Background: Patients with schizophrenia often suffer from cognitive deficits and negative symptoms that are poorly responsive to antipsychotics including clozapine. Clozapine-induced sedation can worsen cognition and impair social and occupational functioning.
Objectives: To evaluate the efficacy, tolerability, and safety of modafinil for negative symptoms, cognition, and wakefulness/fatigue in DSM-IV-diagnosed schizophrenia patients treated with clozapine.
Method: A double-blind, placebo-controlled, flexible-dosed 8-week pilot trial was conducted between September 2003 and September 2007, adding modafinil up to 300 mg/d to stabilized schizophrenia outpatients receiving clozapine.
Psychopathology, cognition, and wakefulness/fatigue were assessed with standard rating scales.
Results: Thirty-five patients were randomly assigned to treatment with study drug and included in the analysis. Modafinil did not reduce negative symptoms or wakefulness/fatigue or improve cognition compared to placebo. Modafinil was
well tolerated and did not worsen psychosis.
Conclusions: Results of this pilot trial do not support routine use of modafinil to treat negative symptoms, cognitive deficits, or wakefulness/fatigue in patients on clozapine. However, given our limited power to detect a treatment effect and the clear possibility of a type II error, larger trials are needed to resolve or refute a potential therapeutic effect of uncertain magnitude.
Trial Registration: clinicaltrials.gov Identifier: NCT00573417
Submitted: September 6, 2008; accepted November 11, 2008.
Online ahead of print: August 11, 2009.
Corresponding author: Oliver Freudenreich, MD, MGH Schizophrenia Program, Freedom Trail Clinic, 25 Staniford St, 2nd Floor, Boston, MA 02114 ([email protected]).
Modafinil for Clozapine-Treated Schizophrenia Patients: A Double-Blind, Placebo-Controlled Pilot Trial
Background: Patients with schizophrenia often suffer from cognitive deficits and negative symptoms that are poorly responsive to antipsychotics including clozapine. Clozapine-induced sedation can worsen cognition and impair social and occupational functioning.
Objectives: To evaluate the efficacy, tolerability, and safety of modafinil for negative symptoms, cognition, and wakefulness/fatigue in DSM-IV-diagnosed schizophrenia patients treated with clozapine.
Method: A double-blind, placebo-controlled, flexible-dosed 8-week pilot trial was conducted between September 2003 and September 2007, adding modafinil up to 300 mg/d to stabilized schizophrenia outpatients receiving clozapine. Psychopathology, cognition, and wakefulness/fatigue were assessed with standard rating scales.
Results: Thirty-five patients were randomly assigned to treatment with study drug and included in the analysis. Modafinil did not reduce negative symptoms or wakefulness/fatigue or improve cognition compared to placebo. Modafinil was well tolerated and did not worsen psychosis.
Conclusions: Results of this pilot trial do not support routine use of modafinil to treat negative symptoms, cognitive deficits, or wakefulness/fatigue in patients on clozapine. However, given our limited power to detect a treatment effect and the clear possibility of a type II error, larger trials are needed to resolve or refute a potential therapeutic effect of uncertain magnitude.
Trial Registration: clinicaltrials.gov Identifier: NCT00573417
J Clin Psychiatry 2009;70(12):1674-1680
© Copyright 2009 Physicians Postgraduate Press, Inc.
Submitted: September 6, 2008; accepted November 11, 2008.
Online ahead of print: August 11, 2009 (doi:10.4088/JCP.08m04683).
Corresponding author: Oliver Freudenreich, MD, MGH Schizophrenia Program, Freedom Trail Clinic, 25 Staniford St, 2nd Floor, Boston, MA 02114 ([email protected]).
C lozapine represented a significant advance in the treatment of schizophrenia. However, clozapine is not broadly effective against all psychopathological domains of schizophrenia, and its use is limited by tolerability. Like all antipsychotics, clozapine mostly treats positive symptoms, with meager efficacy at best for primary negative symptoms1 and cognitive deficits.2 This is of considerable clinical importance since negative symptoms and cognitive deficits are major determinants of the poor social and occupational functioning3 that is characteristic of many patients with schizophrenia.4 In addition, clozapine can be difficult for patients to tolerate, with sedation being one of the most problematic side effects,5 adding insult to injury.
Stimulants are sometimes used to increase wakefulness in schizophrenia,6 but they can worsen psychosis7,8 and have a high potential for abuse. A chemically different medication, modafinil is a wakefulness-promoting agent that is indicated for the treatment of several sleep disorders associated with excessive sleepiness, including narcolepsy, obstructive sleep apnea, and shift work sleep disorder.9 A typical dose of modafinil for these conditions is 200 mg/d. Modafinil’s mechanism of action remains incompletely understood but is believed to differ from stimulants like amphetamine by more selective activation of hypothalamic regions that promote wakefulness, including the tuberomammillary nucleus and orexin (hypocretin) neurons.10 Consequently, modafinil is thought to have a low potential for abuse and a low propensity to induce psychosis. It is also advantageous in not adversely affecting cardiovascular or sleep parameters.11
Three controlled trials in schizophrenia have investigated the efficacy and safety of modafinil add-on therapy, with inconsistent results. Turner and colleagues12 reported improved attentional set shifting and short-term verbal memory span following a single dose of 200 mg/d modafinil in a crossover trial of 20 subjects, mostly treated with clozapine. This trial did not examine effects on negative symptoms or fatigue. Cognitive benefit was not detected in two 8-week placebo-controlled, parallel-group trials13, 14 of modafinil, up to 200 mg/d in samples of 24 and 20 antipsychotic-treated schizophrenia patients, respectively, nor were effects on negative symptoms found. Sevy and colleagues13 also failed to demonstrate an effect of modafinil on fatigue when assessed with the Fatigue Severity Scale (FSS)15 and a visual analog fatigue scale.
