Objective: To evaluate aripiprazole once-monthly (AOM), a long-acting injectable suspension of aripiprazole, as acute treatment in patients with schizophrenia (DSM-IV-TR).
Method: Adults experiencing an acute psychotic episode were randomized to 12 weeks of double-blind treatment with AOM 400 mg or placebo (October 2012-August 2013). The primary efficacy outcome was change from baseline to endpoint (week 10) in Positive and Negative Syndrome Scale (PANSS) total score. The key secondary efficacy outcome was change from baseline in Clinical Global Impressions-Severity of Illness scale (CGI-S) score. Secondary efficacy outcomes included change from baseline in PANSS positive and negative subscale and Personal and Social Performance Scale (PSP) scores. The study took place from October 2012 through August 2013.
Results: Patients (N = 340; 79% male, 66% black) were randomized to AOM (n = 168) or placebo (n = 172). Least squares (LS) mean change from baseline to endpoint (week 10) favored AOM versus placebo in PANSS total (treatment difference, −15.1 [95% CI, −19.4 to −10.8]; P < .0001) and CGI-S (treatment difference, −0.8 [95% CI, −1.1 to −0.6]; P < .0001) scores, as it did at all other timepoints through 12 weeks (all P ≤ .0005). LS mean change from baseline in PANSS positive and negative subscale and PSP scores favored AOM versus placebo (P < .0001). Common (> 10%) treatment-emergent adverse events (AOM vs placebo) were increased weight (16.8% vs 7.0%), headache (14.4% vs 16.3%), and akathisia (11.4% vs 3.5%).
Conclusions: Symptoms and functioning improved with AOM 400 mg versus placebo in patients with acute schizophrenia, with acceptable safety and tolerability. These data suggest that AOM 400 mg is a viable treatment option for patients experiencing an acute schizophrenia episode.
Trial Registration: ClinicalTrials.gov identifier: NCT01663532
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Aripiprazole Once-Monthly in the Acute Treatment of Schizophrenia: Findings From a 12-Week, Randomized, Double-Blind, Placebo-Controlled Study

ABSTRACT
Objective: To evaluate aripiprazole once-monthly (AOM), a long-acting injectable suspension of aripiprazole, as acute treatment in patients with schizophrenia (DSM-IV-TR).
Method: Adults experiencing an acute psychotic episode were randomized to 12 weeks of double-blind treatment with AOM 400 mg or placebo (October 2012-August 2013). The primary efficacy outcome was change from baseline to endpoint (week 10) in Positive and Negative Syndrome Scale (PANSS) total score. The key secondary efficacy outcome was change from baseline in Clinical Global Impressions-Severity of Illness scale (CGI-S) score. Secondary efficacy outcomes included change from baseline in PANSS positive and negative subscale and Personal and Social Performance Scale (PSP) scores. The study took place from October 2012 through August 2013.
Results: Patients (N = 340; 79% male, 66% black) were randomized to AOM (n = 168) or placebo (n = 172). Least squares (LS) mean change from baseline to endpoint (week 10) favored AOM versus placebo in PANSS total (treatment difference, −15.1 [95% CI, −19.4 to −10.8]; P < .0001) and CGI-S (treatment difference, −0.8 [95% CI, −1.1 to −0.6]; P < .0001) scores, as it did at all other timepoints through 12 weeks (all P ≤ .0005). LS mean change from baseline in PANSS positive and negative subscale and PSP scores favored AOM versus placebo (P < .0001). Common (> 10%) treatment-emergent adverse events (AOM vs placebo) were increased weight (16.8% vs 7.0%), headache (14.4% vs 16.3%), and akathisia (11.4% vs 3.5%).
Conclusions: Symptoms and functioning improved with AOM 400 mg versus placebo in patients with acute schizophrenia, with acceptable safety and tolerability. These data suggest that AOM 400 mg is a viable treatment option for patients experiencing an acute schizophrenia episode.
Trial Registration: ClinicalTrials.gov identifier: NCT01663532
J Clin Psychiatry 2014;75(11):1254-1260
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: March 28, 2014; accepted July 8, 2014.
Online ahead of print: August 19, 2014 (doi:10.4088/JCP.14m09168).
Corresponding author: John M. Kane, MD, The Zucker Hillside Hospital, 75-59 263rd St, Kaufmann Bldg, Ste 103, Glen Oaks, NY 11004-1100 ([email protected]).
Oral antipsychotics are effective for treating patients with schizophrenia; however, nonadherence to prescribed oral antipsychotic medication is common1,2 and is associated with more hospitalizations, longer hospital stays, higher health care costs,1 increased risk of suicide, and potential emergence of treatment resistance.3 Discontinuation of antipsychotic treatment is a strong predictor of relapse.4 Compared with oral antipsychotics, long-acting injectables (LAIs) have been shown in naturalistic5 and mirror-image6,7 studies to reduce rates of relapse and rehospitalization. Results from controlled trials and meta-analyses of such trials are mixed,8,9 possibly reflecting methodological challenges in studying nonadherence in randomized controlled trials.
Aripiprazole once-monthly (AOM), an extended-release injectable suspension for intramuscular use, is the first dopamine partial agonist available as an LAI and is approved for the treatment of schizophrenia.10-12 Findings from 2 randomized controlled trials demonstrated that maintenance treatment with AOM reduces the rate of impending relapse compared with placebo or a subtherapeutic dose of AOM13,14 and could be a valuable addition to the current options for maintenance therapy.
The question remains whether AOM can be used in acute settings. An acute exacerbation usually indicates that prior therapy is not working or not being taken, providing rationale for initiating a new therapy. The initial treatment to which a patient responds is often the treatment of choice for ongoing therapy. For patients with acute exacerbations, LAIs may be an important treatment option for ensuring continuous treatment exposure, regardless of the reason for relapse. This randomized, double-blind, placebo-controlled study evaluated the efficacy, safety, and tolerability of AOM for the treatment of acute exacerbations of psychotic symptoms in adults with schizophrenia.
