International Consensus Statement on Major Depressive Disorder
Because considerable variability exists between countries in the management of major depressive disorder, experts in psychiatry gathered for the International Consensus Group on Depression to outline a universal treatment algorithm for this illness. The experts decided to adapt the existing treatment algorithm developed in Japan and discuss strategies for clinical issues that have been problematic in some countries. Specific recommendations were made by the consensus group for screening for, diagnosing, and treating depression, which include periodically screening all patients for depression, completing a differential diagnosis of depression, referring to a psychiatric specialist if needed, establishing a therapeutic alliance with patients and their families, choosing and optimizing the dose of appropriate antidepressants based on individual patient’s needs, and incorporating nonpharmacologic treatment strategies as necessary.
(J Clin Psychiatry 2010;71[suppl E1]:e08)
From the Neuropsychopharmacology Unit, Division of Experimental Medicine, Imperial College, London, United Kingdom (Professor Nutt); Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Durham, North Carolina, United States (Dr Davidson); Healthcare Technology Systems, Inc, and the Department of Psychiatry, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States (Dr Gelenberg, who is now with the Department of Psychiatry, Penn State University Milton S. Hershey Medical Center, Hershey, Pennsylvania); National Center of Neurology and Psychiatry, Tokyo, Japan (Dr Higuchi); Department of Neuropsychiatry, University of Kyushu Graduate School of Medical Sciences, Higashi, Fukuoka, Japan (Dr Kanba); Department of Psychiatry, ÅžiÅŸli Etfal Teaching and Research Hospital, Istanbul, Turkey (Dr Karamustafalıoğlu); Department of Psychiatry, Harvard Medical School and Massachusetts General Hospital, Boston, Massachusetts, United States (Dr Papakostas); Department of Psychiatry, Tokyo Women’s Medical University, Tokyo, Japan (Dr Sakamoto); Department of Neuropsychiatry, Oita University Faculty of Medicine, Yufu, Oita, Japan (Dr Terao); and Shanghai Jiaotong University and Shanghai Mental Health Center, Shanghai, China (Dr Zhang).
This article is derived from the roundtable discussion "International Consensus Group on Depression," which was held on September 1, 2009, in Tokyo, Japan, and supported by an educational grant from GlaxoSmithKline.
Professor Nutt is a consultant for GlaxoSmithKline, Lundbeck, Pfizer, Servier, and sanofi-aventis; has received grant/research support from Organon and P1vital; and is a member of the speakers/advisory boards for Organon, GlaxoSmithKline, Lundbeck, Servier, and Bristol-Myers Squibb. Dr Davidson is an advisor for AstraZeneca, Transcept, and ZARS; has received honoraria as a speaker from DCH Regional Medical Center (Alabama), GlaxoSmithKline, the American Psychiatric Association (APA), AstraZeneca, the Japanese Society of Anxiety Disorders Research, the Tokyo Academy of Cognitive Therapy, Sharp HealthCare, USC School of Medicine, and the University of New Mexico; and has received royalties/license fees from MultiHealth Systems Inc, Guilford Publications, APA, the Connor-Davidson Resilience Scale, and the Social Phobia Inventory. Dr Gelenberg is a consultant for Eli Lilly, Pfizer, Best Practice Project Management, AstraZeneca, Wyeth, Cyberonics, Novartis, Forest, GlaxoSmithKline, ZARS, Jazz, Lundbeck, Takeda, and eResearch Technology; has received research grant funding from Eli Lilly; and has major stock ownership of Healthcare Technology Systems, Inc. Dr Kanba is a consultant and a member of the speakers/advisory boards for Eli Lilly, GlaxoSmithKline, Pfizer, Mitsubishi, Ono, Astellas, Asahi Kasei, Shionogi, and Otsuka; has received grant/research support from Eli Lilly, GlaxoSmithKline, Pfizer, Asahi Kasei, Janssen, Tsumura, Ajinomoto, Yoshitomi, Meiji, Kyowa Hakko, Dainippon Sumitomo, Organon, and Otsuka; and has received honoraria from and is a member of the speakers/advisory boards for Eli Lilly, GlaxoSmithKline, Pfizer, Asahi Kasei, Janssen, Tsumura, Ajinomoto, Yoshitomi, Meiji, Kyowa Hakko, Dainippon Sumitomo, Organon, Otsuka, and Astellas. Dr Karamustafalıoğlu has received grant/research support from Pfizer and Sanovel; has received honoraria from Lundbeck, Wyeth, Bristol-Myers Squibb, Abdi Ä°brahim, Egis, and Eczacıbaşı-Zentiva; and is a member of the speakers/advisory boards for Johnson & Johnson, GlaxoSmithKline, Eli Lilly, and Pfizer. Dr Papakostas has served as an advisor/consultant for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Eli Lilly, Evotec, Inflabloc, Jazz, Shire, Titan, Otsuka, Pfizer, Pierre Fabre, Wyeth, and Pamlab; has received grant/research support from the National Institute of Mental Health, Pamlab, Pfizer, Forest, Precision Human Biolaboratories, and Bristol-Myers Squibb; and has received honoraria from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Eli Lilly, Evotec, Inflabloc, Jazz, Shire, Otsuka, Pierre Fabre, Pfizer, Pamlab, Titan, Wyeth, and Lundbeck. Dr Terao has received grant/research support from and/or is a member of the speakers/advisory boards for GlaxoSmithKline, Eli Lilly, Janssen, Pfizer, Sanofi-Aventis, Kyowa Hakko Kirin, Dainippon Sumitomo, Otsuka, Astellas, Schering-Plough, Meiji, Taisho Toyama, Asahi Kasei, and Yoshitomi Yakuhin. Drs Higuchi, Sakamoto, and Zhang have no personal financial relationships to disclose relative to this article.
Corresponding author: Professor David J. Nutt, Imperial College London, Burlington Danes Bldg, Hammersmith Campus, 160 Du Cane Rd, London W12 0NN ([email protected]).
doi:10.4088/JCP.9058se1c.08gry
© Copyright 2010 Physicians Postgraduate Press, Inc.
