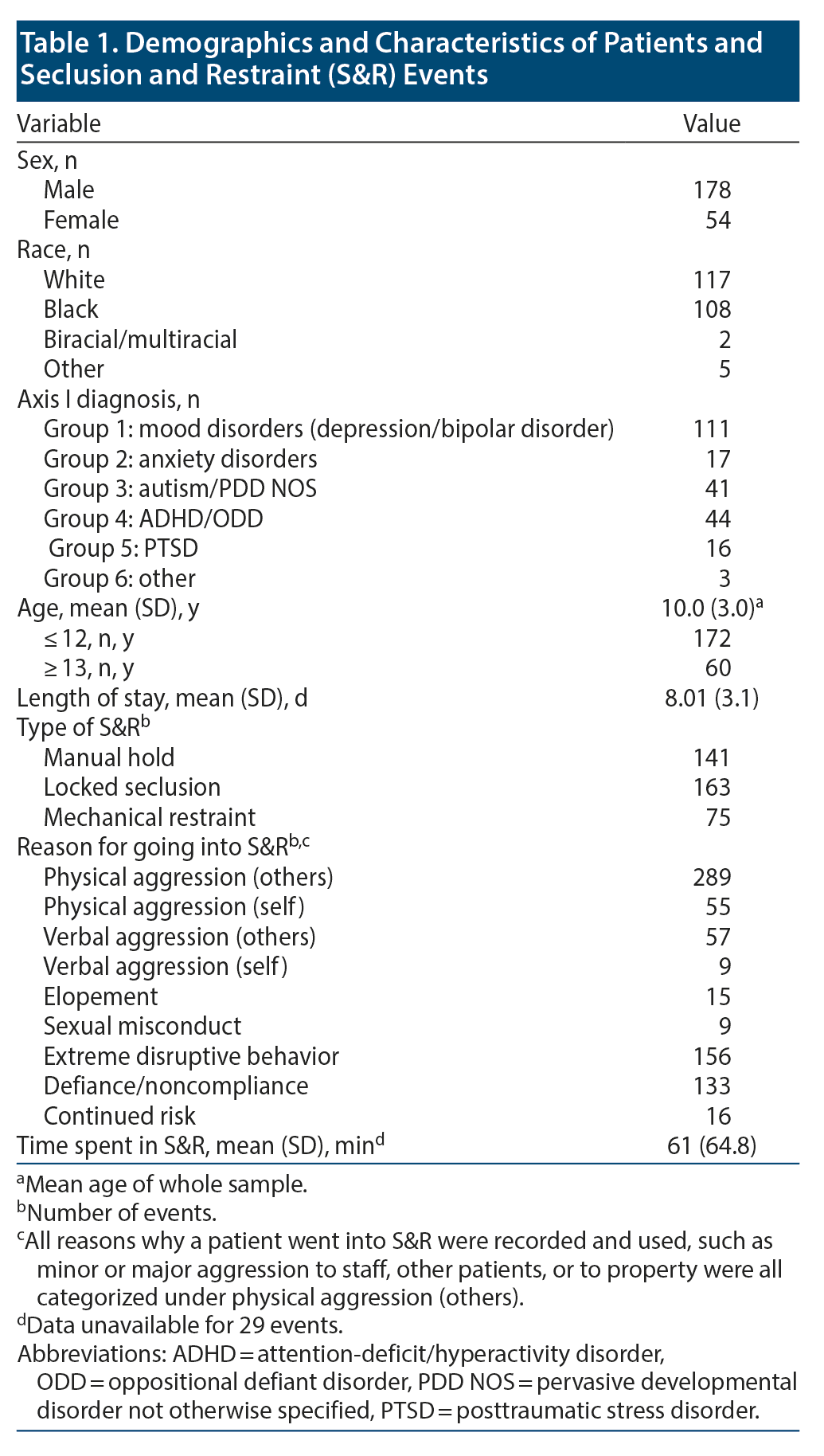
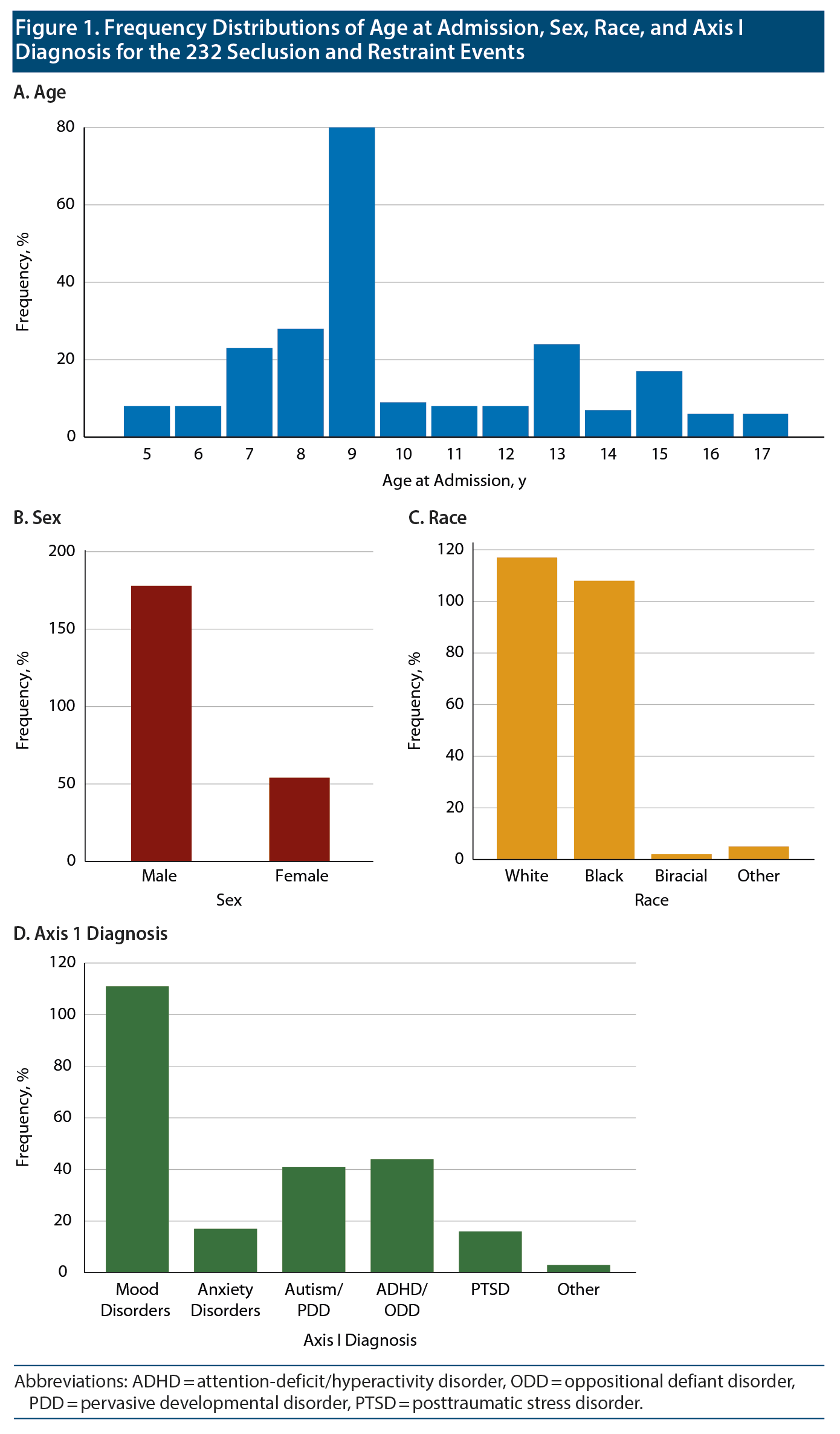
During 2012-2014, 232 S&R events occurred, all of which were used in this stage of the study. The mean (SD) age of the patients was 10 (3.0) years (range, 5-17 years). Of the incidents, 178 involved male patients, while 54 involved female patients; 117 white patients and 108 black patients were placed in S&R. Data showed that 111 of the incidents involved patients with a primary Axis I diagnosis of mood disorders and 172 involved patients who were aged ≤12 years. Table 1 and Figure 1 provide more details on the patients.