METHOD
Study Design
In a phase 3, multicenter, randomized, double-blind, placebo-controlled trial in adult patients with schizophrenia (NCT01663532), patients were enrolled from 41 trial sites (United States, 37; Croatia, 2; Latvia, 2). The study protocol was approved by the institutional review board/independent ethics committee of each site, and all patients provided written informed consent. The study took place from October 2012 through August 2013.
The study included 3 phases. Patients entered the screening phase for up to 13 days to assess eligibility. Patients with prior aripiprazole exposure entered a 7-day washout period from prior antipsychotics and other prohibited concomitant medications. Patients without prior exposure received open-label treatment with oral aripiprazole 10 mg/d for 3 days to establish tolerability before the 7-day washout period. Hospitalization was required during the entire screening period. Patients who met the entrance criteria enrolled in the 12-week acute treatment phase.

- Initial treatment of acute exacerbation of schizophrenia with a long-acting injectable antipsychotic may enable long-term continuity of treatment, potentially improving adherence and outcomes.
- This randomized, controlled study demonstrated the efficacy, safety, and tolerability of aripiprazole once-monthly 400 mg for acute treatment of schizophrenia exacerbation.
- Patients with acute exacerbation of schizophrenia should be monitored for known and potential adverse events when initiating treatment with aripiprazole once-monthly or other long-acting injectable antipsychotics.
At baseline, patients were randomized 1:1 in a double-blind fashion to either AOM 400 mg (injected into a gluteal muscle once every 4 weeks) or placebo. Because the AOM 400 mg and placebo solutions had different appearances, injections were administered by an independent, unblinded drug manager at each study site so that investigators remained blinded to study treatment. A single dose decrease to AOM 300 mg was permitted for tolerability per the investigator’s judgment; a single return to the 400-mg dose was permitted for efficacy. On the basis of simulation data, median plasma levels of aripiprazole reach a therapeutic level ~ 3 days after injection of aripiprazole 400 mg.15 Because therapeutic levels may take longer to achieve in some patients, patients randomized to AOM received concomitant oral aripiprazole (10-20 mg/d, dose based on clinical judgment), and patients randomized to placebo received concomitant oral placebo for 14 days beginning on the day of the first injection. Patients were randomized via an interactive voice response system following computer-generated randomization codes. During the first 2 weeks of the acute treatment phase, patients were required to remain as inpatients, after which hospitalization status was determined on the basis of the investigator’s judgment of the patient’s clinical status. Outpatients returned to the clinic for study visits biweekly through week 12 (even-numbered weeks) and were contacted by phone biweekly (odd-numbered weeks). During the safety follow-up phase, all patients were contacted by phone 14 (± 2) days after the final study visit.
Patients
Eligible patients were 18 to 65 years of age and diagnosed with schizophrenia as defined by the DSM-IV-TR and confirmed by the Mini-International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorders Studies.16 All patients were currently experiencing an acute psychotic episode at screening and baseline, defined as (1) acute exacerbation of psychotic symptoms accompanied by significant deterioration in clinical and/or functional status from their baseline clinical presentation with a Positive and Negative Syndrome Scale (PANSS)17 total score ≥ 80 and (2) specific psychotic symptoms on the PANSS with a score > 4 on each of 4 specific items (conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, unusual thought content; possible scores ranged from 1 to 7 for each item). All patients resided in a stable living environment when not hospitalized, had demonstrated a good response to antipsychotic treatment (other than clozapine) in the last 12 months (investigator’s opinion), and were not treatment resistant.
Patients with a first episode of schizophrenia, who had been hospitalized for ≥ 30 days of the 90 days preceding the screening visit, had a comorbid DSM-IV-TR Axis I diagnosis (eg, major depressive disorder or bipolar disorder), or had evidence of substance abuse were excluded. Patients were also excluded if total PANSS score improved by ≥ 30% between screening and baseline.
Assessments
The primary efficacy outcome was change from baseline to endpoint (week 10) in PANSS total score17; the key secondary efficacy outcome was change from baseline to endpoint in Clinical Global Impressions-Severity of Illness scale (CGI-S)18 score. Week 10 was chosen as the primary study endpoint (blinded to patients and investigators) to minimize potential rater/scoring bias associated with a final study visit; week 12 efficacy assessment data are also presented.
Other secondary efficacy outcomes included change from baseline to endpoint in PANSS positive and negative subscale scores, PANSS responder rate (≥ 30% reduction in PANSS total score) at endpoint, change from baseline to endpoint in Personal and Social Performance Scale (PSP) score,19 Clinical Global Impressions-Improvement scale (CGI-I)18 score at endpoint, and time to all-cause discontinuation. After patients entered the acute treatment phase, PANSS and CGI-S assessments were conducted at weeks 1 and 2 and every 2 weeks thereafter until week 12 (last scheduled visit) and/or last visit (last assessment visit in the acute treatment phase [scheduled or unscheduled]). PSP total score was assessed at endpoint and week 12 or last visit.
Safety variables included treatment-emergent adverse events (TEAEs), extrapyramidal symptoms (EPS; Simpson Angus Scale [SAS],20 Abnormal Involuntary Movement Scale [AIMS],21 and Barnes Akathisia Rating Scale [BARS]22), weight, clinical laboratory assessments, (eg, metabolic parameters [glucose, cholesterol, triglycerides], prolactin), vital signs, and electrocardiograms (ECGs).
The correlation between patient in-hospital days and weight gain at week 12 was evaluated as an exploratory post hoc analysis.
Statistical Analyses
An estimated sample size of 310 patients for randomization (1:1) was determined to achieve at least 90% power at an α level of .05 (2-sided z test) to detect a treatment difference of -7.5 points with a standard deviation (SD) of 20 points in change from baseline in PANSS total score between AOM 400 mg and placebo.