In addition to the treatment guidelines for major depressive disorder (MDD) published in the United States and Europe, progress has been made in developing MDD treatment guidelines in Japan, China, and the Middle East. However, a general consensus has been needed to merge the evidence base and standardize clinical practice in each region to ensure that all patients with MDD receive optimal treatment and have the opportunity to reach remission. According to existing treatment guidelines from various countries,1-6 the following basic principles apply to treating patients with MDD:
- Establish a correct diagnosis by using screening and diagnostic tools in conjunction with a full clinical psychiatric assessment.
- Assess the patient’s depression severity, current stressors, comorbidities, and suicide risk.
- Select a treatment setting for the patient (ie, inpatient versus outpatient) depending on the severity of depression.
- Establish a therapeutic alliance and educate the patient and his or her family about depression and its treatment.
- Choose appropriate, individualized treatments of an adequate dose and duration to help the patient achieve remission.
Experts in psychiatry gathered for the International Consensus Group on Depression to outline a universal treatment algorithm for depression. To avoid duplicating previous guidelines, the consensus group decided to adapt Japan’s existing treatment algorithm for depression7 and address specific issues as they pertain to the way care is provided from country to country. The topics addressed included screening for and diagnosing depression, treating depression in primary care or referring to a specialist, and using preferred treatments for depression while considering treatment availability, cost-effectiveness, and optimal dosing strategies.
Screening for Depression
The consensus group recommended that clinicians periodically screen all patients for depression to identify those who may need additional evaluation. Failing to screen on a routine basis may result in unrecognized cases of depression, which substantially impacts patients as well as society,8 and inaccurate or overdiagnoses of depression, which may lead to inadvertent patient mistreatment.9 One widely accepted, simple, and cost-effective method of screening for depression is administering the 2-item Patient Health Questionnaire (PHQ-2),10 which consists of asking a patient 2 questions: "Over the past 2 weeks, how often have you had little interest or pleasure in doing things?" and "Over the past 2 weeks, how often have you felt down, depressed, or hopeless?" Then, score the patient’s responses to each question as follows: not at all = 0, several days = 1, more than half the days = 2, or nearly every day = 3. If the patient scores a total of 3 or less, then the screen is negative for depression. However, if the patient scores 4 or more, then an in-depth diagnostic assessment for depression should be given and a differential diagnosis should be completed.
For Clinical Use
- Periodically screen all patients for depression using measurement-based tools such as the 2-item Patient Health Questionnaire (PHQ-2).
- Use measurement-based tools to differentially diagnose depression and assess the level of depression severity, the presence of any co-occurring medical or psychiatric disorders, and the risk of suicide or harm to self or others.
- Refer patients with bipolar disorder, psychotic symptoms, a substance use disorder, a risk of suicide or harm to self or others, severe comorbid disorders, or treatment resistance to a psychiatric specialist.
- Educate patients about the disorder and its treatment and establish a positive relationship to improve treatment adherence as well as overall treatment outcomes.
- Begin treatment with antidepressant monotherapy, such as a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), or norepinephrine-dopamine reuptake inhibitor (NDRI), using therapeutic doses. Benzodiazepine monotherapy should be avoided.
- Treat patients to remission and monitor patients’ progress after remission has been achieved.
Some concern has arisen about the reliability and validity of US-designed screening instruments when used outside of the United States. Although the PHQ-2 items regarding mood and interest may successfully screen for depression in the United States, patients in other regions may present differently (for instance, being more likely to report somatic rather than cognitive/emotional symptoms). For example, in locations where depression carries great social stigma, patients may prefer to describe physical symptoms rather than mood symptoms to their physicians. Physicians in some countries initially inquire about the patient’s sleep and sexual functioning. Not only may a preliminary indication of depression be uncovered, but other psychiatric disorders may be identified as well. If the patient answers positively to these 2 questions, then clinicians should, again, complete an in-depth diagnostic assessment for depression and a differential diagnosis.
Although several screening tools for depression are available, more research is needed in developing reliable, valid, and sensitive screening tools specifically for the Middle East and Central, Southern, and Pacific Asian regions, as well as other areas of the world in order to better recognize depression. For more information on screening tools, see the article in this supplement by Alan J. Gelenberg, MD, "Using Assessment Tools to Screen for, Diagnose, and Treat Major Depressive Disorder in Clinical Practice."11
Diagnosing Depression
Using Measurement-Based Diagnostic Tools
The consensus group recommended that physicians use measurement-based tools to diagnose depression and to assess the level of depressive severity, the presence of any co-occurring psychiatric or medical disorders, and the risk of suicidality. Unfortunately, some well-established diagnostic tools are expensive and time-consuming, hindering busy general psychiatrists’ and family practitioners’ ability to make an accurate diagnosis in the brief amount of time spent with a patient. However, other available diagnostic instruments are inexpensive or free and are easy to administer in a primary care setting.
The 9-item Patient Health Questionnaire (PHQ-9)12 is a brief expansion of the PHQ-2 that probes into the patient’s level of anhedonia and depressed mood as well as sleeping and eating habits, self-esteem, psychomotor retardation, and self-harm/suicidal thoughts over the previous 2 weeks. If the patient scores a total of 9 or less, then the patient does not have MDD. However, if the patient scores 10 or more, then a diagnosis of depression can be made, in conjunction with a clinical interview and differential diagnosis.
The Hospital Anxiety and Depression Scale (HADS)13 is a short, reliable, patient-rated questionnaire designed to detect depression and anxiety in primary care. Like the PHQ-9, the 7-item HADS depression subscale measures the patient’s level of anhedonia, depressed mood, and psychomotor retardation. The items are rated from 0 to 3 with scores of 10 or less indicating unlikely cases of depression and scores of 11 or more indicating a definite diagnosis of depression.