We report the results of a double-blind, placebo-controlled, flexible-dose pilot trial of modafinil up to 300 mg/d added for 8 weeks to a stable dose of clozapine in patients with schizophrenia. The trial was designed to assess tolerability and safety of modafinil as well as efficacy for negative and cognitive symptoms and effect on wakefulness and fatigue.
METHOD
Study Setting and Sponsorship
The study was conducted between September 2003 and September 2007 at the Clozapine Program of the Freedom Trail Clinic, a community mental health center clinic in downtown Boston that serves as a referral clinic for schizophrenia patients who require clozapine treatment. The study was an investigator-initiated trial sponsored by the manufacturer of modafinil, Cephalon Inc.
Participant Selection
Patients were referred for this study by their clinicians. The research team assessed competency to participate in clinical research with a semistructured interview (available upon request). All patients or their guardians provided written informed consent to participate in this trial. The study was approved by the responsible institutional review boards.
Characteristics of Subjects
Patients had schizophrenia or schizoaffective disorder as their primary diagnosis, were clinically stable for at least 3 months, and had been taking clozapine for at least 6 months, with a stable dose for at least 1 month. Diagnoses were based on physician interview and chart review (O.F.), using the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) criteria.16
Patients were excluded if they had an active substance use disorder (other than nicotine dependence), epilepsy or a serious medical illness, or suicidal ideation. Current treatment with a psychostimulant led to exclusion as well. Patients had to be able to complete the neuropsychological battery.
Study Design and Procedures
The study design was a parallel-group, placebo-controlled, double-blind escalating-dose trial in which patients were randomly assigned in a 1:1 ratio to placebo or up to 300 mg/d of modafinil added to ongoing clozapine treatment. In this 8-week study, patients were seen every 2 weeks for vital signs, hematology, and side effect monitoring. A clozapine level was drawn at the beginning of the study and at 4 and 8 weeks after initiating study treatment. Patients were assessed with scales (see below) for wakefulness, psychopathology, and side effects at screening and baseline and after weeks 2, 4, and 8. Cognition was assessed with a cognitive battery (see below) at baseline, and the assessment was repeated after 4 and 8 weeks. Time of testing was not controlled with regard to time of modafinil administration.
Drug Regimen
An independent research pharmacy prepared matching capsules that contained either 100 mg of modafinil or placebo and randomized subjects in blocks of 4. Patients initiated treatment with 1 capsule (100 mg) per day for 2 weeks, the dose could be increased to 2 capsules (200 mg) per day after 2 weeks, and a maximum of 3 capsules (300 mg/day) after 4 weeks. Dose was adjusted upward unless tolerability problems developed. After 8 weeks, study medication was discontinued. Adherence to study medication was assessed every 2 weeks by pill count. We chose 75% adherence by pill count as evidence of satisfactory adherence.
Ratings
Wakefulness. Modafinil’s wakefulness-promoting properties were assessed with 2 self-rating scales: the Epworth Sleepiness Scale (ESS),17 to quantify propensity to fall asleep due to excessive sleepiness, and the Fatigue Severity Scale (FSS),15 which is a subjective measure of the feeling of fatigue.
Clinical psychopathology. Clinical psychopathology was assessed with well-established rating scales for schizophrenia: the Positive and Negative Syndrome Scale (PANSS)18 for a global measure of psychopathology (PANSS total score) and psychosis (PANSS positive symptoms subscale score); the Scale for the Assessment of Negative Symptoms (SANS)19,20 for negative symptoms; and the Global Assessment of Functioning (GAF)16 and the Heinrichs-Carpenter Quality of Life Scale21 for an overall measure of functioning and well-being, respectively. In the presence of missing item scores, total scores for these instruments were calculated as reweighted sums of the present responses if the rate of missing items was less than 25%. Side effects were collected with the Systematic Assessment for Treatment Emergent Events (SAFTEE),22 a rating scale that contains both open-ended and closed inquiries.
Cognitive measures. The North American Adult Reading Test (NAART)23 was used to estimate premorbid intelligence. The cognitive battery assessed several cognitive domains and included the following 8 tests, which were included to calculate a psychometric battery composite score: the Degraded Stimulus Continuous Performance Test (DS-CPT)24 to assess sustained attention and vigilance; the Hopkins Verbal Learning Test (HVLT)25 to assess secondary verbal memory; the Faces and Family Pictures subtests from the Wechsler Memory Scale-III (WMS-III)26 to assess visual memory; the Wisconsin Card Sorting Test (WCST)27 to assess executive function/problem solving; the Trail-Making Test to assess executive functioning/set shifting; the Letter-Number Sequencing subtest (WAIS-III)26 to assess working memory; the Letter and Category Fluency (LCF)28 to assess semantic fluency; and the Grooved Pegboard (model 32025, Lafayette Instrument Company) to assess psychomotor speed.