The efficacy sample was defined as the modified intent-to-treat (mITT) population, which included all randomized patients who received ≥ 1 injection of AOM or placebo and had ≥ 1 postbaseline efficacy assessment. The safety sample included all patients who received ≥ 1 injection of AOM or placebo. Baseline values were defined as the last observation before the first injection of study medication.
The primary and key secondary efficacy outcome comparisons were performed using a mixed-model repeated measures (MMRM) analysis with an unstructured variance-covariance matrix for the repeated measures based on the observed cases (OC) dataset. This model included fixed class effect terms for treatment, geographic region, visit week, and an interaction term of treatment by visit week (covariate). Sensitivity analyses were performed using the pattern mixture approach with multiple imputations (based on the discontinuation reasons) under the assumption of missing not at random.
Changes from baseline in PANSS positive and negative subscale scores were compared using the same analytic approach described for the primary efficacy outcome (MMRM; OC dataset). Responder rate (≥ 30% reduction in PANSS total score) was analyzed in the mITT population with the Fisher exact test (last observation carried forward [LOCF]). CGI-I score was analyzed in the mITT population with the Cochran-Mantel-Haenszel row mean score test (van Elteren test) controlling for geographical region (LOCF). Change from baseline in PSP total score was analyzed using analysis of covariance (ANCOVA) with treatment group as a factor and baseline as a covariate (LOCF). Time to all-cause discontinuation was estimated using Kaplan-Meier and compared with a log-rank test.
Descriptive statistics were generated for all safety variables. Changes from baseline to week 12 (OC dataset) in EPS scale scores (SAS, AIMS, BARS), body weight, and prolactin levels were analyzed using ANCOVA with treatment group as a factor and baseline as a covariate.
Because observed weight changes did not align with those from earlier long-term outpatient studies, correlations between patient in-hospital days and weight gain in the safety sample at week 12 were explored using Pearson correlation coefficients.
RESULTS
Patient Disposition and Characteristics
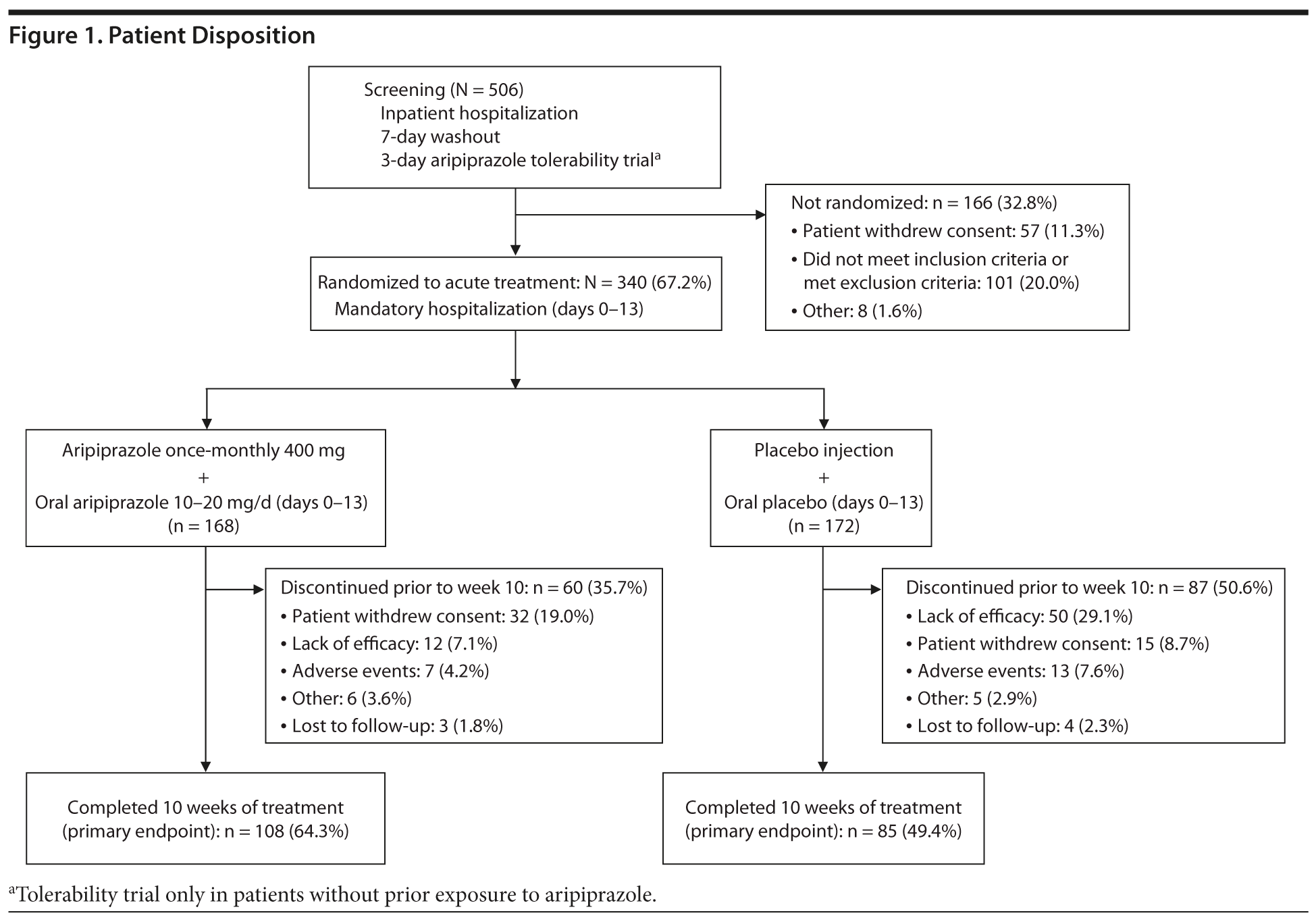
Of 506 patients screened, 340 patients were randomized to double-blind treatment with AOM 400 mg (n = 168) or placebo (n = 172; Figure 1); no patient failed screening due to intolerance of aripiprazole. A total of 64.3% (AOM 400 mg) and 49.4% (placebo) of patients completed 10 weeks of treatment. The most common reason for discontinuation was patient withdrawal of consent in the AOM group (n = 32/168 [19.0%]) and lack of efficacy in the placebo group (n = 50/172 [29.1%]; Figure 1).