The use of diagnostic assessment tools should be accompanied by a clinical interview and differential diagnosis to ensure a correct diagnosis. These tools help quantify the severity of patients’ depressive symptoms as well as their symptomatic improvement and should supplement, not replace, clinicians’ experience and judgment. For more information on diagnostic tools, see the article in this supplement by Alan J. Gelenberg, MD, "Using Assessment Tools to Screen for, Diagnose, and Treat Major Depressive Disorder in Clinical Practice."11
Completing a Differential Diagnosis
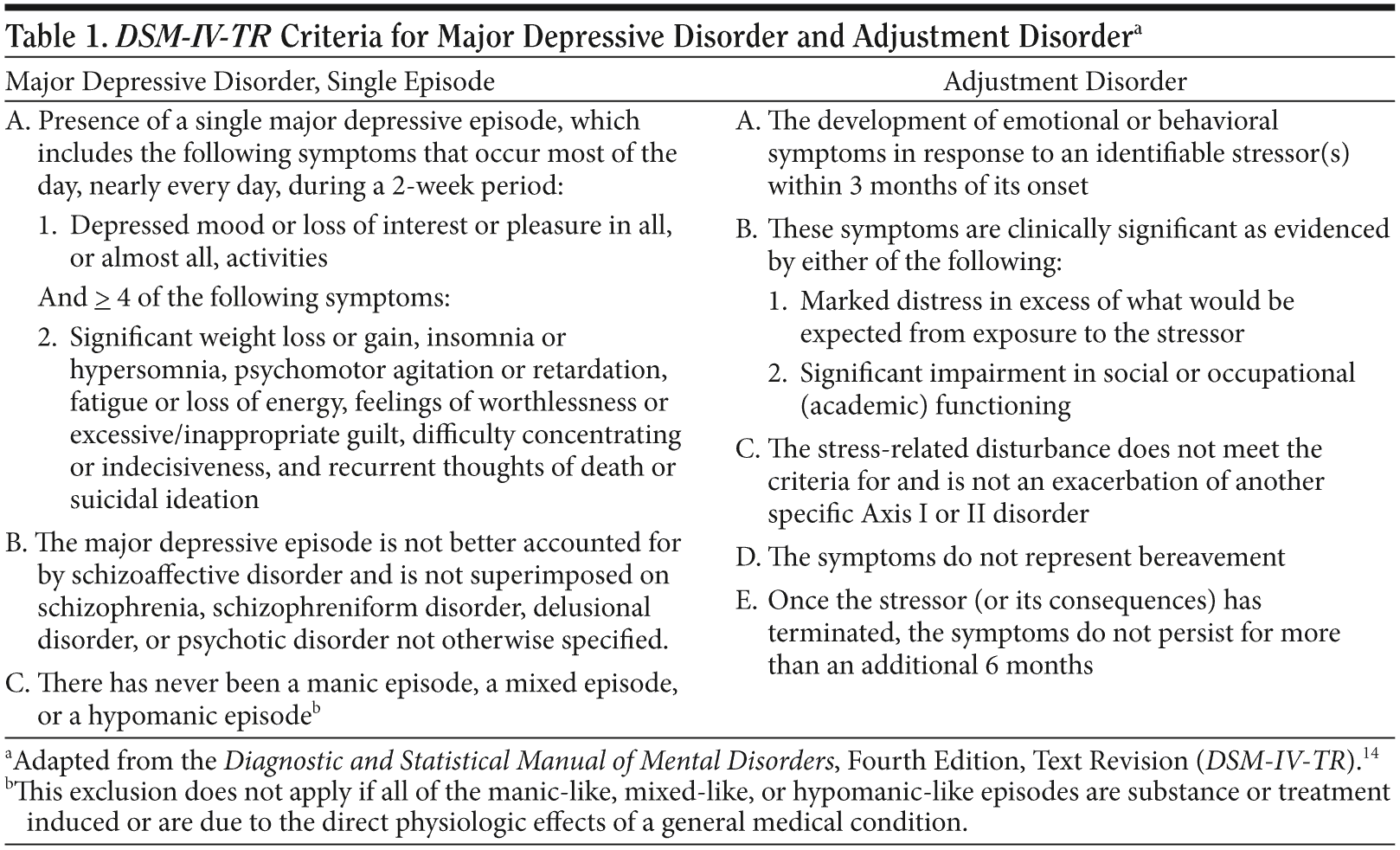
To ensure an accurate diagnosis and to avoid misdiagnosing depression, physicians should always assess patients for psychiatric and medical conditions that may be mistaken for major depression. Assessments should be systematically completed using diagnostic criteria such as those in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)14 or the International Classification of Diseases, Tenth Revision (ICD-10).15
Adjustment disorder is often misdiagnosed as depression because patients exhibit depressive symptoms such as depressed mood, tearfulness, or feelings of hopelessness.14 However, adjustment disorder is a response to a clearly identifiable stressor within 3 months of its occurrence, and the symptoms do not meet the criteria for another psychiatric disorder (Table 1). Similarly, if a patient who has lost a job meets the criteria for a major depressive episode, then adjustment disorder is not a correct diagnosis even though a stressor existed.14
Another disorder often initially misdiagnosed as depression is bipolar disorder. Patients with bipolar disorder frequently experience depressive rather than manic or hypomanic symptoms16,17; thus, they tend to present to physicians with predominantly depressive symptoms. The key differentiating factor between bipolar disorder and MDD is a history of at least 1 manic or hypomanic episode, which indicates bipolar disorder. Therefore, when depression is suspected, clinicians should always ask patients if they have experienced the following symptoms: persistently elevated or irritable mood, increased self-esteem or grandiosity, decreased need for sleep, talkativeness, racing thoughts, distractibility, or increased involvement in goal-directed or pleasurable activities.14
Other conditions that should be assessed before making a definitive diagnosis of major depression are psychosis, substance abuse or misuse, and medical illnesses, particularly thyroid conditions. Finally, if the diagnosis of MDD has been established, the presence of psychotic symptoms should be assessed because therapeutic management differs between MDD and MDD with psychotic features.
Referring Patients to a Psychiatrist
In some countries, relatively few psychiatrists are available, and most patients visit primary care physicians. Because of the lack of psychiatrists, the appropriate time to refer a patient to a specialist has not always been clearly defined and, in some cases, referral is not possible. However, the consensus group described specific indicators for when a patient should be treated in secondary care, if at all possible. If, after completing the differential diagnosis, the clinician finds that a patient has bipolar disorder (such as a history of manic/hypomanic episodes), psychotic symptoms, a substance use disorder, risk of suicide or harm to self or others, severe comorbid psychiatric or medical illnesses, or treatment resistance (ie, a history of multiple unsuccessful treatment attempts), then that patient should be referred to a specialist.1 Further, a patient with extremely severe MDD should be hospitalized.