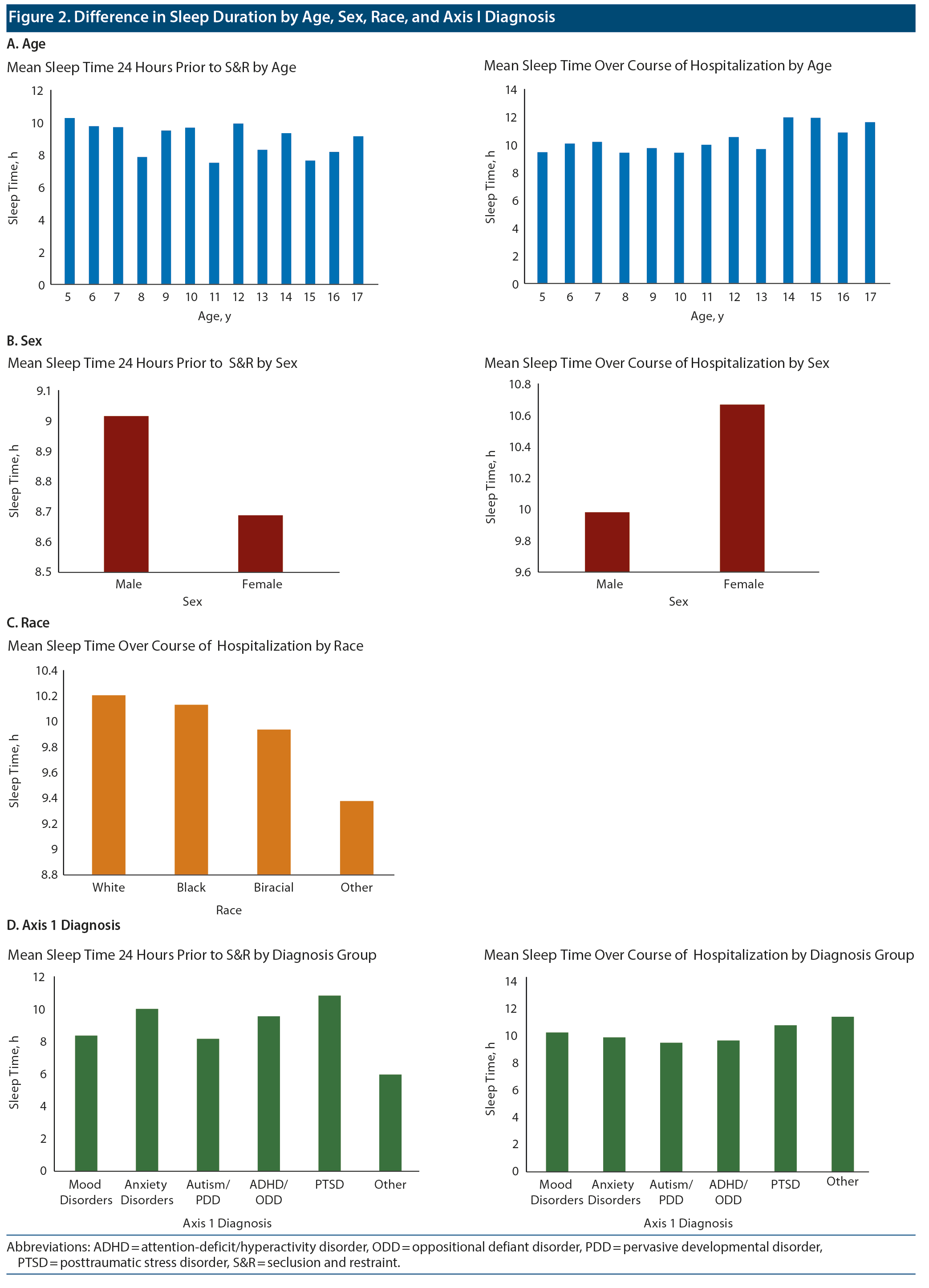
Data on sleep duration 24 hours prior to an S&R event were available for 206 S&R cases recorded between 2012 and 2014, while mean sleep duration during hospitalization data were available for 232 S&R cases. Data on sleep times after S&R were available in 198 cases. Sleep time data that differed significantly based on age, sex, race, and diagnosis are displayed in Figure 2. A paired sample t test revealed a significant mean (SD) decrease in sleep time prior to S&R (9.5 [2.24]) compared to average sleep time during hospitalization (10.07 [1.08], t205 = −3.722, P < .01). Significant differences were also observed in average sleep time during hospitalization and sleep time post S&R event and between sleep time prior to and post S&R event (Table 2, Figure 3).
DISCUSSION
The objective of the current study was to determine whether there were differences in sleep time 24 hours prior to and after an S&R event compared to mean sleep time during hospitalization. We found a statistically significant decrease in sleep of approximately 30 minutes that occurs the night prior to S&R when compared to average sleep time during hospitalization.