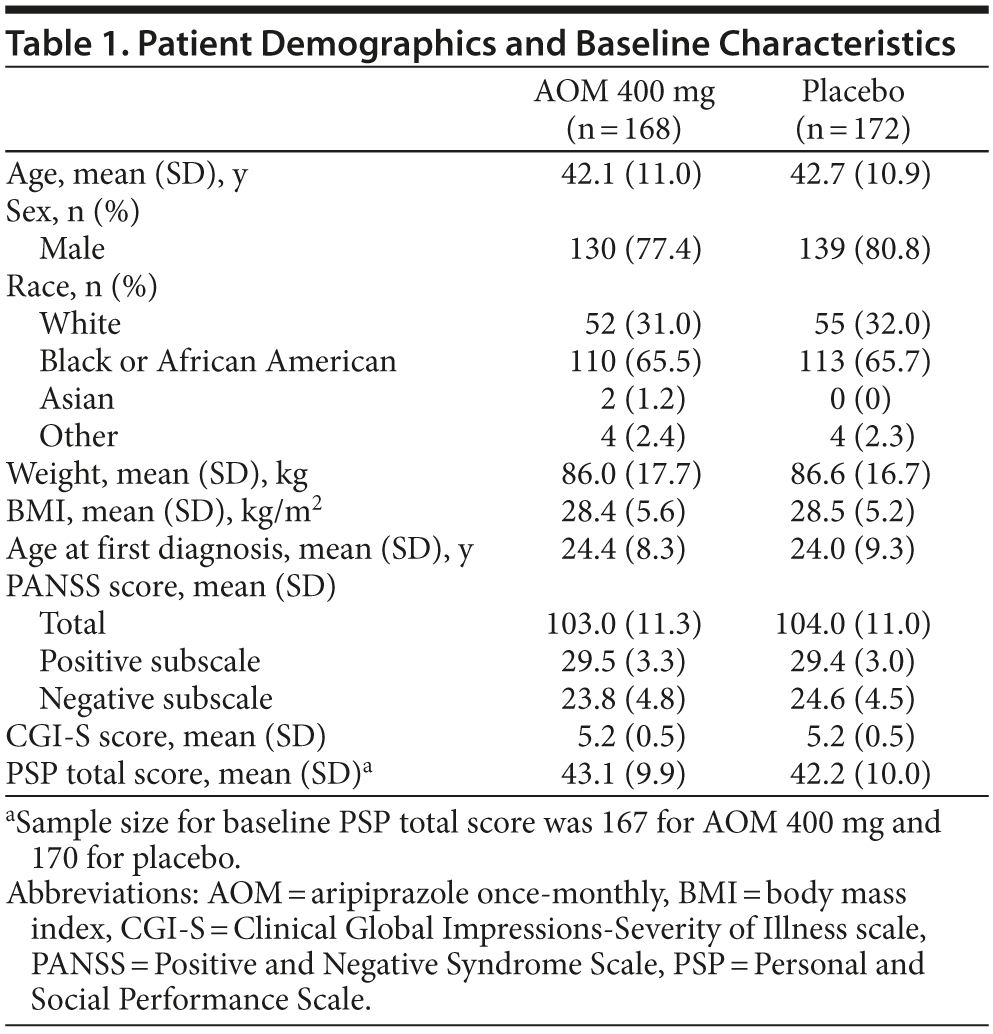
Baseline demographic and disease characteristics were similar between treatment groups in the randomized population (Table 1). Mean patient age was approximately 42 years; ~ 79% were men, and ~ 66% were black. Before enrollment, 6.5% and 6.4% of patients randomized to AOM and placebo, respectively, had taken oral aripiprazole.
Treatment Exposure
Of 340 randomized patients, 195 received oral aripiprazole (10 mg/d for 3 days) during the screening phase to establish tolerability to aripiprazole. During the first 2 weeks of the acute treatment phase, patients randomized to AOM received a mean (SD) average daily dose of 12.8 (3.2) mg oral aripiprazole. Almost all patients remained at their starting injection dose (AOM 400 mg, 161/167 [96.4%]; placebo, 168/172 [97.7%]). Use of concomitant benzodiazepines was comparable between treatment arms (139/167 [83.2%] AOM, 144/172 [83.7%] placebo), with similar mean average daily doses.