Treating Depression
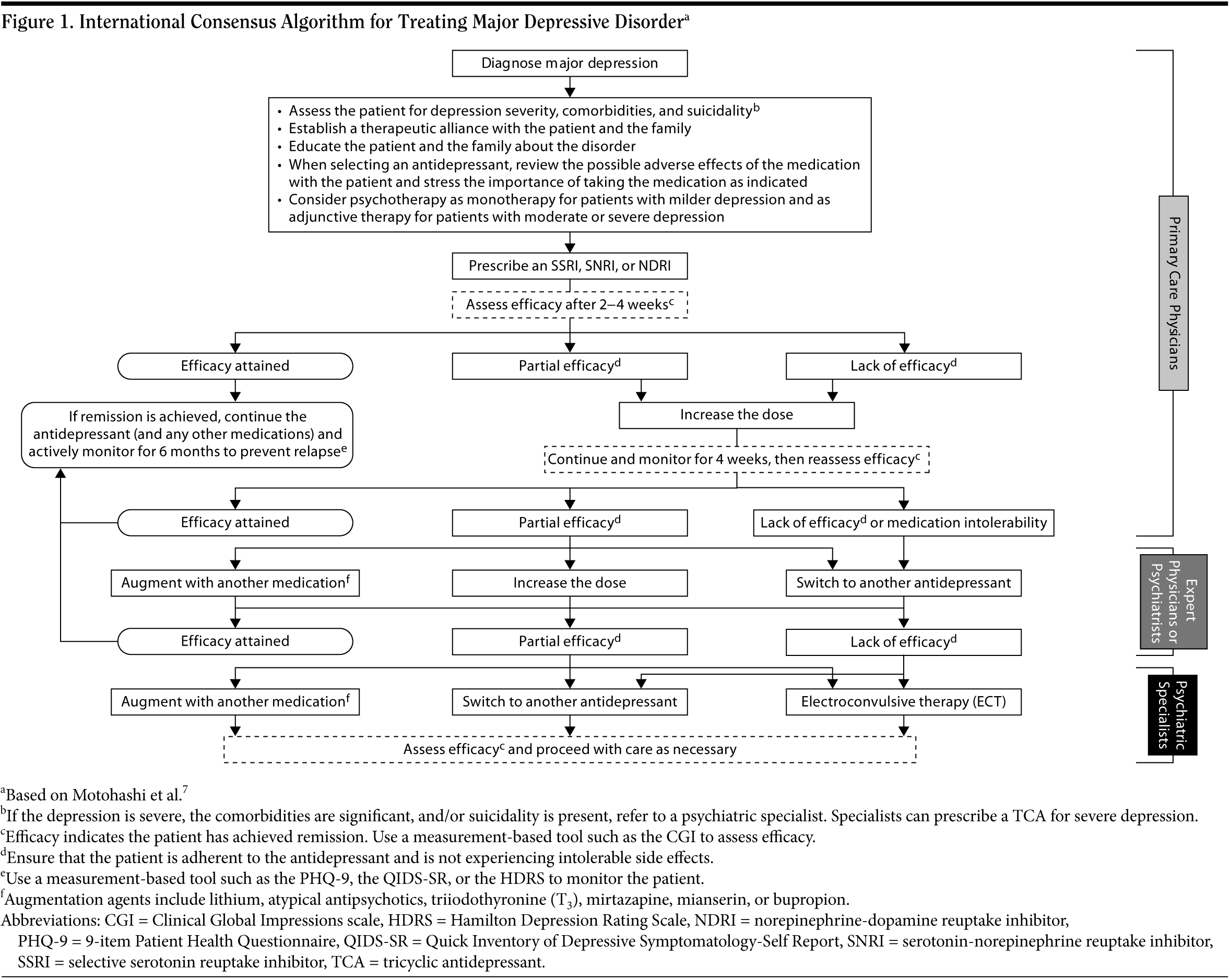
The goal of treating depression is dual: achieving symptomatic remission as well as full functional recovery. The consensus group created an algorithm of recommended treatment practices for physicians to use in order to help patients with MDD achieve remission. This was based on the existing Japanese treatment algorithm for depression (Figure 1).7
After confirming a diagnosis of MDD, clinicians should first determine the severity of the present episode. If the depression is severe, patients should be treated by a psychiatric specialist and may require hospitalization. If the depression is mild or moderate, then clinicians should choose an appropriate treatment based on the individual patient’s needs and preferences while considering treatment availability, cost-effectiveness, safety, and optimal dosing strategies. Additionally, establishing a therapeutic alliance and educating patients are important early steps in the treatment of depression.
Establishing a Therapeutic Alliance and Educating Patients
During the initial clinical visits, physicians should establish a positive working relationship with patients and their families and educate them about depression, its treatment, and the goals of treatment. Patients need to be aware that depression is a chronic and often lifelong illness that needs to be managed accordingly. They also need to be informed of their treatment options, particularly of antidepressants, including the potential side effects and drug-drug interactions, that antidepressants need to be taken daily for 2 to 4 weeks to feel an effect, and of the importance of taking the medication as prescribed and not discontinuing the antidepressant without talking with the physician, regardless of symptomatic improvement.18 Recommending that patients schedule enjoyable activities can also be helpful.18
Clinicians should immediately respond to any worries the patient has about taking medication, such as fears about experiencing intolerable side effects, developing medication dependence, or just taking medication in general. Describe the follow-up plan and reassure the patient that, as treatment progresses, any problems that arise will receive immediate attention and will be addressed appropriately. Patients perceive the thoughtfulness of the physician as he or she answers their questions, and a reciprocal effect occurs in which patients want to cooperate with the physician’s treatment guidance. The physician’s attitude must be one of understanding, compassion, support, and listening. Developing the therapeutic alliance helps to improve patients’ treatment adherence, quality of care, and overall outcomes.2
Byrne et al19 indicated that establishing a collaborative dialogue about medication and involving the patient in the decision-making process is the most effective strategy to improve treatment adherence for patients with mood disorders. Additionally, a study20 of patients with depression found that, as the therapeutic alliance becomes stronger, the odds for patients achieving remission substantially increase, regardless of the type of treatment intervention.