A composite score of neurocognitive battery (COGBAT) was calculated as the mean of the internally standardized scores (all study values adjusted to mean = 0 and standard deviation [SD] = 1 separately for each measure) from the following 8 assessments: sensitivity from the DS-CPT, total item recall from the HVLT, immediate face recall from the WMS-III, the mean of categories completed and 64 − perseverative errors from the WCST, Trails B scores (see below), the mean of recall with and without reordering from the WAIS-III, the mean total FAS letter word count (in which subjects name, in 1 minute for each, as many words as possible that begin with the letters ‘ F,’ ‘ A,’ and ‘ S’ ) and animal word count (in which subjects name as many animals as possible in 1 minute) from the LCF, and the negative dominant-hand pegboard time. Trails B scores were derived by inverting Trails B times and normalizing to Trails A times as follows: using only baseline observations, a regression of Trails B time versus Trails A time was estimated. Using the coefficients from this regression, predicted Trails B times were estimated from observed Trails A times for all observations. Trails B scores were calculated as predicted Trails B times minus observed Trails B times. Larger Trails B scores indicate faster Trails B execution (lower Trails B times) relative to that expected from an individual’s Trails A execution speed.
Data Analysis
Baseline comparisons were made by t test or Fisher exact test for continuous and categorical variables, respectively. Serum clozapine levels were log-transformed prior to analysis. Our primary aims were to examine the effects of modafinil on wakefulness/fatigue, negative symptoms, and cognition. Effect of modafinil versus placebo on change in clinical variables was analyzed in a mixed-model analysis of variance with fixed terms for treatment group, visit, and treatment ×— visit interaction and random participant-specific intercepts and slopes. The random slopes structure was chosen as optimal by Bayes information criterion over models with random intercepts only, random visits, and first-order auto-regressive errors. Treatment effect was summarized as the difference in mean slopes over time estimated from linear contrasts on the visit least square means. Effect sizes were calculated for the difference in slopes using a pooled standard deviation inferred from the standard error for the treatment contrast. All tests were 2-tailed, with level of significance set at .05. Both uncorrected P values and P values corrected for multiple comparisons by Hommel’s closed testing procedure29 are reported where applicable. All analyses were performed using SAS version 9.1.3 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
Study Population
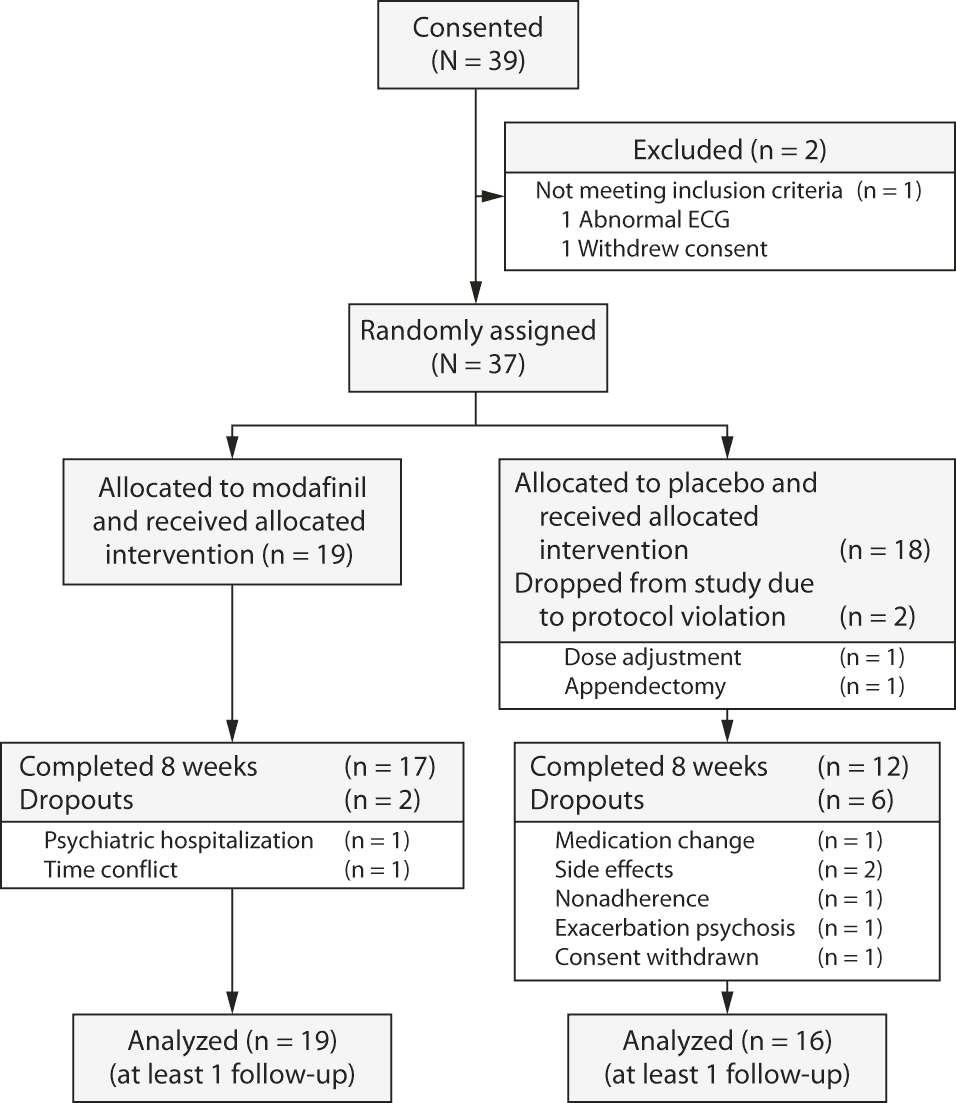
Thirty-nine patients consented to the study and 37 were randomly assigned (1 patient withdrew consent and 1 patient had an abnormal electrocardiogram leading to exclusion prior to random assignment). Two patients in the placebo group were terminated immediately after random assignment (1 hospitalization for appendicitis, 1 protocol violation because the clozapine dose was adjusted) and were excluded from analysis. Of the final sample of 35 patients, 16 were randomly assigned to placebo and 19 to modafinil. Six patients in the placebo group and 2 patients in the modafinil group did not finish the trial (33% and 11%, respectively). All 35 patients took at least 1 dose of study medication and completed the first follow-up visit after 2 weeks. All analyses are based on the final sample of 35 patients. Further details regarding patient flow in the trial are provided in Figure 1.