Primary and Key Secondary Efficacy Outcomes
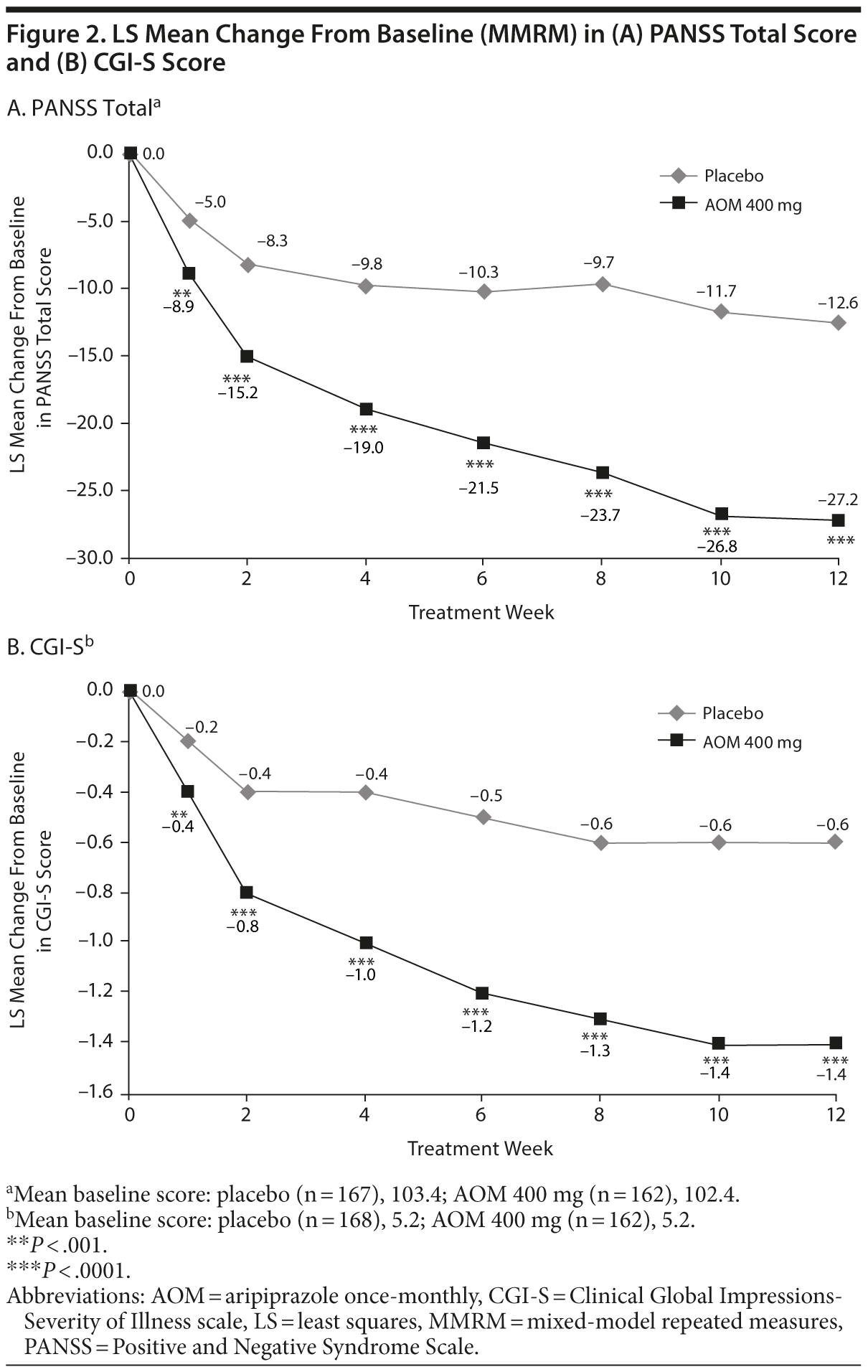
Least squares (LS) mean change from baseline at endpoint was significantly greater with AOM versus placebo for PANSS total score (Figure 2A; treatment difference, −15.1 [95% CI, −19.4 to −10.8]; P < .0001) and for CGI-S (Figure 2B; treatment difference, −0.8 [95% CI, −1.1 to −0.6]; P < .0001). MMRM results were confirmed by ANCOVA with both OC and LOCF data. Sensitivity analyses demonstrated that the superiority of AOM to placebo at endpoint was maintained up to a deduction of 140% (PANSS total) and 160% (CGI-S) estimated treated difference from the imputed data after discontinuation for all discontinued patients in the AOM group. Subgroup analysis by sex showed a similar pattern of improvement at endpoint with AOM on primary and key secondary efficacy measures for men (PANSS total score and CGI-S, AOM vs placebo, both P < .05) and women (PANSS, P = .053; CGI-S, P < .05). At all time points assessed, AOM significantly improved both PANSS total and CGI-S scores (Figure 2A and 2B).
Secondary Efficacy Outcomes
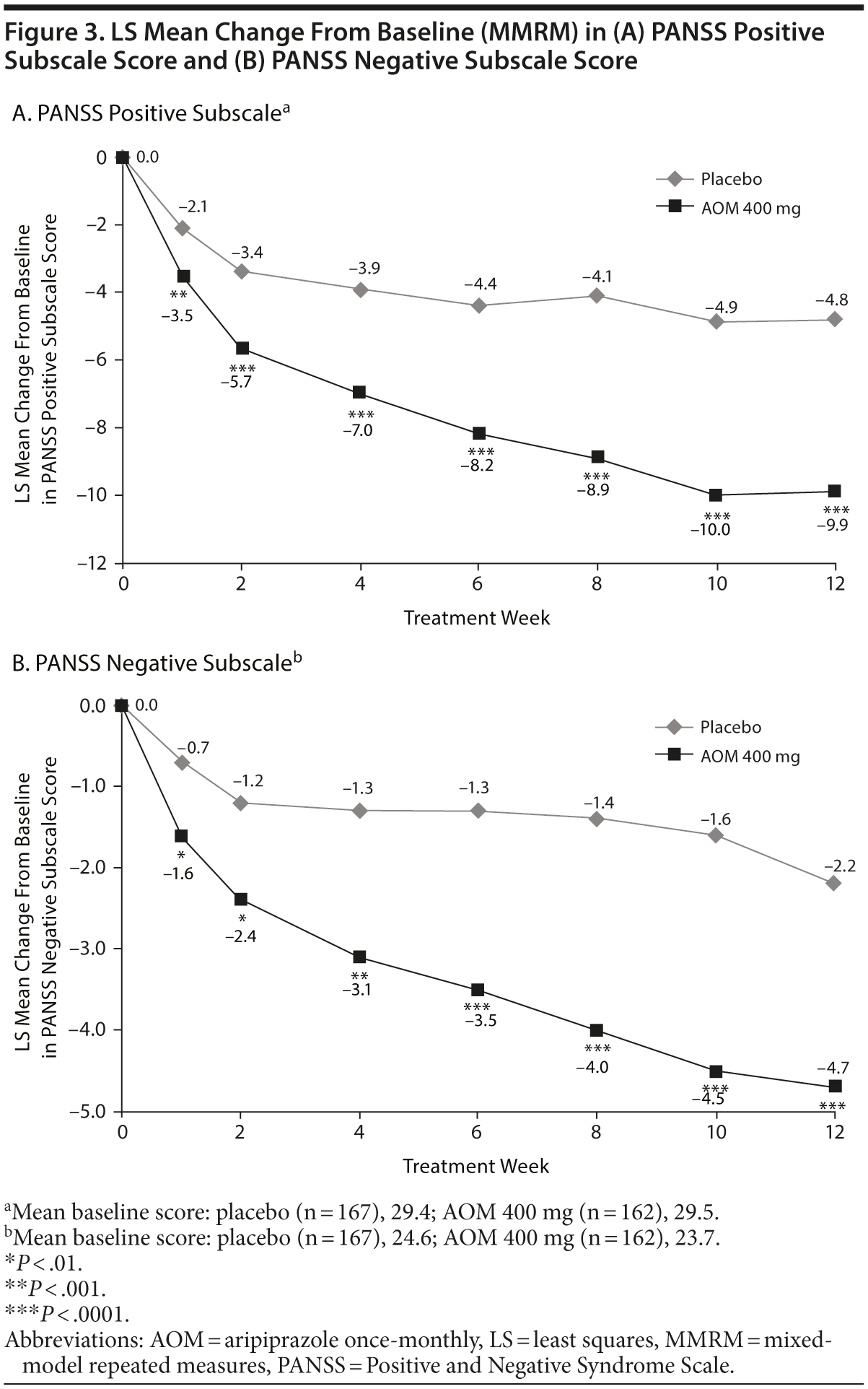
LS mean change from baseline in PANSS positive and negative subscale scores was significantly improved with AOM versus placebo at each time point (P < .0001; Figure 3A and 3B). PANSS responder rate (≥ 30% reduction in total score) was significantly higher with AOM versus placebo at endpoint (60/162 [37.0%] vs 24/167 [14.4%]; P < .0001) and week 12 (56/162 [34.6%] vs 26/167 [15.6%]; P < .0001).