While it is not possible to know from this study what exact role this sleep change has in the subsequent S&R events, there is evidence that sleep disturbances can increase aggression and other disruptive behaviors in children. A study30 of hospitalized patients with autism found that higher Aberrant Behavior Checklist-Community scores (irritability, stereotypy, and hyperactivity subscales) at admission were significantly associated with fewer minutes slept during the last 5 nights of hospitalization. A study31 of normally developing preschoolers showed that chronic shortened sleep duration was associated with increasing hyperactivity and impulsivity by school age. Given these study results, there is some suggestion of a link between sleep changes and impulsivity and aggression, which are common in S&R events that occur on a child and adolescent psychiatry inpatient unit. However, it is not possible to tell if this decrease in sleep is because these patients are becoming agitated due to their psychopathology, leading to the later S&R, or if sleeping poorly is the cause of their later S&R.
This study was an exploratory chart review that has several limitations. Since this study was retrospective, sleep data were limited to a subjective report made by nursing staff and reported in 8-hour aggregates. Ideally, future studies should utilize more objective methods of data collection such as actigraphs to measure sleep time and quality. It would have been ideal to have more information about patients’ sleeping patterns over time, including out of the hospital setting, and total number of hospitalizations. Another limitation of this study is that the sample consisted only of patients that had S&R events. Sleep time of the patients was compared to their own average over the course of the hospitalization, but there were no outside controls. It is possible that sleep times for patients who have an S&R event may be significantly different from other patients on the unit that did not have this event, including those who had similar behaviors but were able to be redirected and did not require S&R. Finally, our study does not account for all possible sedating medications that patients might be exposed to during the hospitalization including acute agitation and scheduled medications. Ideally, future studies would account for these factors.
When comparing our patient sample to other studies looking at risk factors for S&R, it is notable that our sample seems to have more males and a younger age. It is important to note with regard to age that this may simply be because it is not clear if the units described in other studies accepted preadolescent patients. The diagnoses of our sample appear to be similar to others. Although many studies did not provide information on race, given the geographical spread of our sample compared to others, there likely are racial differences related to local population.23-25,32,33
Despite these limitations, these preliminary data when compared to other studies do suggest that there is some link between changes in sleep and S&R events, which may provide a future symptomatic target to reduce use of S&R in the inpatient setting. Ideally, in future studies, we would like to compare sleep times (subjective and objective) and behavioral measures between different patient groups (with S&R vs without S&R). Also, to draw stronger conclusions, larger multisite epidemiologic studies are warranted. Such studies would also address biases regarding individual institutional policies around S&R and, to a certain extent, possible staff perceptional biases as well.
Submitted: March 5, 2020; accepted July 9, 2020.
Published online: December 23, 2020.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: Poster presented at the 66th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Chicago, IL; October 18, 2019.
REFERENCES
1.Pilkington S. Causes and consequences of sleep deprivation in hospitalised patients. Nurs Stand. 2013;27(49):35-42. PubMed CrossRef
2.Linder LA, Christian BJ. Nighttime sleep disruptions, the hospital care environment, and symptoms in elementary school-age children with cancer. Oncol Nurs Forum. 2012;39(6):553-561. PubMed CrossRef
3.Crawford S, Utt A, Beebe C, et al. Quality of sleep in a pediatric hospital: a descriptive study based on an assessment of interruptions, perceptions, and the environment. J Nurs Adm. 2019;49(5):273-279. PubMed CrossRef
4.Tarokh L, Hamann C, Schimmelmann BG. Sleep in child and adolescent psychiatry: overlooked and underappreciated. Eur Child Adolesc Psychiatry. 2014;23(6):369-372. PubMed CrossRef
5.Raniti MB, Allen NB, Schwartz O, et al. Sleep duration and sleep quality: associations with depressive symptoms across adolescence. Behav Sleep Med. 2017;15(3):198-215. PubMed CrossRef
6.Aronen ET, Lampenius T, Fontell T, et al. Sleep in children with disruptive behavioral disorders. Behav Sleep Med. 2014;12(5):373-388. PubMed CrossRef
7.Mazurek MO, Sohl K. Sleep and behavioral problems in children with autism spectrum disorder. J Autism Dev Disord. 2016;46(6):1906-1915. PubMed CrossRef
8.Bordoloi M, Ramtekkar U. Relationship between sleep and psychosis in the pediatric population: a brief review. Med Sci (Basel). 2018;6(3):76. PubMed
9.Yates NJ. Schizophrenia: the role of sleep and circadian rhythms in regulating dopamine and psychosis. Rev Neurosci. 2016;27(7):669-687. PubMed CrossRef
10.Poon YPY, Kan CK, Yeung WF, et al. Delayed sleep-wake phase disorder and delayed sleep-wake phase in schizophrenia: clinical and functional correlates. Schizophr Res. 2018;202:412-413. PubMed CrossRef
11.Mullin BC, Pyle L, Haraden D, et al. A preliminary multimethod comparison of sleep among adolescents with and without generalized anxiety disorder. J Clin Child Adolesc Psychol. 2017;46(2):198-210. PubMed CrossRef
12.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20(4):267-277. PubMed
13.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10(1):679-708. PubMed CrossRef
14.Weiss EM. Deadly restraint: a nationwide pattern of death. Hartford Courant. October 1998:11-15. https://www.courant.com/news/connecticut/hc-xpm-1998-10-11-9810090779-story.html. Accessed October 27, 2020.