Treatment Availability and Cost-Effectiveness
Because the availability of specific antidepressants varies from country to country (ie, not all agents are approved for the treatment of MDD in all countries of the world), treatments were discussed on a class level rather than an agent level.
Additionally, the cost of antidepressants varies from country to country, which may also dictate availability and use patterns. For example, some governments in countries with universal health care may not pay for certain antidepressants except under specific circumstances. Patients who have the financial resources may be allowed to pay the difference for a certain medication if it is not covered by governmental insurance. Regardless, the tricyclic antidepressants (TCAs) are generally less expensive than antidepressants belonging to newer classes, although their inferior tolerability and safety profiles may adversely impact compliance as well as morbidity and mortality.
Nondrug therapies such as psychotherapy are available to many patients with MDD. However, some governments may not recognize the administration of psychotherapy as a legitimate professional activity, and so, some countries with social insurance do not cover psychotherapy as a treatment for depression, and patients must pay for the treatments themselves. Depending on the country, psychotherapy may be administered by psychiatrists rather than psychologists or may be offered in tertiary care settings such as university hospitals rather than secondary care settings. To make psychotherapy readily available to and cost-effective for patients, research is currently being conducted on implementing computer-aided psychotherapy21 and Internet-based interventions for depression.22 However, more research and development are needed in translating and administering cognitive-behavioral therapy (CBT) online to international audiences.
Selecting Antidepressants
If clinicians are concerned that a patient may have mild depression or depression precipitated by an acute stressor and that the condition may improve without medication at the time of assessment, then the patient should receive supportive or psychosocial therapy and be re-evaluated after 2 weeks. If no improvement in depression occurs during this period of active monitoring, then initiating treatment with an antidepressant and/or psychotherapy is appropriate.14
For patients who meet criteria for moderate or severe depression, clinicians should discuss initiating antidepressant therapy with those patients. Because of their superior tolerability and safety profiles, the use of newer antidepressants such as the selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or the norepinephrine-dopamine reuptake inhibitors (NDRIs) is encouraged over the use of monoamine oxidase inhibitors (MAOIs) or TCAs, that is, if costs permit (see Figure 1). Although numerous agents belonging to each of these classes are currently available, robust differences in overall efficacy between contemporary antidepressants do not appear to exist.1 However, during the course of therapy, patients may require treatment with more than 1 antidepressant (sequentially) in order to achieve remission. Therefore, the consensus group recommended that clinicians become familiar with 2 or 3 antidepressants from each class, including knowing the side effect profiles, dosing strategies, and drug-drug interactions, which will help in selecting an appropriate medication based on each individual patient’s needs. Finally, clinicians should be aware that although benzodiazepine monotherapy is commonly practiced for MDD in some regions, it is not a standard treatment approach and should be discouraged. For more information on the use of benzodiazepines in depression, see the article in this supplement by David Nutt, "Rationale for, Barriers to, and Appropriate Medication for the Long-Term Treatment of Depression."23