Figure 1. Patient Flow Through a Double-Blind, Randomized Trial of Added Modafinil to Clozapine-Treated Patients With Schizophrenia
Abbreviation: ECG = electrocardiogram.
Click figure to enlarge
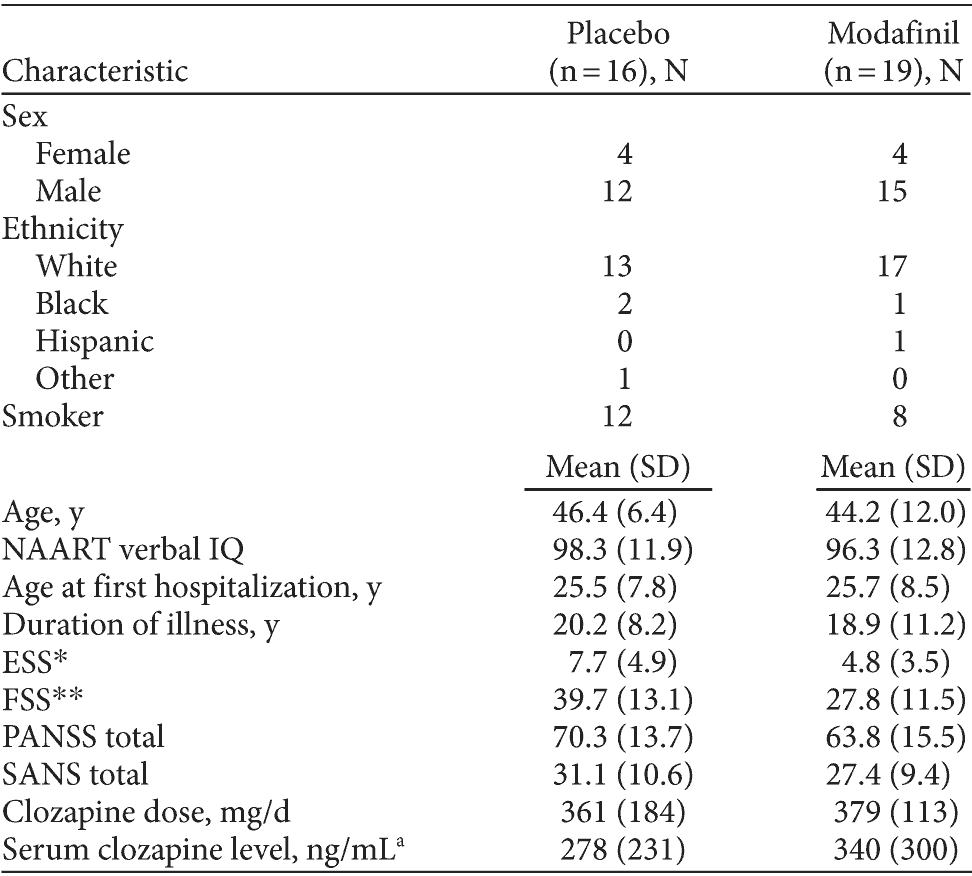
The majority of patients were male (77%), with an average age of 45.2 ± 9.7 years (range 20-64 years). The mean (± SD) age of first hospitalization was 25.6 ± 8.0 years (range, 15-50 years), and patients had been ill for 19.5 ± 9.8 years (range, 2-35 years). The average PANSS total score at baseline was 66.7 ± 14.8 (range, 39-94), reflecting mild-to-moderate psychopathology. Average GAF score was 58.9 ± 10.6 (range, 40-90), and average Heinrichs-Carpenter Quality of Life scale score was 74.8 ± 15.1 (range, 42-109). By chance, both measures of fatigue (FSS and visual analog fatigue scale) were significantly higher at baseline among participants randomly assigned to the placebo group (see Table 1).
With few exceptions, pill count adherence was over 75% for both groups; 2 patients in the placebo group were discovered to have extended periods of nonadherence. Twenty-four (69%) patients reached a dose of 300 mg/d of study drug, including 17 (49%) who remained at 300 mg/d at the end of treatment (11 [58%] on modafinil and 6 [38%] on placebo, P = .48). Five patients taking modafinil (26%) versus 4 patients on placebo (25%) at least temporarily reduced their dose of study drug (P = 1.0). The average dose of modafinil at the end of treatment was 250 mg/d and 216 mg/d for placebo.
Table 1. Baseline Demographic and Clinical Characteristics of Participants With Clozapine-Treated Schizophrenia in a Modafinil Add-On Trial
aMedian and width of interquartile range.
*P = .06.
**P < .01.
Abbreviations: ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, NAART = North American Adult Reading Test, PANSS = Positive and Negative Syndrome Scale, SANS = Scale for the Assessment of Negative Symptoms.