LS mean (SE) change from baseline in PSP total score was significantly greater with AOM versus placebo at endpoint (12.3 [1.2] vs 5.2 [1.2], respectively; P < .0001) and week 12 (13.0 [1.2] vs 5.5 [1.2]; P < .0001). Mean (SD) CGI-I score was significantly better with AOM versus placebo at each assessment (P < .0001), including endpoint (2.7 [1.2] vs 3.7 [1.3]) and week 12 (2.6 [1.2] vs 3.7 [1.3]).
Estimated median time to discontinuation due to all reasons was longer in the AOM 400 mg group than in the placebo group (92 vs 60 days; P = .0029).
Exploratory Post Hoc Analysis
A statistically significant correlation was found between in-hospital days and weight change at week 12 with AOM (r = 0.2340; P = .0197) but not placebo (r = 0.1496; P = .2306).
Safety and Tolerability
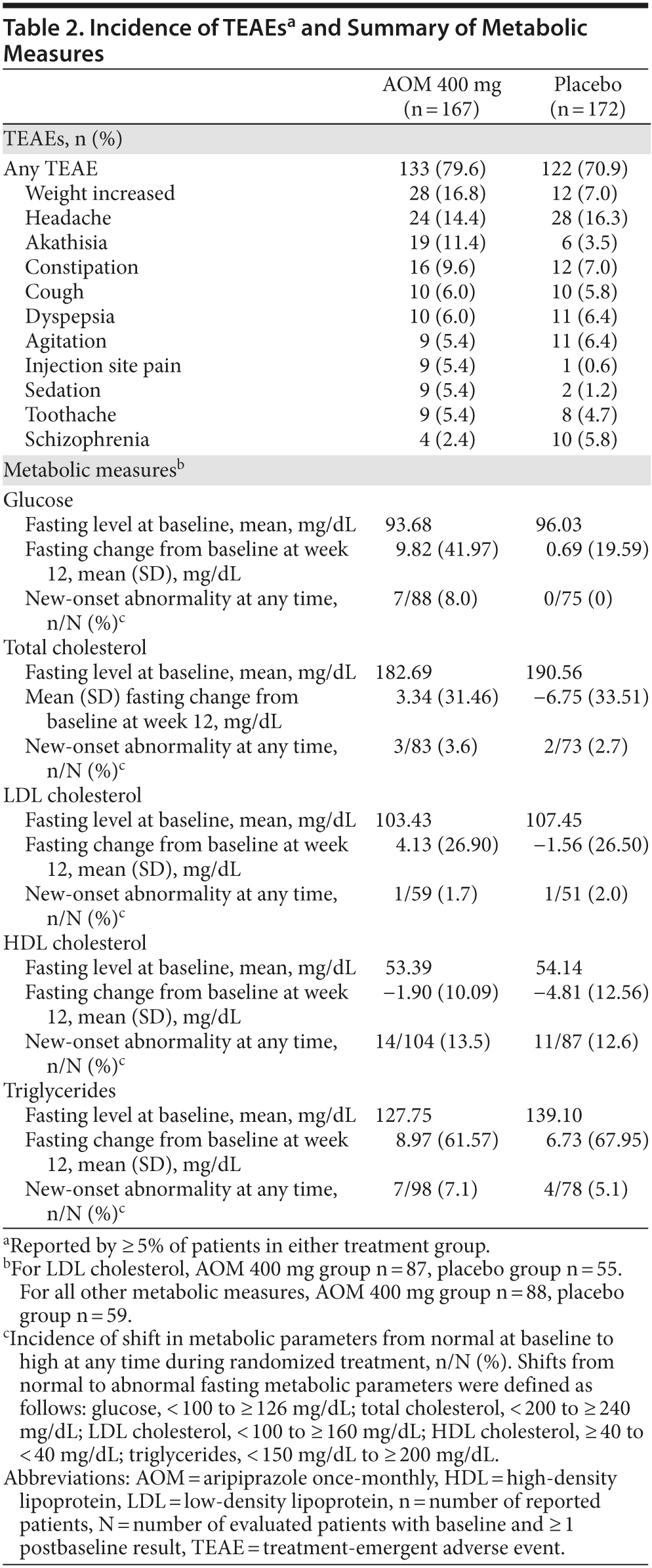
TEAEs through week 12 are summarized in Table 2; the most common (> 10%) were weight increase, headache, and akathisia in the AOM group and headache in the placebo group. No patient discontinued due to weight gain, headache, or akathisia. Serious TEAEs were observed in 8/167 (4.8%) patients in the AOM group and 6/172 (3.5%) in the placebo group. Serious TEAEs observed in ≥ 1% of patients were schizophrenia (AOM, 3/167 [1.8%]; placebo, 3/172 [1.7%]) and psychotic disorder (AOM, 2/167 [1.2%]; placebo, 2/172 [1.2%]). No other serious TEAEs were observed in > 1 patient in either group, and all serious TEAEs resolved. No deaths occurred.