Antidepressant Safety and Tolerability
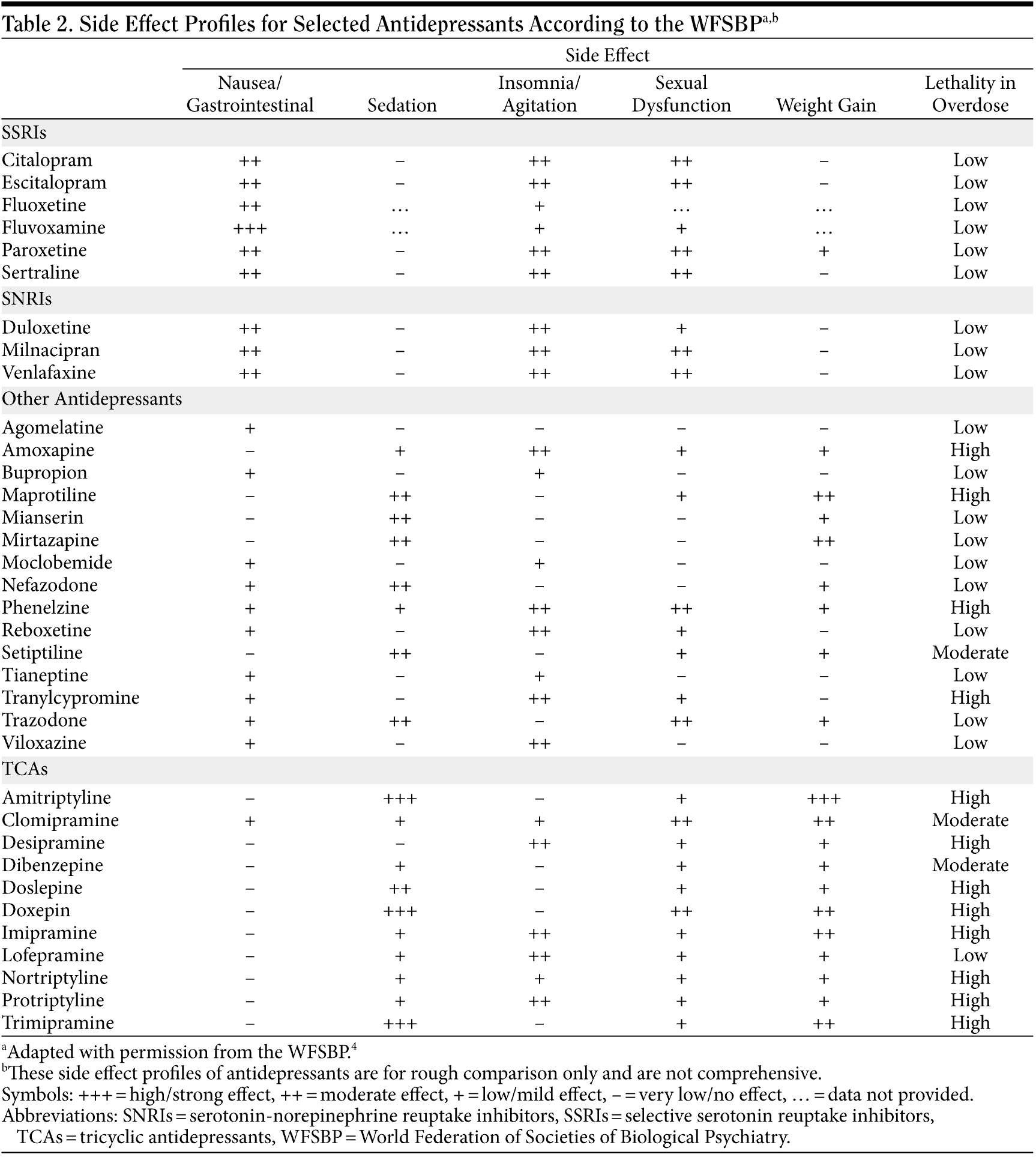
All antidepressants carry some risk of side effects, including nausea, sedation, insomnia, sexual dysfunction, and weight gain. The tendency of specific antidepressants to result in particular side effects are outlined by the World Federation of Societies of Biologic Psychiatry (WFSBP)4 in Table 2. Clinicians should disclose the potential for the emergence and/or worsening of suicidal ideation during antidepressant therapy for patients aged younger than 25 years. While the presence of these side effects may deter some patients from seeking or accepting treatment for their depression, clinicians should stress to patients that the risks of untreated depression, on average, greatly outweigh the risks associated with antidepressant therapy (including the risk of emergent/worsening suicidal ideation). In short, clinicians should routinely recommend antidepressant therapy for their patients with moderate or severe depression and, upon initiation of therapy, closely monitor those patients for treatment-emergent side effects including worsening suicidal ideation.
In order to improve tolerability, clinicians may choose to initiate antidepressant therapy at subtherapeutic doses and then gradually titrate up to a therapeutic level. Should side effects become intolerable, clinicians should consider lowering the dose of the antidepressant (if the dose is already within a therapeutic range) and then measuring the patient’s side effects 2 weeks later. If the intolerable side effects persist, clinicians should consider switching the antidepressant and refer the patient to a specialist, if possible.
For more in-depth information on antidepressants, see the article in this supplement by George I. Papakostas, MD, "The Efficacy, Tolerability, and Safety of Contemporary Antidepressants."24
Optimizing Antidepressant Dosages
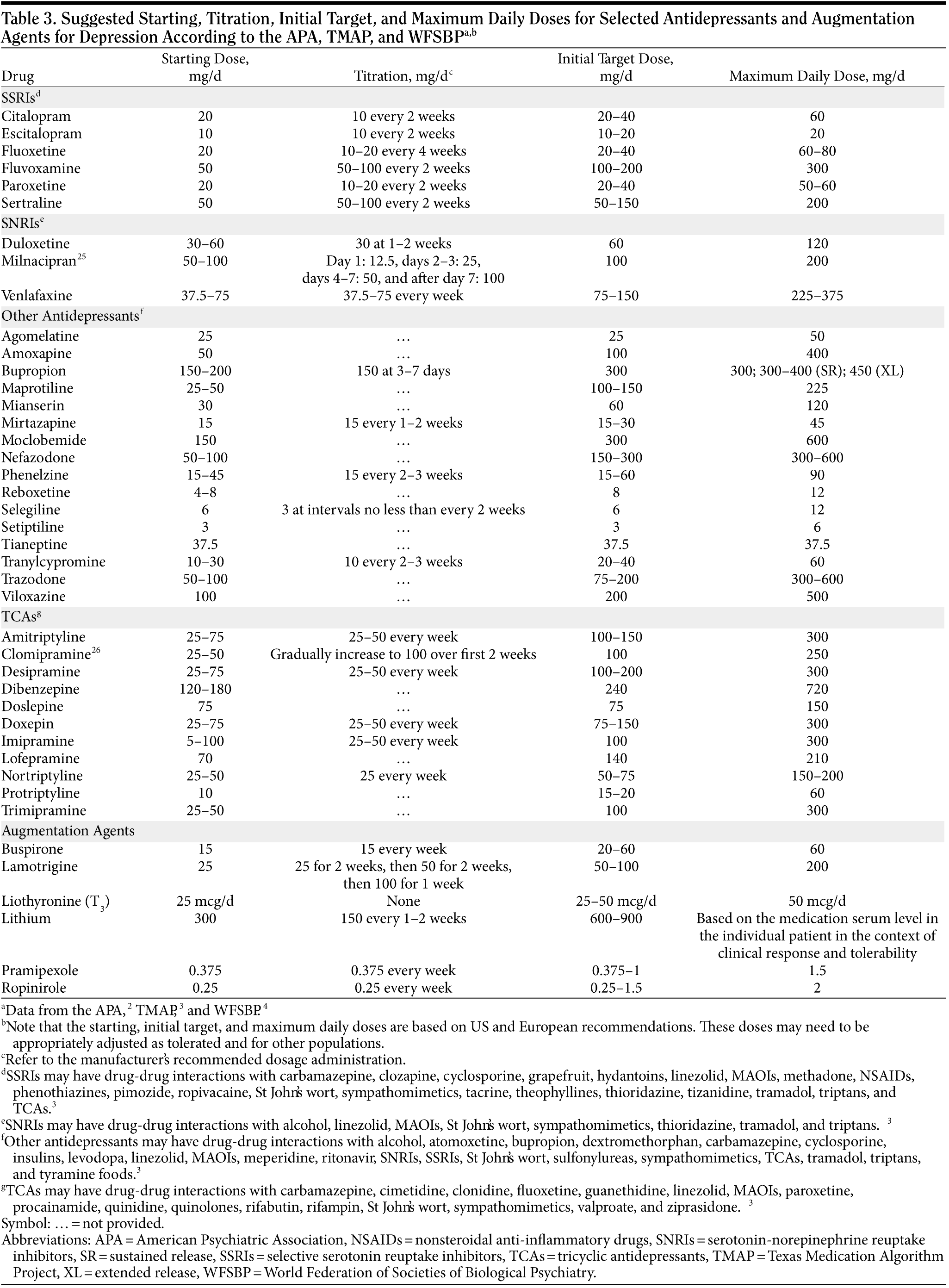
After an antidepressant has been selected, the dosing strategy should be chosen. The goal of a successful dosing strategy for MDD is to achieve a minimally effective dose as soon as possible while optimizing tolerability. The use of multiple agents at subtherapeutic doses, while occasionally a last resort, should not constitute the standard of care. Upon reaching a minimally effective dose, clinicians should wait approximately 2 to 4 weeks to assess symptomatic improvement and tolerability before deciding to increase the dose (in cases of inadequate improvement but acceptable tolerability), maintain the current dose (in cases of adequate improvement and acceptable tolerability), decrease the dose (in cases of adequate improvement but poor tolerability), or switch the patient to a different agent (in cases of inadequate improvement and poor tolerability). Table 325,26 outlines the starting, initial target, and maximum daily doses as recommended by the American Psychiatric Association (APA),2 the Texas Medication Algorithm Project (TMAP),3 and the WFSBP.4 Titration schedules are also recommended in Table 3 to help patients reach therapeutic dosages of the antidepressant.