Click figure to enlarge
Outcome Measures
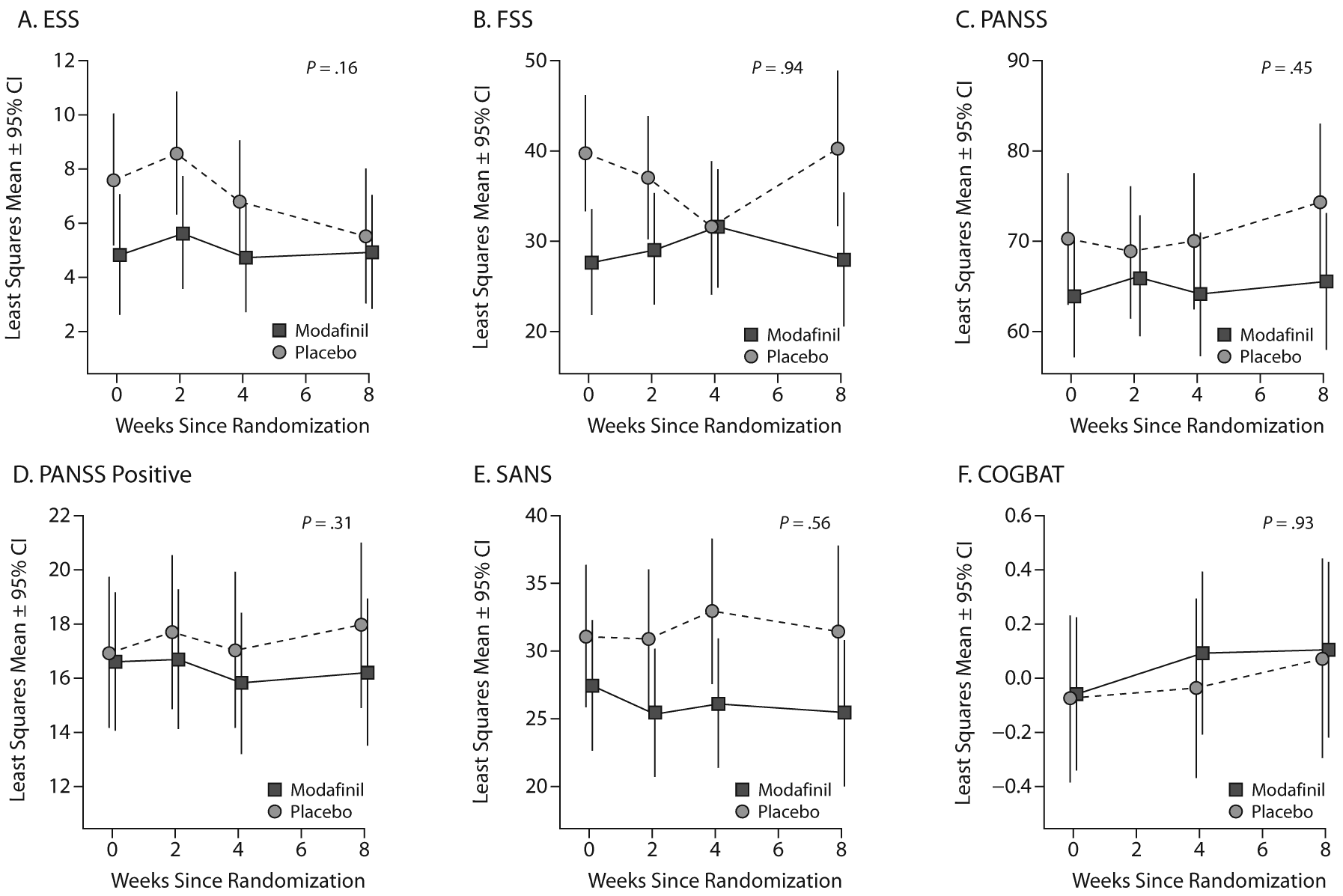
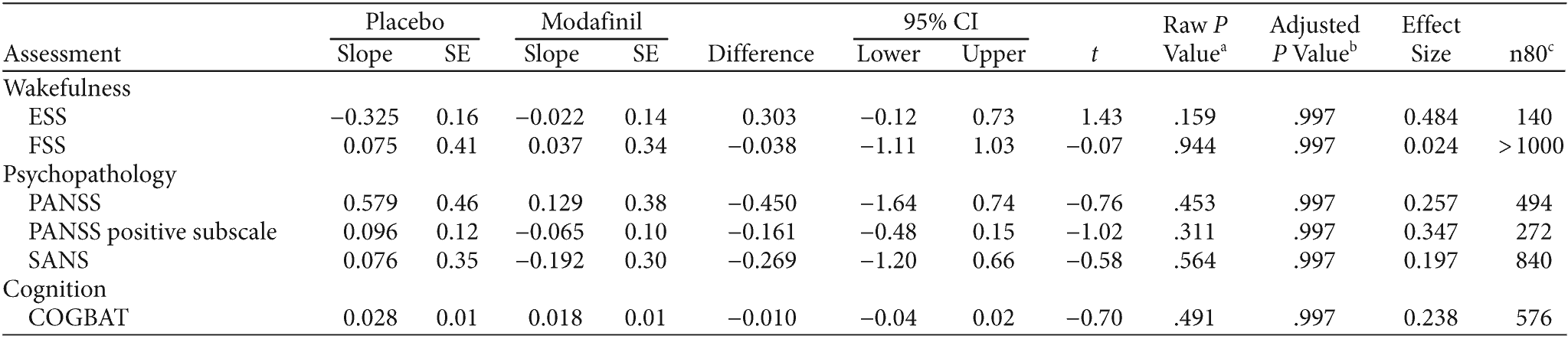
We observed no significant effect of modafinil on measures of wakefulness/fatigue, psychopathology, or cognition (see Figure 2 and Table 2). Among our primary outcomes, the possible benefit of modafinil was greatest for PANSS positive subscale scores (effect size = 0.35), but no treatment comparisons were significant and in favor of modafinil even without correction for multiple comparisons (data not shown). Among a total of 28 outcome measures, including individual scores on cognitive tests, trajectories differed significantly between treatment groups only for the Grooved Pegboard, which showed a greater improvement among subjects randomly assigned to placebo. There was no evidence for improved wakefulness with modafinil; ESS scores improved nonsignificantly more in the placebo group, with a 95% confidence interval that spans only modest benefit from modafinil. Although the estimated modafinil effect was modest at best for our outcomes of interest, the 95% confidence intervals do include potentially large beneficial effect of modafinil outcomes other than ESS (expressing the upper 95% confidence bound as an effect size in units of SD): FSS = 0.70, PANSS = 0.94, PANSS positive subscale = 1.03, SANS total = 0.88, COGBAT = 0.95.