On average, minimal changes from baseline to week 12 in EPS scales were observed, with no statistically significant differences between groups. EPS scale LS (SE) mean changes from baseline to week 12 for the AOM and placebo groups were as follows: SAS total score, −1.0 (0.1) and 0.1 (0.2), respectively; AIMS movement rating score, 0.1 (0.1) and 0.1 (0.1); and BARS global score, −0.1 (0.1) and −0.1 (0.1).
Mean (SD) weight increases from baseline to week 12 (completer analysis) were significantly larger with AOM (3.5 [5.8] kg) versus placebo (0.8 [4.3] kg; P = .0018). The incidence of potentially clinically relevant weight gain (≥ 7% change from baseline) at any time during the acute treatment phase was 31/144 (21.5%) for AOM and 12/141 (8.5%) for placebo. At week 12, African American patients receiving AOM (n = 59) gained a mean (SD) of 4.9 (5.4) kg, whereas white patients (n = 34) gained 0.7 (5.6) kg. Mean changes from baseline to week 12 in fasting metabolic parameters and shifts in metabolic parameters from normal levels at baseline to abnormal at any time during treatment were generally comparable between groups, although fasting glucose levels showed numerically greater changes with AOM versus placebo (Table 2).
Mean prolactin levels decreased in both groups; the mean (SD) reduction from baseline to week 12 was significantly larger with AOM (−6.4 [13.5]) than placebo (−1.1 [14.5]; P = .0176).
No clinically relevant mean changes from baseline were observed in vital signs or ECG parameters (data not shown).
DISCUSSION
These data demonstrate the efficacy, safety, and tolerability of AOM 400 mg with concomitant oral aripiprazole for the first 2 weeks for the treatment of adult patients with schizophrenia experiencing an acute exacerbation of psychosis. Both primary (PANSS total score change) and key secondary (CGI-S change) study endpoints were superior for AOM 400 mg over placebo. Results from other placebo-controlled studies of atypical LAIs were comparable to the present study.23,24 The significant reduction in both PANSS subscale scores as early as week 1 demonstrates that AOM may rapidly resolve not only positive symptoms but also negative symptoms. Negative symptoms are often associated with functional deficits,25 and low PSP scores at baseline in the present study indicated marked functional impairment. Improvements in PSP scores and PANSS negative symptoms indicated clinically significant improvement in patient function. Together with data showing that AOM 400 mg maintains stability of both symptoms and function over long-term treatment,13,14 the present data demonstrate the utility of AOM for acute treatment.
The overall safety and tolerability profile of AOM 400 mg in the current study was generally consistent with that observed in previous double-blind phase 3 studies.13,14 EPS and metabolic issues are important considerations with any antipsychotic treatment.26 EPS scale scores indicated no significant difference between AOM and placebo, yet EPS-related TEAEs should be continuously monitored.
Mean change in weight and the incidence of clinically significant weight change were higher with AOM in the present study versus placebo and were higher with AOM in the present study compared with previous long-term AOM studies.13,14 Mean changes from baseline in AOM 400 mg treatment groups during the double-blind phase of a 52-week study14 and a 38-week study13 were -0.2 kg and +0.1 kg, respectively, suggesting that long-term outpatient treatment with AOM 400 mg is not associated with notable weight gain in most patients. One explanation for the difference between the current study and previous long-term studies, supported by the observed positive correlation between inpatient days and weight gain, could be that hospitalized patients receiving AOM experienced symptom improvements that potentially led to increased food intake given the provision of regular meals in the hospital setting. This may be particularly true for these patients who exhibited a full range of positive symptoms at enrollment. Another explanation may relate to patient demographics; ~ 66% of patients in the current study were African American compared with ~ 20% in previous AOM studies, and mean weight gain at 12 weeks was numerically greater in African American than in white patients.13,14 Increased risk for obesity and weight gain has been observed in African Americans with severe mental illness.27 The prevalence of a risk allele (rs489693, genotype A/A) associated with antipsychotic drug-induced weight gain28 is approximately twice as high in people of African ancestry compared with those of European ancestry.29 Regardless of reason, continued treatment of patients experiencing weight gain should be based on clinical assessment of the patient in the context of the overall benefits and risks of treatment. All patients being treated with atypical antipsychotics should be monitored for changes in glucose, lipids, and body weight.30
Several important study features may limit the generalizability of these findings to all patients treated in real-world clinical practice. These include the exclusion of patients with comorbid DSM-IV-TR Axis I diagnoses or substance abuse, the intense weekly follow-up, and the use of objective assessments.