Assessment of symptomatic improvement and tolerability should occur every 2 weeks (see Figure 1). At each assessment, reasonable options include modifying the antidepressant dose, switching to a different antidepressant, or augmentation/combination strategies. Augmentation/combination strategies include, but are not limited to, lithium, atypical antipsychotics, triiodothyronine (T3), mirtazapine, mianserin, or bupropion.27 Before employing augmentation or switching strategies, if possible, refer the patient to an expert primary care physician or psychiatric specialist who is accustomed to and comfortable with implementing complex treatment strategies for patients with depression. During all phases of treatment, clinicians should actively monitor patients’ course of illness with measurement-based assessments such as the PHQ-9,12 the Clinical Global Impressions (CGI) scale,28 the Hamilton Depression Rating Scale (HDRS),29 or the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR).30 For more information on monitoring tools, see the article in this supplement by Alan J. Gelenberg, MD, "Using Assessment Tools to Screen for, Diagnose, and Treat Major Depressive Disorder in Clinical Practice."11 In general, physicians should choose a tool that is convenient and inexpensive and then use it consistently throughout treatment; patient-rated scales save time and can be completed on paper, by telephone, or by computer.
If remission is achieved during the course of therapy, then (in light of acceptable tolerability) it is recommended that the medication regimen be continued for at least 6 months to help maintain remission and prevent relapse.
Nonpharmacologic Treatments for Depression
When educating patients about treatment options, clinicians should inform patients of pharmacologic as well as nonpharmacologic treatments. Although antidepressants are considered the first-line treatment for patients with depression, nonpharmacologic adjunctive treatments and alternatives to medication are available, including psychotherapy, electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS), all of which should be administered by a psychiatric specialist.
Psychotherapy is an integral part of patients’ treatment and should be considered for initial therapy for mild depression and as augmentation to an antidepressant for moderate or severe depression or for patients with adherence problems.1-4 Overall, psychotherapy has been shown to be effective for acute depression, particularly in combination with antidepressants, and can help in preventing depressive relapse and recurrence.4
Via electric currents or magnetic pulses, ECT, rTMS, VNS, and DBS stimulate brain activity by inducing neurophysiologic effects. ECT should be considered only for patients with severe depression or psychotic symptoms or other special cases or emergency situations.1-4 rTMS is not recommended as a first-line treatment and should be considered only when other, more effective acute treatments are not available.1 VNS is not recommended as a first-line or acute treatment for depression but may be effective for patients with treatment resistance.1,3 Currently, DBS is not recommended for treating depression due to a paucity of data, but research is being conducted in this area.
Summary
This International Consensus Statement on Major Depressive Disorder outlines the basic strategies for detecting, diagnosing, and treating acute MDD. Of critical importance are screening all patients for depression, accurately diagnosing the disorder using appropriate tools and clinical judgment, and treating patients with depression with an antidepressant. Physicians need to become very familiar with the dosing, potential side effects, and costs of a few antidepressants in each class that are available in their countries.
Physicians should develop a therapeutic alliance with each patient and educate patients and their families about the disorder and its treatment. Physicians should be sure to use therapeutic doses of antidepressants, and benzodiazepine monotherapy should be avoided. Psychosocial support and nonpharmacologic therapies may also be needed.
Patients who are resistant to treatment or have complexities such as suicidality, severe depression, or significant comorbidities should be referred to psychiatric specialists or a hospital; if psychiatrists are unavailable, consult with expert general practitioners.
To ensure that patients reach and maintain remission, clinicians should systematically use monitoring tools to assess patients’ treatment response and side effects at regular intervals. Many tools are available that are inexpensive, brief, offered in many languages, and easy to use.
Drug Names: atomoxetine (Strattera), bupropion (Aplenzin, Wellbutrin, and others), buspirone (BuSpar and others), carbamazepine (Carbatrol, Equetro, and others), cimetidine (Tagamet and others), citalopram (Celexa and others), clomipramine (Anafranil and others), clonidine (Catapres, Duraclon, and others), clozapine (FazaClo, Clozaril, and others), cyclosporine (Gengraf, Restasis, and others), desipramine (Norpramin and others), doxepin (Zonalon and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), imipramine (Tofranil and others), insulins (Humalog, Lantus, and others), lamotrigine (Lamictal and others), linezolid (Zyvox), liothyronine (Cytomel, Triostat, and others), lithium (Lithobid and others), meperidine (Demerol and others), methadone (Methadose and others), milnacipran (Savella), mirtazapine (Remeron and others), nortriptyline (Pamelor, Aventyl, and others), paroxetine (Paxil, Pexeva, and others), phenelzine (Nardil), pimozide (Orap), pramipexole (Mirapex and others), protriptyline (Vivactil and others), rifabutin (Mycobutin), rifampin (Rifadin, Rimactane, and others), ritonavir (Norvir), ropinirole (Requip and others), ropivacaine (Naropin), selegiline (Emsam, Zelapar, and others), sertraline (Zoloft and others), tacrine (Cognex), theophylline (Elixophyllin, Theocron, and others), tizanidine (Zanaflex and others), tramadol (Ryzolt, Ultram, and others), tranylcypromine (Parnate and others), trimipramine (Surmontil and others), valproate (Depacon and others), venlafaxine (Effexor and others), ziprasidone (Geodon).