Figure 2. Treatment-Specific Trajectories of Six Measures of Fatigue, Positive and Negative Symptoms, and Cognitive Functiona
aEach panel displays least squares means with 95% confidence intervals and the nominal P-value from a test of treatment-dependent differences in the mean rate of change of each measure from the mixed model analysis described in the text.
Abbreviations: COGBAT = composite score of neurocognitive battery, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, PANSS = Positive and Negative Syndrome Scale, SANS = Scale for the Assessment of Negative Symptoms.
Click figure to enlarge
Table 2. Effects of Modafinil on Wakefulness, Psychopathology, and Cognition in an 8-Week, Placebo-Controlled, Double-Blind Trial of Clozapine-Treated Patients
aComparison-wise P value.
bMultiple-comparison-corrected P value for 28 comparisons using Hommel’s closed testing procedure.
cTotal sample size required for 80% power if the same trial were repeated under the assumption of a parallel-group design with equal allocation, equivalent measurement schedule, equivalent visit completion rates, and analysis by the same mixed-model analysis of variance with 2-tailed comparison of slopes estimated by linear contrasts at α = .05.
Abbreviations: COGBAT = composite score of neurocognitive battery, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, PANSS = Positive and Negative Syndrome Scale, SANS = Scale for the Assessment of Negative Symptoms.
Click figure to enlarge
Laboratory Values
Serum clozapine levels increased from a geometric mean of 330 ng/mL at baseline to 377 ng/mL at week 8 in the modafinil group and decreased from 330 ng/mL to 260 ng/mL in the placebo group. The 8-week change in serum clozapine concentrations did not differ significantly between treatments (mean ratio of change with modafinil versus change with placebo = 154%, 95% CI, 77%-308%, t14 = 1.34; P = .20.
Safety and Tolerability
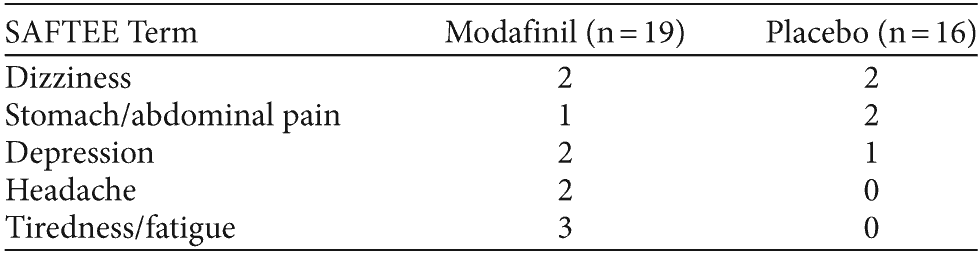
Table 3. Side Effects in a Double-Blind, Placebo-Controlled Pilot Trial of Modafinil Added to Clozapine in 35 Patients With Schizophrenia as Assessed by SAFTEEa
aEvents reported by 10% or greater of either treatment group.
Abbreviation: SAFTEE = Systematic Assessment for Treatment Emergent Events.
Click figure to enlarge
Modafinil was well tolerated. Dose reductions were less frequent among patients treated with modafinil than among those receiving placebo. There were 2 serious adverse events, 1 psychiatric hospitalization in a modafinil-treated subject and worsening of psychosis in a placebo-treated subject. Treatment-emergent or worsening side effects as collected with the SAFTEE were infrequent and not significantly different between the treatment group and placebo group (see Table 3).
DISCUSSION
We could not confirm benefit from modafinil for cognition, negative symptoms, or wakefulness/fatigue when added to ongoing treatment with clozapine in patients with schizophrenia. Unlike the only controlled trial that reported cognitive benefits from a single dose of 200 mg/d modafinil,12 we did not see improvement with repeated administration of modafinil in attentional set shifting and short-term verbal memory. Single-dose administration was also reported to increase activation of the anterior cingulate30 and the dorsolateral prefrontal cortex31 in functional magnetic imaging studies of patients with poor baseline executive function. The disparity in results between our study and studies of single-dose administration raise the question of whether tachyphylaxis may occur with chronic dosing. In addition, timing of testing might be important. The positive Turner et al study12 tested patients 2 hours after modafinil administration, while neither the Sevy et al study13 nor our own controlled for time of testing in relation to drug administration.
While modafinil was reported to improve antipsychotic-related sedation in 3 patients,32 the only controlled trial that specifically measured fatigue did not find such benefit,13 consistent with our results. It is also possible that effects of modafinil might be most pronounced in those patients with the greatest fatigue; we did not specify a threshold of severity for fatigue to enter the trial and did not find any interaction between baseline fatigue, treatment group, and rate of change in fatigue. Specifically targeting patients with impaired wakefulness or fatigue based on rating scale scores might be necessary to show drug effect (such as reported in cases selected for sedation32). However, in our representative sample of clozapine patients, few patients showed such serious impairment. Moreover, regression to the mean will exaggerate drug efficacy in samples selected for symptom severity.