In conclusion, treatment of acute schizophrenia with AOM 400 mg with 2 weeks of concomitant oral aripiprazole significantly improved patient symptoms and functioning compared with placebo. Treatment discontinuation in the AOM group was notably lower than in the placebo group, and almost all patients stayed on their starting dose of 400 mg. Safety and tolerability were generally as expected on the basis of previous studies of AOM in stable patients with schizophrenia.13,14 Collectively, the results suggest that AOM 400 mg is a viable treatment option for patients who experience an acute exacerbation of schizophrenia.
Drug names: aripiprazole once-monthly (Abilify Maintena), clozapine (Clozaril, FazaClo, and others).
Author affiliations: The Zucker Hillside Hospital, Glen Oaks, and the Hofstra North Shore-LIJ School of Medicine, Hempstead, New York (Dr Kane); Otsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey (Drs Peters-Strickland, Baker, McQuade, Carson, and Sanchez and Mss Perry and Gara); H. Lundbeck A/S, Copenhagen-Valby, Denmark (Dr Hertel); Lundbeck LLC, Deerfield, Illinois (Dr Eramo); and Otsuka Pharmaceutical Development & Commercialization, Inc, Rockville, Maryland (Ms Jin).
Potential conflicts of interest: Dr Kane has received honoraria for lectures and/or consulting from Alkermes, Amgen, Bristol-Myers Squibb, Cephalon, Eisai, Boehringer Ingelheim, Eli Lilly, Forest, Genentech, Intracellular Therapeutics, Janssen, Johnson & Johnson, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Pierre Fabre, Proteus, Reviva, Roche, Sunovion, and Targacept and is a shareholder of MedAvante. Drs Peters-Strickland, Baker, McQuade, Carson, and Sanchez and Mss Jin, Perry, and Gara are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Dr Hertel is an employee of H. Lundbeck A/S. Dr Eramo is an employee of Lundbeck LLC.
Funding/support: This research was supported by Otsuka Pharmaceutical Development & Commercialization, Inc, and H. Lundbeck A/S.
Acknowledgments: Editorial support for the preparation of this manuscript was provided by C4 MedSolutions, LLC, a CHC Group company (Yardley, Pennsylvania) with funding from Otsuka Pharmaceutical Commercialization & Development, Inc, and H. Lundbeck A/S.
REFERENCES
1. Offord S, Lin J, Mirski D, et al. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286-297. PubMed doi:10.1007/s12325-013-0016-5
2. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263. PubMed doi:10.1097/JCP.0b013e31828568bc
3. Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200-218. PubMed doi:10.1177/2045125312474019
4. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247. PubMed doi:10.1001/archpsyc.56.3.241
5. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. PubMed doi:10.1176/appi.ajp.2011.10081224
6. Kane JM, Sanchez R, Zhao J, et al. Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Media Econ. 2013;16(7):917-925. PubMed doi:10.3111/13696998.2013.804411
7. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. PubMed doi:10.4088/JCP.13r08440
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213. PubMed doi:10.1093/schbul/sbs150
9. Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1-3):83-92. PubMed doi:10.1016/j.schres.2010.11.020
10. Abilify Maintena US [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Co, Ltd; 2013.
11. Abilify Maintena EU [package insert]. Wexham, UK: Otsuka Pharmaceutical Europe Ltd; 2013.
12. Abilify Maintena CAN [package insert]. Saint-Laurent, QC, Canada: Otsuka Canada Pharmaceutical Inc; 2014.
13. Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study [published online ahead of print June 12, 2014]. Br J Psychiatry. PubMed doi:10.1192/bjp.bp.113.134213
14. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617-624. PubMed doi:10.4088/JCP.11m07530
15. Committee for Medicinal Products for Human Use. Abilify Maintena EMA Public Assessment Report (EPAR). European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/
document_library/EPAR_-_Public_assessment_report/human/002755/WC500156105.pdf. Accessed June 6, 2014.
16. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
17. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
18. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:217-222.
19. Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323-329. PubMed
20. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;45(S212):11-19. PubMed doi:10.1111/j.1600-0447.1970.tb02066.x
21. Guy W. Abnormal Involuntary Movement Scale (AIMS). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:534-537.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed doi:10.1192/bjp.154.5.672
23. Alphs L, Bossie CA, Sliwa JK, et al. Onset of efficacy with acute long-acting injectable paliperidone palmitate treatment in markedly to severely ill patients with schizophrenia: post hoc analysis of a randomized, double-blind clinical trial. Ann Gen Psychiatry. 2011;10(1):12. PubMed doi:10.1186/1744-859X-10-12
24. Gopal S, Pandina G, Lane R, et al. A post-hoc comparison of paliperidone palmitate to oral risperidone during initiation of long-acting risperidone injection in patients with acute schizophrenia. Innov Clin Neurosci. 2011;8(8):26-33. PubMed
25. Rabinowitz J, Levine SZ, Garibaldi G, et al. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res. 2012;137(1-3):147-150. PubMed doi:10.1016/j.schres.2012.01.015
26. Hasan A, Falkai P, Wobrock T, et al; World Federation Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-378. PubMed doi:10.3109/15622975.2012.696143
27. Carliner H, Collins PY, Cabassa LJ, et al. Prevalence of cardiovascular risk factors among racial and ethnic minorities with schizophrenia spectrum and bipolar disorders: a critical literature review. Compr Psychiatry. 2014;55(2):233-247. PubMed doi:10.1016/j.comppsych.2013.09.009
28. Malhotra AK, Correll CU, Chowdhury NI, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch Gen Psychiatry. 2012;69(9):904-912. PubMed doi:10.1001/archgenpsychiatry.2012.191
29. National Center for Biotechnology Information. dbSNP short genetic variations. http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=489693. Accessed March 24, 2014.
30. Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients With Schizophrenia, Second Edition. Am J Psychiatry. 2004;161(2, suppl):1-56.
This PDF is free for all visitors!