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, agomelatine, clomipramine, dibenzepine, doslepine, doxepin, fluvoxamine, lofepramine, maprotiline, mianserin, milnacipran, moclobemide, reboxetine, setiptiline, tianeptine, and viloxazine are not approved by the US Food and Drug Administration for the treatment of major depression, and buspirone, lamotrigine, liothyronine, lithium, pramipexole, ropinirole, and triiodothyronine are not approved as augmentation agents for major depression.
References
1. Anderson IM, Ferrier IN, Baldwin RC, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22(4):343-396. http://www.bap.org.uk/docsbycategory.php?docCatID=2. Accessed March 3, 2010. doi:10.1177/0269881107088441 PubMed
2. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 2nd ed. http://www.psychiatryonline.com/pracGuide/pracGuideTopic_7.aspx. Published 2000. Accessed March 3, 2010.
3. Suehs BT, Argo TR, Bendele SD, et al. Texas Medication Algorithm Project Procedural Manual: Major Depressive Disorder Algorithms. Austin, TX: Texas Department of State Health Services; 2008. http://www.dshs.state.tx.us/mhprograms/disclaimer.shtm. Accessed March 3, 2010.
4. Bauer M, Bschor T, Pfennig A, et al, for the WFSBP Task Force on Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders in Primary Care. World J Biol Psychiatry. 2007;8(2):67-104. http://www.wfsbp.org/treatment-guidelines/unipolar-depressive-disorder.html. Accessed March 3, 2010. doi:10.1080/15622970701227829 PubMed
5. National Institute for Health and Clinical Excellence. Depression: the Treatment and Management of Depression in Adults. www.nice.org.uk/CG90. Published October 28, 2009. Accessed March 3, 2010.
6. Kennedy SH, Lam RW, Parikh SV, et al, for the Canadian Network for Mood and Anxiety Treatments. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults: introduction. J Affect Disord. 2009;117(suppl 1):S1-S2. doi:10.1016/j.jad.2009.06.043 PubMed
7. Motohashi N, Shioe K, Nakamura J, et al. Revised psychopharmacological algorithms for the treatment of mood disorders in Japan. Int J Psychiatry Clin Pract. 2008;12(1):11-18. doi:10.1080/13651500701330791
8. World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: WHO Press; 2008. http://www.who.int/healthinfo/global_burden_disease/en/. Accessed March 3, 2010.
9. Aragonרs E, Piױol JL, Labad A. The overdiagnosis of depression in non-depressed patients in primary care. Fam Pract. 2006;23(3):363-368. doi:10.1093/fampra/cmi120 PubMed
10. Pfizer Incorporated. Patient Health Questionnaire-2 (PHQ-2). http://www.cqaimh.org/pdf/tool_phq2.pdf. Published 1999. Accessed March 3, 2010.
11. Gelenberg AJ. Using assessment tools to screen for, diagnose, and treat major depressive disorder in clinical practice. J Clin Psychiatry. 2010;71(suppl E1):e01.
12. Pfizer Incorporated. Patient Health Questionnaire (PHQ-9). http://www.cqaimh.org/pdf/tool_phq9.pdf. Published 2005. Accessed March 3, 2010.
13. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi:10.1111/j.1600-0447.1983.tb09716.x PubMed
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
15. The World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva, Switzerland: The World Health Organization; 2007. http://www.who.int/classifications/icd/en/. Accessed March 3, 2010.
16. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. doi:10.1001/archpsyc.59.6.530 PubMed
17. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269. doi:10.1001/archpsyc.60.3.261 PubMed
18. Lin EH, Von Korff M, Katon W, et al. The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care. 1995;33(1):67-74. doi:10.1097/00005650-199501000-00006 PubMed
19. Byrne N, Regan C, Livingston G. Adherence to treatment in mood disorders. Curr Opin Psychiatry. 2006;19(1):44-49. doi:10.1097/01.yco.0000191501.54034.7c PubMed
20. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1996;64(3):532-539. doi:10.1037/0022-006X.64.3.532 PubMed
21. Marks I, Cavanagh K. Computer-aided psychological treatments: evolving issues. Annu Rev Clin Psychol. 2009;5(1):121-141. doi:10.1146/annurev.clinpsy.032408.153538 PubMed
22. Donker T, van Straten A, Riper H, et al. Implementation of Internet-based preventive interventions for depression and anxiety: role of support? The design of a randomized controlled trial. Trials. 2009;10(1):59. doi:10.1186/1745-6215-10-59 PubMed
23. Nutt DJ. Rationale for, barriers to, and appropriate medication for the long-term treatment of depression. J Clin Psychiatry. 2010;71(suppl E1):e02.
24. Papakostas GI. The effiacy, tolerability, and safety of contemporary antidepressants. J Clin Psychiatry. 2010;71(suppl E1):e03.
25. Milnacipran [package insert]. New York, NY: Forest Pharmaceuticals, Inc; 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022256s001lbl.pdf. Accessed March 3, 2010.
26. Clomipramine [package insert]. Hazelwood, MO: Mallinckrodt, Inc; 2007. http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019906s34lbl.pdf. Accessed March 3, 2010.
27. Lam RW, Kennedy SH, Grigoriadis S, et al, for the Canadian Network for Mood and Anxiety Treatments (CANMAT). Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults, III: pharmacotherapy. J Affect Disord. 2009;117(suppl 1):S26-S43. doi:10.1016/j.jad.2009.06.041 PubMed
28. Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976.
29. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. doi:10.1136/jnnp.23.1.56 PubMed
30. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. doi:10.1016/S0006-3223(02)01866-8 PubMed