Because the mean daily modafinil dose by end of treatment was 250 mg/d, and compliance, as estimated by pill counts, was quite good, it is unlikely that underdosing explains our negative results. Modafinil 200 mg/d is a standard dose for narcolepsy and other disorders of wakefulness and was the dose used in the positive, single-dose trial in schizophrenia patients reported by Turner and colleagues.12 Whereas tachyphylaxis as seen with other cognitive enhancing drugs (eg, D-cycloserine33) may account for the lack of cognitive benefit, an open-label, 12-week trial of modafinil as an adjunct to nasal continuous positive airway pressure in 125 patients with obstructive sleep apnea and residual fatigue found no evidence of tolerance to modafinil.34 Since we relied on an "honor system" pill count, we cannot exclude that underdosing from poor adherence occurred.
Our power to detect differences between modafinil and placebo was limited by sample size. While the estimated modafinil effect was at best modest for our outcomes of interest (effect size ≤ 0.35 in favor of modafinil), the 95% confidence intervals were wide and included potentially large beneficial effects of modafinil for outcomes other than ESS. Larger trials will be needed to better resolve the true therapeutic effect, if any, of modafinil in this population. For each variable, Table 2 shows total sample size required for 80% power if the same trial were repeated.
Modafinil was well tolerated, with no clear tolerability problem in this small sample. We did not detect worsening of psychosis, one of the safety concerns that has been raised with modafinil. According to the package insert35 and a case report,36 modafinil can in rare instances induce psychosis in individuals without a history of mental illness. Previous modafinil add-on trials also have not reported worsening of psychosis in stabilized patients with schizophrenia.13,14 However, a case report suggests that it remains a possibility.37 An additional, recent safety concern has been the reporting of serious rashes in the worldwide, postmarketing phase since modafinil’s release. The package insert contains a warning about rare, life-threatening rashes including Stevens-Johnson syndrome. None were observed in our study.
We also examined drug-drug interactions between modafinil and clozapine. In vitro studies have shown that modafinil in addition to inhibiting cytochrome P450 (CYP)2C19 can lead to a small, concentration-dependent induction of (CYP)1A2, (CYP)2B6, and (CYP)3A4.38,39 Clinical studies in healthy volunteers have confirmed the potential of modafinil to lower plasma concentrations of alprazolam and ethinyl estradiol, both medications with 3A4-dependent metabolism.40 While clozapine is a substrate of (CYP)1A2, with minor contributions from other enzymes including (CYP)2C19 and (CYP)3A4,41 we did not observe clinically meaningful changes in plasma clozapine levels from the addition of modafinil in our patients. However, increased plasma clozapine levels as a result of (CYP)2C19 inhibition by modafinil was thought to have been the cause of clozapine toxicity in a case report.42 Because individual differences in clozapine metabolism might make it difficult to predict drug interactions with clozapine,43 plasma clozapine level monitoring should be considered.
Future analyses should focus on the effects of clozapine on weight and metabolic parameters. Perhaps an important effect of modafinil is missed by only examining rating scales of negative symptoms. Using a double-blind, crossover design, Farrow and colleagues44 detected increased motor activity (using a wrist-worn device to record motor activity) in patients following a single dose of 100 mg/d modafinil. If sustained, such an effect on motor activity could lead to accrued health benefits over time by reducing the time spent sedated. Henderson and colleagues45 reported a case of significant weight loss in a clozapine-treated patient after addition of modafinil; this was attributed to less fatigue and increased activity during the day. Their case suggests that at least in individual patients, modafinil might have clear benefits that are not generalizable to all patients with schizophrenia.
While modafinil was well tolerated, our pilot trial could not confirm efficacy for modafinil when added to clozapine for the chronic treatment of wakefulness/fatigue, negative symptoms, or cognitive symptoms. However, we had limited power to detect a treatment effect, and a type II error is clearly possible in our small sample. If clinicians nevertheless consider prescribing modafinil on a case-by-case basis for an off-label indication like clozapine-induced sedation or negative symptoms, a recent warning regarding Stevens-Johnson syndrome must be taken into account.
Drug names: alprazolam (Xanax, Niravam, and others), amphetamine (Adderall, Desoxyn, and others), clozapine (FazaClo, Clozaril, and others), ethinyl estradiol (Ocella, Yasmin, and others), modafinil (Provigil).
Author affiliations: Massachusetts General Hospital Schizophrenia Program (Drs Freudenreich, Henderson, Evins, Fan, Cather, and Goff and Mr Walsh); Harvard Medical School (all authors); Boston University School of Medicine (Dr Freudenreich); and Massachusetts General Hospital Biostatistics Center (Dr Macklin), Boston, Massachusetts.
Financial disclosure: Dr Freudenreich receives grant/research support from Cephalon. Dr Henderson is a consultant for Covance, receives research support from Solvay and Takeda, and has received honoraria from Janssen, Bristol-Meyers Squibb, and Pfizer. Dr Goff receives grant/research support from Cephalon. Drs Macklin, Evins, Fan, Cather, and Mr Walsh report no financial or other relationships relevant to the subject of this article.
Funding/support: Funding for this trial was provided by an investigator-initiated grant from Cephalon awarded to Dr Goff.