15.Berzlanovich AM, Schöpfer J, Keil W. Deaths due to physical restraint. Dtsch Arztebl Int. 2012;109(3):27-32. PubMed
16.LeBel J, Stromberg N, Duckworth K, et al. Child and adolescent inpatient restraint reduction: a state initiative to promote strength-based care. J Am Acad Child Adolesc Psychiatry. 2004;43(1):37-45. PubMed CrossRef
17.Mohr WK, Petti TA, Mohr BD. Adverse effects associated with physical restraint. Can J Psychiatry. 2003;48(5):330-337. PubMed CrossRef
18.Sethi F, Parkes J, Baskind E, et al. Restraint in mental health settings: is it time to declare a position? Br J Psychiatry. 2018;212(3):137-141. PubMed CrossRef
19.De Hert M, Einfinger G, Scherpenberg E, et al. The prevention of deep venous thrombosis in physically restrained patients with schizophrenia. Int J Clin Pract. 2010;64(8):1109-1115. PubMed CrossRef
20.Day DM. Examining the therapeutic utility of restraints and seclusion with children and youth: the role of theory and research in practice. Am J Orthopsychiatry. 2002;72(2):266-278. PubMed CrossRef
21.Dazzi F, Tarsitani L, Di Nunzio M, et al. Psychopathological assessment of risk of restraint in acute psychiatric patients. J Nerv Ment Dis. 2017;205(6):458-465. PubMed CrossRef
22.Georgieva I, Vesselinov R, Mulder CL. Early detection of risk factors for seclusion and restraint: a prospective study. Early Interv Psychiatry. 2012;6(4):415-422. PubMed CrossRef
23.Furre A, Falk RS, Sandvik L, et al. Characteristics of adolescents frequently restrained in acute psychiatric units in Norway: a nationwide study. Child Adolesc Psychiatry Ment Health. 2017;11(1):3. PubMed CrossRef
24.Furre A, Sandvik L, Friis S, et al. A nationwide study of why and how acute adolescent psychiatric units use restraint. Psychiatry Res. 2016;237:60-66. PubMed CrossRef
25.Pogge DL, Pappalardo S, Buccolo M, et al. Prevalence and precursors of the use of restraint and seclusion in a private psychiatric hospital: comparison of child and adolescent patients. Adm Policy Ment Health. 2013;40(3):224-231. PubMed CrossRef
26.Flammer E, Steinert T. Association between restriction of involuntary medication and frequency of coercive measures and violent incidents. Psychiatr Serv. 2016;67(12):1315-1320. PubMed CrossRef
27.Green-Hennessy S, Hennessy KD. Predictors of seclusion or restraint use within residential treatment centers for children and adolescents. Psychiatr Q. 2015;86(4):545-554. PubMed CrossRef
28.Masters KJ, Bellonci C, Bernet W, et al. Summary of the practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1356-1358. PubMed CrossRef
29.Beck NC, Hammer JH, Robbins S, et al. Highly aggressive women in a forensic psychiatric hospital. J Am Acad Psychiatry Law. 2017;45(1):17-24. PubMed
30.Sannar EM, Palka T, Beresford C, et al; Autism and Developmental Disorders Inpatient Research Collaborative (ADDIRC). Sleep Problems and their relationship to maladaptive behavior severity in psychiatrically hospitalized children with autism spectrum disorder (ASD). J Autism Dev Disord. 2018;48(11):3720-3726. PubMed CrossRef
31.Touchette E, Petit D, Séguin JR, et al. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30(9):1213-1219. PubMed CrossRef
32.Duke SG, Scott J, Dean AJ. Use of restrictive interventions in a child and adolescent inpatient unit – predictors of use and effect on patient outcomes. Australas Psychiatry. 2014;22(4):360-365. PubMed CrossRef
33.Timbo W, Sriram A, Reynolds EK, et al. Risk factors for seclusion and restraint in a pediatric psychiatry day hospital. Child Psychiatry Hum Dev. 2016;47(5):771-779. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!