REFERENCES
1. Buchanan RW, Breier A, Kirckpatrick B, et al. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155(6):751-760. PubMed
2. Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159(6):1018-1028. PubMed doi:10.1176/appi.ajp.159.6.1018
3. Milev P, Ho BC, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506. PubMed doi:10.1176/appi.ajp.162.3.495
4. Rosenheck R, Leslie D, Keefe R, et al. Barriers to employment for people with schizophrenia. Am J Psychiatry. 2006;163(3):411-417. PubMed doi:10.1176/appi.ajp.163.3.411
5. Safferman A, Lieberman JA, Kane JM, et al. Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17(2):247-261. PubMed
6. Burke M, Sebastian CS. Treatment of clozapine sedation. (letter) Am J Psychiatry. 1993;150(12):1900-1901. PubMed
7. Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196-204. PubMed doi:10.1192/bjp.185.3.196
8. Richard ML, Liskow BI, Perry PJ. Recent psychostimulant use in hospitalized schizophrenics. J Clin Psychiatry. 1985;46(3):79-83. PubMed
9. Valentino RM, Foldvary-Schaefer N. Modafinil in the treatment of excessive daytime sleepiness. Cleve Clin J Med. 2007;74(8):561-566, 568-571. PubMed doi:10.3949/ccjm.74.8.561
10. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628. PubMed
11. Roth T, Schwartz JR, Hirshkowitz M, et al. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med. 2007;3(6):595-602. PubMed
12. Turner DC, Clark I, Pomarol-Clotet E, et al. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004;29(7):1363-1373. PubMed doi:10.1038/sj.npp.1300457
13. Sevy S, Rosenthal MH, Alvir J, et al. Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry. 2005;66(7):839-843. PubMed
14. Pierre JM, Peloian JH, Wirshing DA, et al. A randomized, double-blind, placebo-controlled trial of modafinil for negative symptoms in schizophrenia. J Clin Psychiatry. 2007;68(5):705-710. PubMed
15. Krupp LB, LaRocca NG, Muir-Nash J, et al. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121-1123. PubMed
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
17. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. PubMed
18. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed
19. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39(7):784-788. PubMed
20. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1983.
21. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398. PubMed
22. Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343-381. PubMed
23. Blair J, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3(2):129-136. doi:10.1080/13854048908403285
24. Nuechterlein KH, et al. Developmental processes in schizophrenic disorders: Longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18(3):387-425. PubMed
25. Benedict RHB, Schretlen D, Groninger L, et al. The Hopkins Verbal Learning Test—Revised. Clin Neuropsychol. 1998;12(1):43-55.
26. Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. (WAIS-III). San Antonio, TX: The Psychological Corporation; 1997.
27. Heaton R. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1990.
28. Benton A, Hamsher K. Multilingual Aphasia Examination (Manual Revised). Iowa City: University of Iowa; 1978.
29. Hommel G. A comparison of two modified Bonferroni procedures. Biometrika. 1989;75(2):383-386. doi:10.1093/biomet/75.2.383
30. Spence SA, Green RD, Wilkinson ID, et al. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry. 2005;187:55-61. PubMed doi:10.1192/bjp.187.1.55
31. Hunter MD, Ganesan V, Wilkinson ID, et al. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163(12):2184-2186. PubMed doi:10.1176/appi.ajp.163.12.2184
32. Makela EH, Miller K, Cutlip WD. Three case reports of modafinil use in treating sedation induced by antipsychotic medications. J Clin Psychiatry. 2003;64(4):485-486. PubMed
33. Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83(3):224-231. PubMed doi:10.1016/j.nlm.2005.01.001
34. Schwartz JRL, Hirshkowitz M, Erman MK, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003;124(6):2192-2199. PubMed doi:10.1378/chest.124.6.2192
35. Provigil [package insert]. West Chester, PA: Cephalon; 2008.
36. Mariani JJ, Hart CL. Psychosis associated with modafinil and shift work. (letter) Am J Psychiatry. 2005;162(10):1983. PubMed doi:10.1176/appi.ajp.162.10.1983
37. Narendran R, Young CM, Valenti AM, et al. Is psychosis exacerbated by modafinil? (letter) Arch Gen Psychiatry. 2002;59(3):292-293. PubMed doi:10.1001/archpsyc.59.3.292
38. Robertson P, DeCory HH, Madan A, et al. In vitro inhibition and induction of human hepatic cytochrome P450 enzymes by modafinil. Drug Metab Dispos. 2000;28(6):664-671. PubMed
39. Robertson P Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42(2):123-137. PubMed doi:10.2165/00003088-200342020-00002
40. Robertson P Jr, Hellriegel ET, Arora S, et al. Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers. Clin Pharmacol Ther. 2002;71(1):46-56. PubMed doi:10.1067/mcp.2002.121217
41. Spina E, de Leon J. Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007;100(1):4-22. PubMed doi:10.1111/j.1742-7843.2007.00017.x
42. Dequardo JR. Modafinil-associated clozapine toxicity (comment on Am J Psychiatry 2001;158:970). Am J Psychiatry. 2002;159(7):1243-1244. PubMed doi:10.1176/appi.ajp.159.7.1243-a
43. Chetty M, Murray M. CYP-mediated clozapine interactions: how predictable are they? Curr Drug Metab. 2007;8(4):307-313. PubMed doi:10.2174/138920007780655469
44. Farrow TF, Hunter MD, Haque R, et al. Modafinil and unconstrained motor activity in schizophrenia: double-blind crossover placebo-controlled trial. Br J Psychiatry. 2006;189:461-462. PubMed doi:10.1192/bjp.bp.105.017335
45. Henderson DC, Louie PM, Koul P, et al. Modafinil-associated weight loss in a clozapine-treated schizoaffective disorder patient. Ann Clin Psychiatry. 2005;17(2):95-97. PubMed doi:10.1080/10401230590932407
Enjoy this premium PDF as part of your membership benefits!